Opisthorchis viverrini—Current Understanding of the Neglected Hepatobiliary Parasite
Abstract
1. Introduction
2. Methods
3. Epidemiology
4. Morphology
5. Life Cycle
6. Omics
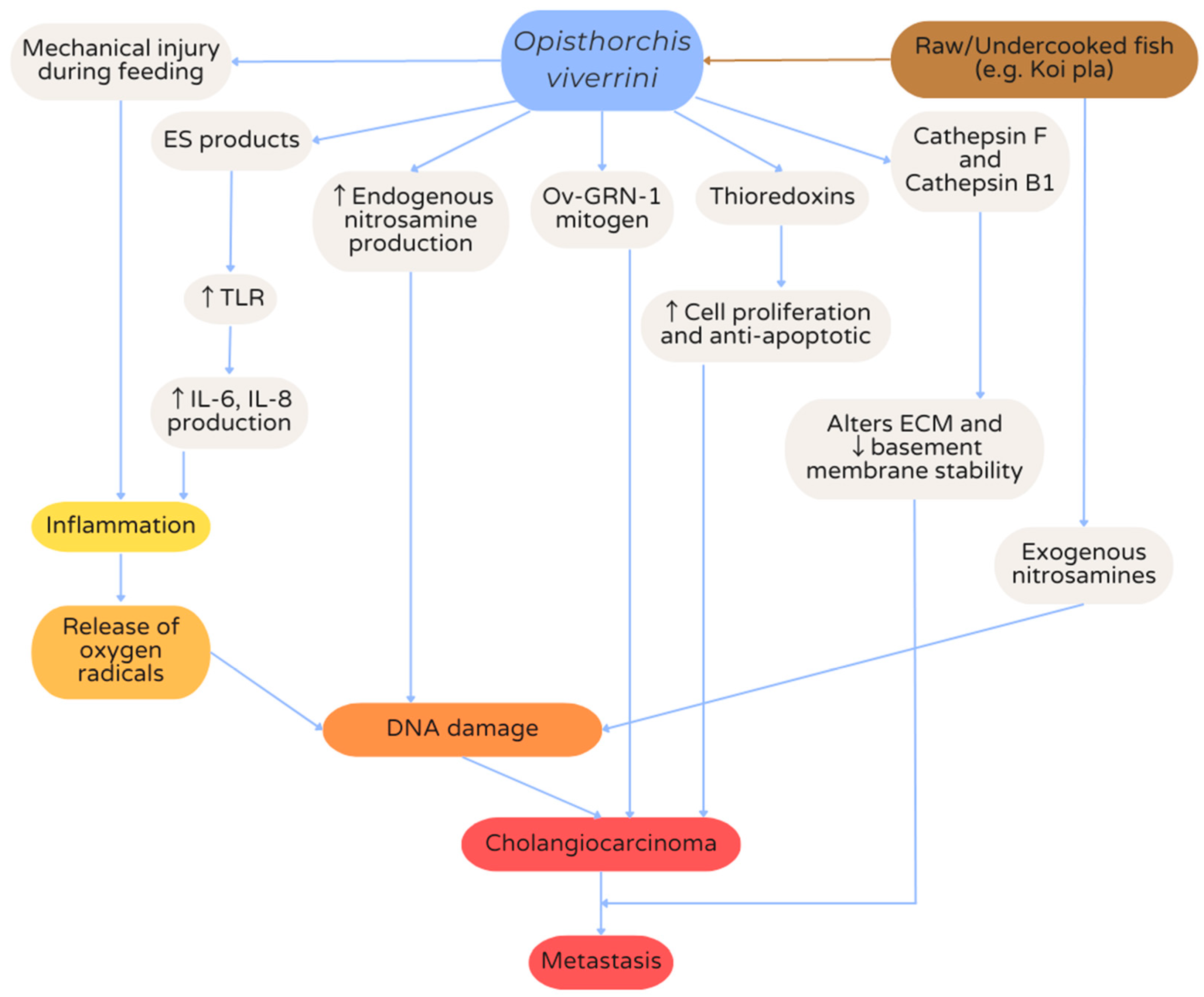
7. Hepatobiliary Opisthorchiasis
8. Diagnosis
8.1. Serological Diagnosis
8.2. Antigenic Diagnosis
8.3. Molecular Diagnosis
8.4. Imaging Modalities
9. Treatment
9.1. Eradication of Parasites
9.2. Treatment of Co-Infection (Helicobacter pylori)
9.3. Treatment of Symptoms
9.4. Treatment of Opisthorchis viverrini-Associated Cholangiocarcinoma
9.4.1. Surgical Resection
9.4.2. Liver Transplantation
9.4.3. Locoregional Therapy
9.4.4. Systemic Therapy
10. Prevention
10.1. Lawa Model
10.2. Vaccines
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaewkes, S. Taxonomy and biology of liver flukes. Acta Trop. 2003, 88, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.H.; Sithithaworn, P.; Petney, T.N. Opisthorchis viverrini: An underestimated parasite in world health. Trends Parasitol. 2008, 24, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Sayasone, S.; Rasphone, O.; Vanmany, M.; Vounatsou, P.; Utzinger, J.; Tanner, M.; Akkhavong, K.; Hatz, C.; Odermatt, P. Severe morbidity due to Opisthorchis viverrini and Schistosoma mekongi infection in Lao People’s Democratic Republic. Clin. Infect. Dis. 2012, 55, e54–e57. [Google Scholar] [CrossRef] [PubMed]
- Khuntikeo, N.; Loilome, W.; Thinkhamrop, B.; Chamadol, N.; Yongvanit, P. A comprehensive public health conceptual framework and strategy to effectively combat cholangiocarcinoma in Thailand. PLoS Negl. Trop. Dis. 2016, 10, e0004293. [Google Scholar] [CrossRef]
- Torgerson, P.R.; Devleesschauwer, B.; Praet, N.; Speybroeck, N.; Willingham, A.L.; Kasuga, F.; Rokni, M.B.; Zhou, X.-N.; Fèvre, E.M.; Sripa, B. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: A data synthesis. PLoS Med. 2015, 12, e1001920. [Google Scholar] [CrossRef]
- Young, N.D.; Nagarajan, N.; Lin, S.J.; Korhonen, P.K.; Jex, A.R.; Hall, R.S.; Safavi-Hemami, H.; Kaewkong, W.; Bertrand, D.; Gao, S. The Opisthorchis viverrini genome provides insights into life in the bile duct. Nat. Commun. 2014, 5, 4378. [Google Scholar] [CrossRef]
- Muller, R.; Wakelin, D. Worms and Human Disease; CABi: Wallingford, UK, 2002. [Google Scholar]
- Khuntikeo, N.; Thinkhamrop, B.; Bundhamcharoen, K.; Andrews, R.H.; Grundy-Warr, C.; Yongvanit, P.; Loilome, W.; Chamadol, N.; Kosuwan, W.; Sithithaworn, P. The socioeconomic burden of cholangiocarcinoma associated with Opisthorchis viverrini sensu lato infection in Northeast Thailand: A preliminary analysis. Adv. Parasitol. 2018, 102, 141–163. [Google Scholar]
- Hong, S.-T. Clonorchis sinensis. In International Handbook of Foodborne Pathogens; CRC Press: Boca Raton, FL, USA, 2003; pp. 601–612. [Google Scholar]
- Pakharukova, M.Y.; Mordvinov, V.A. The liver fluke Opisthorchis felineus: Biology, epidemiology and carcinogenic potential. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 28–36. [Google Scholar] [CrossRef]
- Pakharukova, M.Y.; Mordvinov, V.A. Similarities and differences among the Opisthorchiidae liver flukes: Insights from Opisthorchis felineus. Parasitology 2022, 149, 1306–1318. [Google Scholar] [CrossRef]
- Pakharukova, M.Y.; Zaparina, O.G.; Kapushchak, Y.K.; Baginskaya, N.V.; Mordvinov, V.A. Opisthorchis felineus infection provokes time-dependent accumulation of oxidative hepatobiliary lesions in the injured hamster liver. PLoS ONE 2019, 14, e0216757. [Google Scholar] [CrossRef]
- Pakharukova, M.Y.; da Costa, J.M.C.; Mordvinov, V.A. The liver fluke Opisthorchis felineus as a group III or group I carcinogen. 4open 2019, 2, 23. [Google Scholar] [CrossRef]
- Fedorova, O.S.; Kovshirina, A.E.; Kovshirina, Y.V.; Hattendorf, J.; Onishchenko, S.V.; Katanakhova, L.L.; Taslicki, S.S.; Chizhikov, A.V.; Tataurov, I.A.; Vtorushin, S.V. Opisthorchis felineus infection is a risk factor for cholangiocarcinoma in Western Siberia: A hospital-based case-control study. Clin. Infect. Dis. 2022, 76, e1392–e1398. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Bethony, J.M.; Sithithaworn, P.; Kaewkes, S.; Mairiang, E.; Loukas, A.; Mulvenna, J.; Laha, T.; Hotez, P.J.; Brindley, P.J. Opisthorchiasis and Opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop. 2011, 120, S158–S168. [Google Scholar] [CrossRef] [PubMed]
- Gundamaraju, R.; Vemuri, R.C. Opisthorchis viverrini (liver fluke) as the lot of baleful parasite of tropical region-A replete synopsis. Asian Pac. J. Trop. Dis. 2014, 4, 61–66. [Google Scholar] [CrossRef]
- Suwannatrai, A.; Saichua, P.; Haswell, M. Epidemiology of Opisthorchis viverrini infection. Adv. Parasitol. 2018, 101, 41–67. [Google Scholar] [PubMed]
- Pengput, A.; Schwartz, D.G. Risk factors for Opisthorchis viverrini infection: A systematic review. J. Infect. Public Health 2020, 13, 1265–1273. [Google Scholar] [CrossRef]
- Grundy-Warr, C.; Andrews, R.H.; Sithithaworn, P.; Petney, T.N.; Sripa, B.; Laithavewat, L.; Ziegler, A.D. Raw attitudes, wetland cultures, life-cycles: Socio-cultural dynamics relating to Opisthorchis viverrini in the Mekong Basin. Parasitol. Int. 2012, 61, 65–70. [Google Scholar] [CrossRef]
- Xayaseng, V.; Phongluxa, K.; van Eeuwijk, P.; Akkhavong, K.; Odermatt, P. Raw fish consumption in liver fluke endemic areas in rural southern Laos. Acta Trop. 2013, 127, 105–111. [Google Scholar] [CrossRef]
- Dao, T.T.H.; Van Bui, T.; Abatih, E.N.; Gabriël, S.; Nguyen, T.T.G.; Huynh, Q.H.; Van Nguyen, C.; Dorny, P. Opisthorchis viverrini infections and associated risk factors in a lowland area of Binh Dinh Province, Central Vietnam. Acta Trop. 2016, 157, 151–157. [Google Scholar] [CrossRef]
- Jongsuksuntigul, P.; Imsomboon, T. Opisthorchiasis control in Thailand. Acta Trop. 2003, 88, 229–232. [Google Scholar] [CrossRef]
- Sithithaworn, P.; Andrews, R.H.; Van De, N.; Wongsaroj, T.; Sinuon, M.; Odermatt, P.; Nawa, Y.; Liang, S.; Brindley, P.J.; Sripa, B. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol. Int. 2012, 61, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Srithai, C.; Chuangchaiya, S.; Jaichuang, S.; Idris, Z.M. Prevalence of Opisthorchis viverrini and its associated risk factors in the Phon Sawan District of Nakhon Phanom province, Thailand. Iran. J. Parasitol. 2021, 16, 474. [Google Scholar] [PubMed]
- Saiyachak, K.; Tongsotsang, S.; Saenrueang, T.; Moore, M.A.; Promthet, S. Prevalence and factors associated with Opisthorchis viverrini infection in Khammouane province, Lao PDR. Asian Pac. J. Cancer Prev. 2016, 17, 1589–1593. [Google Scholar] [CrossRef]
- Miyamoto, K.; Kirinoki, M.; Matsuda, H.; Hayashi, N.; Chigusa, Y.; Sinuon, M.; Chuor, C.M.; Kitikoon, V. Field survey focused on Opisthorchis viverrini infection in five provinces of Cambodia. Parasitol. Int. 2014, 63, 366–373. [Google Scholar] [CrossRef]
- Mungmunpuntipantip, R.; Wiwanitkit, V. Relationship Between Prevalence of Opisthorchiasis and Incidence of COVID-19: An Observation. Turk. Parazitol. Derg 2021, 45, 230. [Google Scholar] [CrossRef] [PubMed]
- CDC—Opisthorchis—Biology. Available online: https://www.cdc.gov/parasites/opisthorchis/biology.html (accessed on 4 May 2023).
- Sanpool, O.; Aung, W.P.P.; Rodpai, R.; Maleewong, W.; Intapan, P.M. Human liver fluke Opisthorchis viverrini (Trematoda, Opisthorchiidae) in Central Myanmar: New records of adults and metacercariae identified by morphology and molecular analysis. Acta Trop. 2018, 185, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Harinasuta, C.; Harinasuta, T. Opisthorchis viverrini: Life cycle, intermediate hosts, transmission to man and geographical distribution in Thailand. Arzneim. Forsch. 1984, 34, 1164–1167. [Google Scholar]
- Hoole, D.; Bucke, D.; Burgess, P.; Wellby, I. Diseases of Carp and Other Cyprinid Fishes; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Smout, M.J.; Laha, T.; Mulvenna, J.; Sripa, B.; Suttiprapa, S.; Jones, A.; Brindley, P.J.; Loukas, A. A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini, promotes proliferation of host cells. PLoS Pathog. 2009, 5, e1000611. [Google Scholar] [CrossRef] [PubMed]
- Suttiprapa, S.; Sotillo, J.; Smout, M.; Suyapoh, W.; Chaiyadet, S.; Tripathi, T.; Laha, T.; Loukas, A. Opisthorchis viverrini proteome and host–parasite interactions. Adv. Parasitol. 2018, 102, 45–72. [Google Scholar]
- Sharma, R.; Yang, Y.; Sharma, A.; Awasthi, S.; Awasthi, Y.C. Antioxidant role of glutathione S-transferases: Protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid. Redox Signal. 2004, 6, 289–300. [Google Scholar] [CrossRef]
- Tan, W.B.; Shelat, V.G.; Diddapur, R.K. Oriental liver fluke infestation presenting more than 50 years after immigration. Ann. Acad. Med. Singap. 2009, 38, 735–736. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Jumnainsong, A.; Tangkawattana, S.; Haswell, M.R. Immune response to Opisthorchis viverrini infection and its role in pathology. Adv. Parasitol. 2018, 102, 73–95. [Google Scholar] [PubMed]
- Sripa, B.; Kanla, P.; Sinawat, P.; Haswell-Elkins, M.R. Opisthorchiasis-associated biliary stones: Light and scanning electron microscopic study. World J. Gastroenterol. WJG 2004, 10, 3318. [Google Scholar] [CrossRef] [PubMed]
- Young, N.D.; Gasser, R.B. Opisthorchis viverrini draft genome–Biomedical implications and future avenues. Adv. Parasitol. 2018, 101, 125–148. [Google Scholar]
- Sripa, B.; Thinkhamrop, B.; Mairiang, E.; Laha, T.; Kaewkes, S.; Sithithaworn, P.; Periago, M.V.; Bhudhisawasdi, V.; Yonglitthipagon, P.; Mulvenna, J. Elevated plasma IL-6 associates with increased risk of advanced fibrosis and cholangiocarcinoma in individuals infected by Opisthorchis viverrini. PLoS Negl. Trop. Dis. 2012, 6, e1654. [Google Scholar] [CrossRef]
- Kwan, K.E.L.; Shelat, V.G.; Tan, C.H. Recurrent pyogenic cholangitis: A review of imaging findings and clinical management. Abdom. Radiol. 2017, 42, 46–56. [Google Scholar] [CrossRef]
- Ninlawan, K.; O’Hara, S.P.; Splinter, P.L.; Yongvanit, P.; Kaewkes, S.; Surapaitoon, A.; LaRusso, N.F.; Sripa, B. Opisthorchis viverrini excretory/secretory products induce toll-like receptor 4 upregulation and production of interleukin 6 and 8 in cholangiocyte. Parasitol. Int. 2010, 59, 616–621. [Google Scholar] [CrossRef][Green Version]
- Sripa, B.; Kaewkes, S. Localisation of parasite antigens and inflammatory responses in experimental opisthorchiasis. Int. J. Parasitol. 2000, 30, 735–740. [Google Scholar] [CrossRef]
- Jittimanee, S.; Wongratanacheewin, S.; Kaewraemruaen, C.; Jittimanee, J. Opisthorchis viverrini antigens up-regulates the expression of CD80 and MHC class II in JAWSII mouse dendritic cells and promotes IL-10 and TGF-β secretions. Parasitol. Int. 2021, 84, 102401. [Google Scholar] [CrossRef]
- Mahmood, D.F.D.; Abderrazak, A.; El Hadri, K.; Simmet, T.; Rouis, M. The thioredoxin system as a therapeutic target in human health and disease. Antioxid. Redox Signal. 2013, 19, 1266–1303. [Google Scholar] [CrossRef]
- Miwa, M.; Honjo, S.; You, G.; Tanaka, M.; Uchida, K.; Srivatanakul, P.; Khuhaprema, T.; Loilome, W.; Techasen, A.; Wongkham, C. Genetic and environmental determinants of risk for cholangiocarcinoma in Thailand. World J. Gastrointest. Pathophysiol. 2014, 5, 570. [Google Scholar] [CrossRef] [PubMed]
- Thamavit, W.; Bhamarapravati, N.; Sahaphong, S.; Vajrasthira, S.; Angsubhakorn, S. Effects of dimethylnitrosamine on induction of cholagiocarcinoma in Opisthorchis viverrini-infected Syrian golden hamsters. Cancer Res. 1978, 38, 4634–4639. [Google Scholar] [PubMed]
- Farazi, P.A.; Zeisberg, M.; Glickman, J.; Zhang, Y.; Kalluri, R.; DePinho, R.A. Chronic bile duct injury associated with fibrotic matrix microenvironment provokes cholangiocarcinoma in p53-deficient mice. Cancer Res. 2006, 66, 6622–6627. [Google Scholar] [CrossRef]
- Sithithaworn, P.; Tesana, S.; Pipitgool, V.; Kaewkes, S.; Pairojkul, C.; Sripa, B.; Paupairoj, A.; Thaiklar, K. Relationship between faecal egg count and worm burden of Opisthorchis viverrini in human autopsy cases. Parasitology 1991, 102, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Phupiewkham, W.; Sadaow, L.; Sanpool, O.; Rodpai, R.; Yamasaki, H.; Ittiprasert, W.; Mann, V.H.; Brindley, P.J.; Maleewong, W.; Intapan, P.M. Comparative assessment of immunochromatographic test kits using somatic antigens from adult Opisthorchis viverrini and IgG and IgG4 conjugates for serodiagnosis of human opisthorchiasis. Parasitol. Res. 2021, 120, 2839–2846. [Google Scholar] [CrossRef]
- Sadaow, L.; Sanpool, O.; Rodpai, R.; Yamasaki, H.; Ittiprasert, W.; Mann, V.H.; Brindley, P.J.; Maleewong, W.; Intapan, P.M. Development of an immunochromatographic point-of-care test for serodiagnosis of opisthorchiasis and clonorchiasis. Am. J. Trop. Med. Hyg. 2019, 101, 1156. [Google Scholar] [CrossRef] [PubMed]
- Taron, W.; Phooplub, K.; Sanchimplee, S.; Piyanamvanich, K.; Jamnongkan, W.; Techasen, A.; Phetcharaburanin, J.; Klanrit, P.; Namwat, N.; Khuntikeo, N. Smartphone-based fluorescent ELISA with simple fluorescent enhancement strategy for Opisthorchis viverrini (Ov) antigen detection in urine samples. Sens. Actuators B Chem. 2021, 348, 130705. [Google Scholar] [CrossRef]
- Taron, W.; Jamnongkan, W.; Techasen, A.; Phetcharaburanin, J.; Namwat, N.; Sithithaworn, P.; Khuntikeo, N.; Mukdasai, S.; Sayasone, S.; Loilome, W. AuNPs-LISA, an efficient detection assay for Opisthorchis viverrini (Ov) antigen in urine. Talanta 2020, 209, 120592. [Google Scholar] [CrossRef]
- Phadungsil, W.; Pumpa, S.; Sirisabhabhorn, K.; Geadkaew-Krenc, A.; Grams, R.; Mungthin, M.; Ruang-Areerate, T.; Adisakwattana, P.; Labbunruang, N.; Martviset, P. Efficiency of the Stool-PCR Test Targeting NADH Dehydrogenase (Nad) Subunits for Detection of Opisthorchis viverrini Eggs. J. Trop. Med. 2021, 2021, 3957545. [Google Scholar] [CrossRef]
- Pumpa, S.; Phadungsil, W.; Grams, R.; Martviset, P.; Ruang-Areerate, T.; Mungthin, M.; Geadkaew-Krenc, A. Improvement of a PCR-based method for the detection of Opisthorchis viverrini eggs in human stool samples by targeting internal transcribed spacer-2 (ITS-2), cytochrome oxidase subunit 1 (cox1), and cytochrome b (cyb). J. Parasit. Dis. 2021, 45, 474–478. [Google Scholar] [CrossRef]
- Worasith, C.; Wangboon, C.; Duenngai, K.; Kiatsopit, N.; Kopolrat, K.; Techasen, A.; Sithithaworn, J.; Khuntikeo, N.; Loilome, W.; Namwat, N. Comparing the performance of urine and copro-antigen detection in evaluating Opisthorchis viverrini infection in communities with different transmission levels in Northeast Thailand. PLoS Negl. Trop. Dis. 2019, 13, e0007186. [Google Scholar] [CrossRef] [PubMed]
- Worasith, C.; Wangboon, C.; Kopolrat, K.Y.; Homwong, C.; Sithithaworn, J.; Techasen, A.; Thanan, R.; Khuntikeo, N.; Sithithaworn, P. Application of urine antigen assay to evaluate outcomes of praziquantel treatment and reinfection in opisthorchiasis in northeast Thailand. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Mairiang, E.; Mairiang, P. Clinical manifestation of opisthorchiasis and treatment. Acta Trop. 2003, 88, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Damrongsak, D.; Damrongsak, C.; Bhothisuwan, W.; Chancharoensin, C.; Kruatrachue, C.; Prabhasawat, D. Computed tomography in opisthorchiasis. Comput. Radiol. 1984, 8, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Hanpanich, P.; Pinlaor, S.; Charoensuk, L.; Yongvanit, P.; Thomas, C.; Kothan, S.; Mairiang, E. MRI and 1H MRS evaluation for the serial bile duct changes in hamsters after infection with Opisthorchis viverrini. Magn. Reson. Imaging 2013, 31, 1418–1425. [Google Scholar] [CrossRef]
- Siripongsakun, S.; Sapthanakorn, W.; Mekraksakit, P.; Vichitpunt, S.; Chonyuen, S.; Seetasarn, J.; Bhumiwat, S.; Sricharunrat, T.; Srittanapong, S. Premalignant lesions of cholangiocarcinoma: Characteristics on ultrasonography and MRI. Abdom. Radiol. 2019, 44, 2133–2146. [Google Scholar] [CrossRef]
- Jhaveri, K.S.; Hosseini-Nik, H. MRI of cholangiocarcinoma. J. Magn. Reson. Imaging 2015, 42, 1165–1179. [Google Scholar] [CrossRef]
- Lim, J.H.; Mairiang, E.; Ahn, G.H. Biliary parasitic diseases including clonorchiasis, opisthorchiasis and fascioliasis. Abdom. Imaging 2008, 33, 157–165. [Google Scholar] [CrossRef]
- Foodborne Trematode Infections. Available online: https://www.who.int/news-room/fact-sheets/detail/foodborne-trematode-infections (accessed on 4 May 2023).
- Soukhathammavong, P.; Odermatt, P.; Sayasone, S.; Vonghachack, Y.; Vounatsou, P.; Hatz, C.; Akkhavong, K.; Keiser, J. Efficacy and safety of mefloquine, artesunate, mefloquine–artesunate, tribendimidine, and praziquantel in patients with Opisthorchis viverrini: A randomised, exploratory, open-label, phase 2 trial. Lancet Infect. Dis. 2011, 11, 110–118. [Google Scholar] [CrossRef]
- Sayasone, S.; Keiser, J.; Meister, I.; Vonghachack, Y.; Xayavong, S.; Senggnam, K.; Phongluxa, K.; Hattendorf, J.; Odermatt, P. Efficacy and safety of tribendimidine versus praziquantel against Opisthorchis viverrini in Laos: An open-label, randomised, non-inferiority, phase 2 trial. Lancet Infect. Dis. 2018, 18, 155–161. [Google Scholar] [CrossRef]
- Meister, I.; Assawasuwannakit, P.; Vanobberghen, F.; Penny, M.A.; Odermatt, P.; Sayasone, S.; Huwyler, J.; Tarning, J.; Keiser, J. Pooled population pharmacokinetic analysis of tribendimidine for the treatment of Opisthorchis viverrini infections. Antimicrob. Agents Chemother. 2019, 63, e01391-18. [Google Scholar] [CrossRef] [PubMed]
- Jala, I.; Almanfaluthi, M.L.; Laha, T.; Kanthawong, S.; Tangkawattana, S.; Saichua, P.; Suttiprapa, S.; Sripa, B. Helicobacter pylori GroEL Seropositivity Is Associated with an Increased Risk of Opisthorchis viverrini-Associated Hepatobiliary Abnormalities and Cholangiocarcinoma. Korean J. Parasitol. 2021, 59, 363. [Google Scholar] [CrossRef] [PubMed]
- Phung, H.T.T.; Deenonpoe, R.; Suttiprapa, S.; Mairiang, E.; Edwards, S.W.; Sripa, B. Persistent advanced periductal fibrosis is associated with cagA-positive Helicobacter pylori infection in post-praziquantel treatment of opisthorchiasis. Helicobacter 2022, 27, e12897. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Haswell, M.R. Mast cell hyperplasia in Opisthorchis viverrini-associated cholecystitis. Parasitol. Res. 2021, 120, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, J.R.; Charles, A. Acute cholecystitis: A review. JAMA 2022, 327, 965–975. [Google Scholar] [CrossRef]
- Miura, F.; Takada, T.; Kawarada, Y.; Nimura, Y.; Wada, K.; Hirota, M.; Nagino, M.; Tsuyuguchi, T.; Mayumi, T.; Yoshida, M. Flowcharts for the diagnosis and treatment of acute cholangitis and cholecystitis: Tokyo Guidelines. J. Hepato-Biliary-Pancreat. Surg. 2007, 14, 27–34. [Google Scholar] [CrossRef]
- Wong, R.K.; Peura, D.A.; Mutter, M.L.; Heit, H.A.; Birnsv, M.T.; Johnson, L.F. Hemobilia and liver flukes in a patient from Thailand. Gastroenterology 1985, 88, 1958–1963. [Google Scholar] [CrossRef]
- Singh Rana, S.; Bhasin, D.K.; Nanda, M.; Singh, K. Parasitic infestations of the biliary tract. Curr. Gastroenterol. Rep. 2007, 9, 156–164. [Google Scholar] [CrossRef]
- Blechacz, B.; Komuta, M.; Roskams, T.; Gores, G.J. Clinical diagnosis and staging of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 512–522. [Google Scholar] [CrossRef]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Prim. 2021, 7, 65. [Google Scholar] [CrossRef]
- Weber, S.M.; Jarnagin, W.R.; Klimstra, D.; DeMatteo, R.P.; Fong, Y.; Blumgart, L.H. Intrahepatic cholangiocarcinoma: Resectability, recurrence pattern, and outcomes. J. Am. Coll. Surg. 2001, 193, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Spolverato, G.; Yakoob, M.Y.; Kim, Y.; Alexandrescu, S.; Marques, H.P.; Lamelas, J.; Aldrighetti, L.; Gamblin, T.C.; Maithel, S.K.; Pulitano, C. The impact of surgical margin status on long-term outcome after resection for intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2015, 22, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Nagino, M.; Ebata, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Takahashi, Y.; Nimura, Y. Evolution of surgical treatment for perihilar cholangiocarcinoma: A single-center 34-year review of 574 consecutive resections. Ann. Surg. 2013, 258, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Ebata, T.; Mizuno, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Nagino, M. Surgical resection for Bismuth type IV perihilar cholangiocarcinoma. J. Br. Surg. 2018, 105, 829–838. [Google Scholar] [CrossRef]
- Aphivatanasiri, C.; Sa-ngaimwibool, P.; Sangkhamanon, S.; Intarawichian, P.; Kunprom, W.; Thanee, M.; Prajumwongs, P.; Loilome, W.; Khuntikeo, N.; Titapun, A. Modification of the 8th AJCC/UICC Staging System for Perihilar Cholangiocarcinoma: An Alternative Pathological Staging System from Cholangiocarcinoma-Prevalent Northeast Thailand. Front. Med. 2022, 2022, 2387. [Google Scholar]
- DeOliveira, M.L.; Cunningham, S.C.; Cameron, J.L.; Kamangar, F.; Winter, J.M.; Lillemoe, K.D.; Choti, M.A.; Yeo, C.J.; Schulick, R.D. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 2007, 245, 755. [Google Scholar] [CrossRef]
- Kunprom, W.; Aphivatanasiri, C.; Sa-Ngiamwibool, P.; Sangkhamanon, S.; Intarawichian, P.; Bamrungkit, W.; Thanee, M.; Prajumwongs, P.; Loilome, W.; Khuntikeo, N. Prognostic significance of growth pattern in predicting outcome of opisthorchis viverrini-associated distal cholangiocarcinoma in Thailand. Front. Public Health 2022, 10, 816028. [Google Scholar] [CrossRef]
- Chua, T.C.; Mittal, A.; Arena, J.; Sheen, A.; Gill, A.J.; Samra, J.S. Resection margin influences survival after pancreatoduodenectomy for distal cholangiocarcinoma. Am. J. Surg. 2017, 213, 1072–1076. [Google Scholar] [CrossRef]
- Kodali, S.; Saharia, A.; Ghobrial, R.M. Liver transplantation and intrahepatic cholangiocarcinoma: Time to go forward again? Curr. Opin. Organ Transplant. 2022, 27, 320–328. [Google Scholar] [CrossRef]
- McMillan, R.R.; Javle, M.; Kodali, S.; Saharia, A.; Mobley, C.; Heyne, K.; Hobeika, M.J.; Lunsford, K.E.; Victor III, D.W.; Shetty, A. Survival following liver transplantation for locally advanced, unresectable intrahepatic cholangiocarcinoma. Am. J. Transplant. 2022, 22, 823–832. [Google Scholar] [CrossRef]
- Gulamhusein, A.F.; Sanchez, W. Liver transplantation in the management of perihilar cholangiocarcinoma. Hepatic Oncol. 2015, 2, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Murad, S.D.; Kim, W.R.; Therneau, T.; Gores, G.J.; Rosen, C.B.; Martenson, J.A.; Alberts, S.R.; Heimbach, J.K. Predictors of pretransplant dropout and posttransplant recurrence in patients with perihilar cholangiocarcinoma. Hepatology 2012, 56, 972–981. [Google Scholar] [CrossRef]
- Mauro, E.; Ferrer-Fàbrega, J.; Sauri, T.; Soler, A.; Cobo, A.; Burrel, M.; Iserte, G.; Forner, A. New Challenges in the Management of Cholangiocarcinoma: The Role of Liver Transplantation, Locoregional Therapies, and Systemic Therapy. Cancers 2023, 15, 1244. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Lamarca, A.; McNamara, M.G.; Jacobs, T.; Hubner, R.A.; Palmer, D.; Koerkamp, B.G.; Johnson, P.; Guiu, B.; Valle, J.W. Locoregional therapies in patients with intrahepatic cholangiocarcinoma: A systematic review and pooled analysis. Cancer Treat. Rev. 2021, 99, 102258. [Google Scholar] [CrossRef] [PubMed]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Kennedy, E.B.; Bachini, M.; Bekaii-Saab, T.; Crane, C.; Edeline, J.; El-Khoueiry, A.; Feng, M.; Katz, M.H.; Primrose, J. Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J. Clin. Oncol. 2019, 37, 1015–1027. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K. ABC-06| A randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC+ mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. J. Clin. Oncol. 2019, 37, 4003. [Google Scholar]
- Loilome, W.; Yongvanit, P.; Wongkham, C.; Tepsiri, N.; Sripa, B.; Sithithaworn, P.; Hanai, S.; Miwa, M. Altered gene expression in Opisthorchis viverrini-associated cholangiocarcinoma in hamster model. Mol. Carcinog. Publ. Coop. Univ. Tex. MD Cancer Cent. 2006, 45, 279–287. [Google Scholar]
- Loilome, W.; Yooyuen, S.; Namwat, N.; Sithithaworn, P.; Puapairoj, A.; Kano, J.; Noguchi, M.; Miwa, M.; Yongvanit, P. PRKAR1A overexpression is associated with increased ECPKA autoantibody in liver fluke-associated cholangiocarcinoma: Application for assessment of the risk group. Tumor Biol. 2012, 33, 2289–2298. [Google Scholar] [CrossRef]
- Techasen, A.; Loilome, W.; Namwat, N.; Takahashi, E.; Sugihara, E.; Puapairoj, A.; Miwa, M.; Saya, H.; Yongvanit, P. Myristoylated alanine-rich C kinase substrate phosphorylation promotes cholangiocarcinoma cell migration and metastasis via the protein kinase C-dependent pathway. Cancer Sci. 2010, 101, 658–665. [Google Scholar] [CrossRef]
- Padthaisong, S.; Thanee, M.; Techasen, A.; Namwat, N.; Yongvanit, P.; Liwatthakun, A.; Hankla, K.; Sangkhamanon, S.; Loilome, W. Nimotuzumab inhibits cholangiocarcinoma cell metastasis via suppression of the epithelial–mesenchymal transition process. Anticancer Res. 2017, 37, 3591–3597. [Google Scholar]
- Dokduang, H.; Juntana, S.; Techasen, A.; Namwat, N.; Yongvanit, P.; Khuntikeo, N.; Riggins, G.J.; Loilome, W. Survey of activated kinase proteins reveals potential targets for cholangiocarcinoma treatment. Tumor Biol. 2013, 34, 3519–3528. [Google Scholar] [CrossRef] [PubMed]
- Padthaisong, S.; Dokduang, H.; Yothaisong, S.; Techasen, A.; Namwat, N.; Yongvanit, P.; Khuntikeo, N.; Titapun, A.; Sangkhamanon, S.; Loilome, W. Inhibitory effect of NVP-BKM120 on cholangiocarcinoma cell growth. Oncol. Lett. 2018, 16, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Loilome, W.; Dokduang, H.; Suksawat, M.; Padthaisong, S. Therapeutic challenges at the preclinical level for targeted drug development for Opisthorchis viverrini-associated cholangiocarcinoma. Expert Opin. Investig. Drugs 2021, 30, 985–1006. [Google Scholar] [CrossRef]
- Tangkawattana, S.; Sripa, B. Integrative EcoHealth/One health approach for sustainable liver fluke control: The Lawa model. Adv. Parasitol. 2018, 102, 115–139. [Google Scholar] [PubMed]
- Sripa, B.; Tangkawattana, S.; Sangnikul, T. The Lawa model: A sustainable, integrated opisthorchiasis control program using the EcoHealth approach in the Lawa Lake region of Thailand. Parasitol. Int. 2017, 66, 346–354. [Google Scholar] [CrossRef]
- Sripa, B.; Tangkawattana, S.; Laha, T.; Kaewkes, S.; Mallory, F.F.; Smith, J.F.; Wilcox, B.A. Toward integrated opisthorchiasis control in northeast Thailand: The Lawa project. Acta Trop. 2015, 141, 361–367. [Google Scholar] [CrossRef]
- Chaiyadet, S.; Sotillo, J.; Krueajampa, W.; Thongsen, S.; Brindley, P.J.; Sripa, B.; Loukas, A.; Laha, T. Vaccination of hamsters with Opisthorchis viverrini extracellular vesicles and vesicle-derived recombinant tetraspanins induces antibodies that block vesicle uptake by cholangiocytes and reduce parasite burden after challenge infection. PLoS Negl. Trop. Dis. 2019, 13, e0007450. [Google Scholar] [CrossRef]
- Phung, L.T.; Chaiyadet, S.; Hongsrichan, N.; Sotillo, J.; Dieu, H.D.T.; Tran, C.Q.; Brindley, P.J.; Loukas, A.; Laha, T. Recombinant Opisthorchis viverrini tetraspanin expressed in Pichia pastoris as a potential vaccine candidate for opisthorchiasis. Parasitol. Res. 2019, 118, 3419–3427. [Google Scholar] [CrossRef]
- Phung, L.T.; Chaiyadet, S.; Hongsrichan, N.; Sotillo, J.; Dieu, H.D.T.; Tran, C.Q.; Brindley, P.J.; Loukas, A.; Laha, T. Partial protection with a chimeric tetraspanin-leucine aminopeptidase subunit vaccine against Opisthorchis viverrini infection in hamsters. Acta Trop. 2020, 204, 105355. [Google Scholar] [CrossRef] [PubMed]

| Study | Target | Results |
|---|---|---|
| Serological Diagnostic Tests | ||
| Phupiewkham 2021 [49] | Somatic antigens of adult Opisthorchis viverrini with IgG and IgG4 conjugates | IgG Sample size: 332 Sensitivity: 86.6% Specificity: 89.5% Positive predictive value: 82.9% Negative predictive value: 91.9% IgG4 Sample size: 332 Sensitivity: 75% Specificity: 98.4% Positive predictive value: 96.6% Negative predictive value: 87% |
| Sadaow 2019 [50] | Excretory–secretory antigen from adult Opisthorchis viverrini | Sample size: 236 Sensitivity: 100% Specificity: 98.3% Positive predictive value: 97.9% Negative predictive value: 100% |
| Antigenic Diagnostic Tests | ||
| Taron 2021 [51] | Portable smartphone-based fluorescent enzyme-linked immunosorbent assay | Sample size: 440 Sensitivity: 84.88% Specificity: 89.66% Positive predictive value: 95.82% Negative predictive value: 67.97% |
| Taron 2020 [52] | Enzyme-linked immunosorbent assay enhanced with gold nanoparticles | Sample size: 390 Sensitivity: 93.81% Specificity: 91.34% Positive predictive value: 81.54% Negative predictive value: 97.31% |
| Molecular Diagnostic Tests | ||
| Phadungsil 2021 [53] | Opisthorchis viverrini NADH dehydrogenase subunits (OvNad1, OvNad2, OvNad4 and OvNad5) | Sample size: 75 OvNad1 sensitivity: 64.00% OvNad2 sensitivity: 88.00% OvNad4 sensitivity: 80.00% OvNad5 sensitivity: 100.00% |
| Pumpa 2021 [54] | Internal transcribed spacer-2 (ITS-2), cytochrome oxidase subunit 1 (cox1), and cytochrome b (cyb) | Sample size: 26 ITS-2 sensitivity: 76.9% cox1 sensitivity: 96.2% cyb sensitivity: 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liau, M.Y.Q.; Toh, E.Q.; Shelat, V.G. Opisthorchis viverrini—Current Understanding of the Neglected Hepatobiliary Parasite. Pathogens 2023, 12, 795. https://doi.org/10.3390/pathogens12060795
Liau MYQ, Toh EQ, Shelat VG. Opisthorchis viverrini—Current Understanding of the Neglected Hepatobiliary Parasite. Pathogens. 2023; 12(6):795. https://doi.org/10.3390/pathogens12060795
Chicago/Turabian StyleLiau, Matthias Yi Quan, En Qi Toh, and Vishalkumar Girishchandra Shelat. 2023. "Opisthorchis viverrini—Current Understanding of the Neglected Hepatobiliary Parasite" Pathogens 12, no. 6: 795. https://doi.org/10.3390/pathogens12060795
APA StyleLiau, M. Y. Q., Toh, E. Q., & Shelat, V. G. (2023). Opisthorchis viverrini—Current Understanding of the Neglected Hepatobiliary Parasite. Pathogens, 12(6), 795. https://doi.org/10.3390/pathogens12060795







