Effect of TB Treatment on Neutrophil-Derived Soluble Inflammatory Mediators in TB Patients with and without HIV Coinfection
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Assessment of Lung Function and Damage
2.3. Luminex Assay
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Study Population
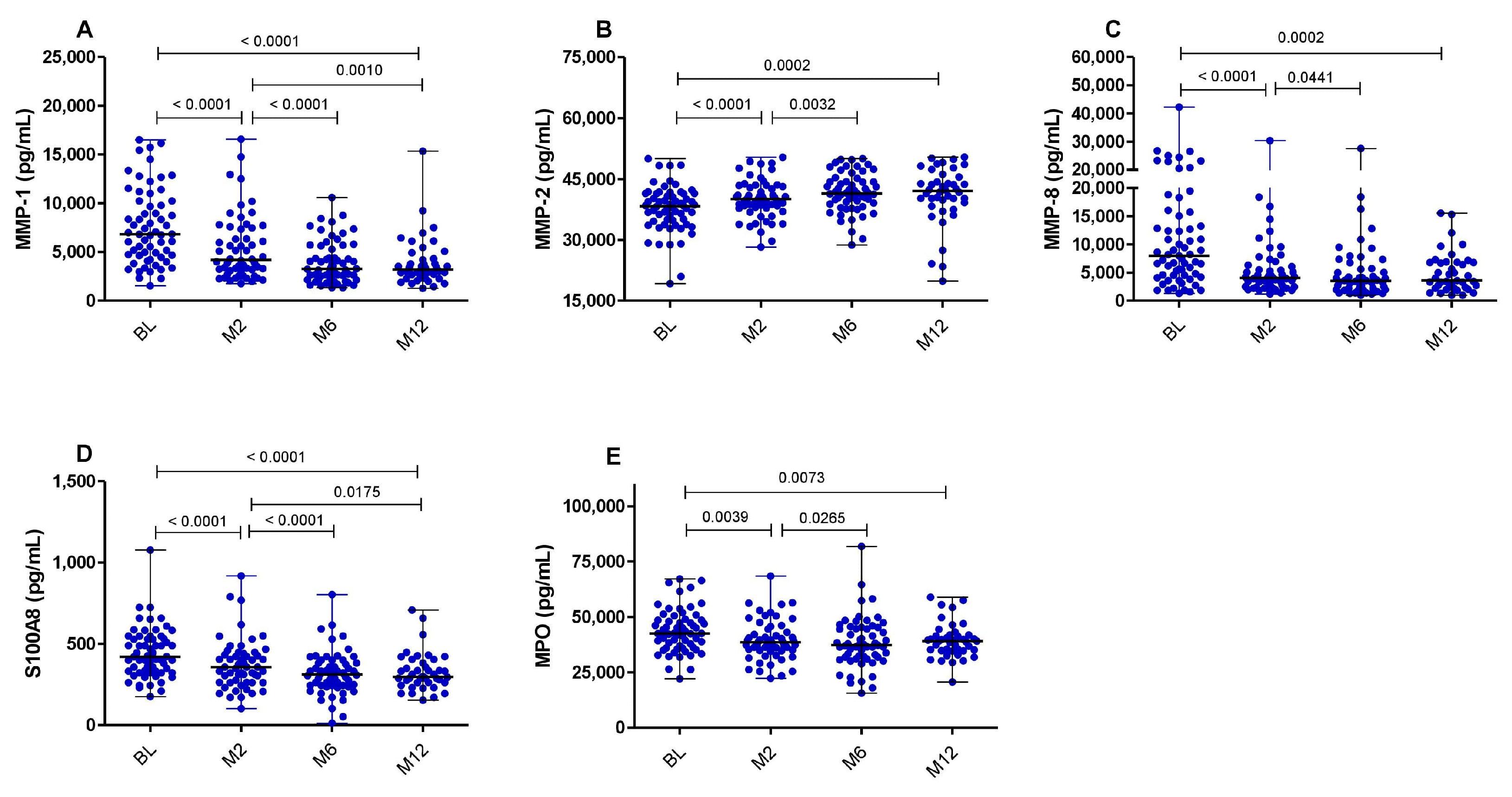
3.2. MMP-2 Plasma Levels Strongly Correlate with Neutrophils and Ralph Score, and Increased after TB Treatment Initiation
3.3. ART-Naïve Patients Living with HIV Coinfected with TB Exhibit Markedly Elevated Levels of MMP-8 and S100A8
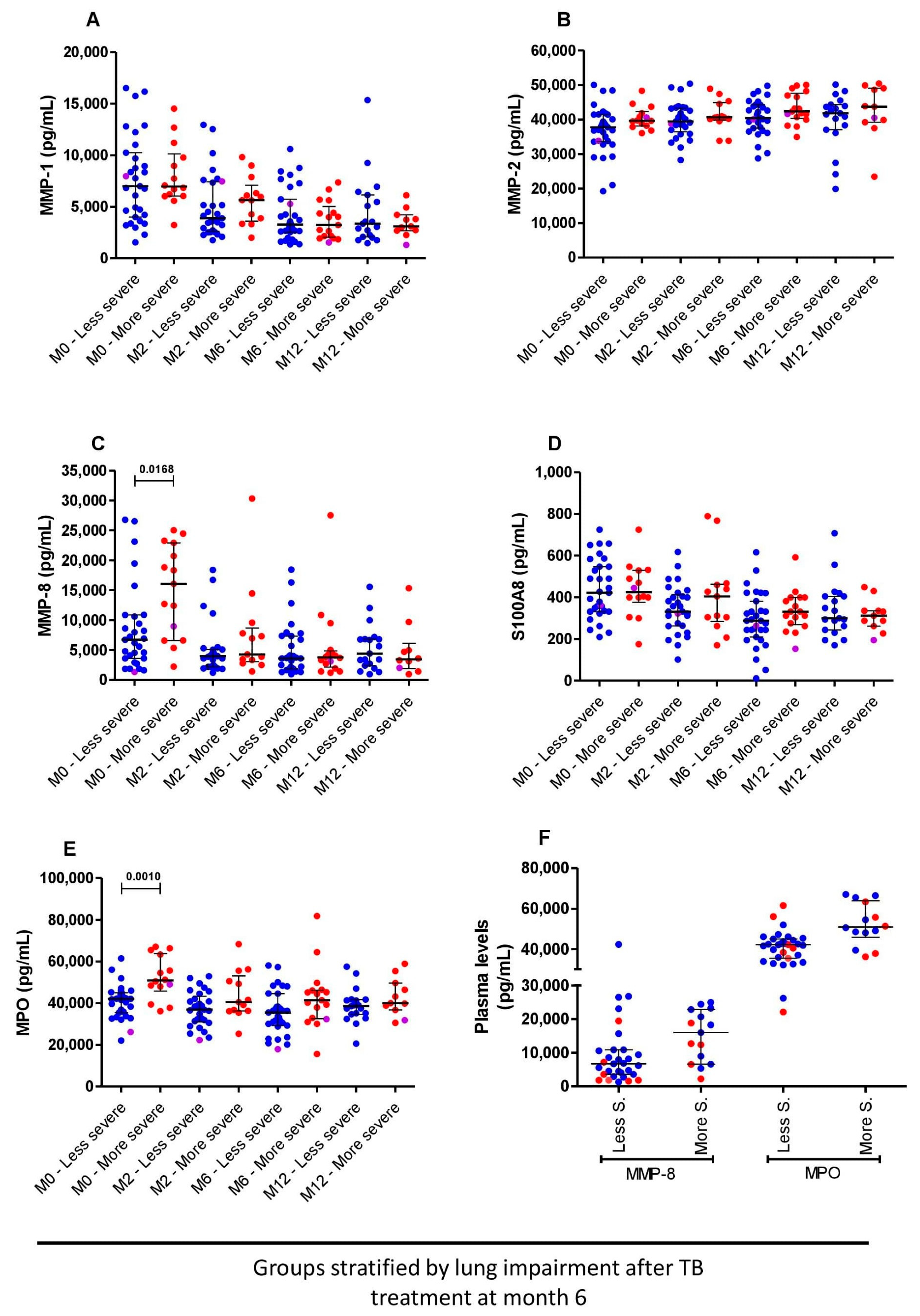
3.4. Higher Levels of MMP-8 and MPO before Treatment Initiation Are Linked to More Severe Lung Impairment at the End of TB Treatment Initiation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Kwan, C.K.; Ernst, J.D. HIV and Tuberculosis: A Deadly Human Syndemic. Clin. Microbiol. Rev. 2011, 24, 351–376. [Google Scholar] [CrossRef]
- Bruchfeld, J.; Correia-Neves, M.; Källenius, G. Tuberculosis and HIV Coinfection. Cold Spring Harb. Perspect. Med. 2015, 5, a017871. [Google Scholar] [CrossRef] [PubMed]
- Pillay, K.; Lewis, L.; Rambaran, S.; Yende-Zuma, N.; Archary, D.; Gengiah, S.; Govender, D.; Hassan-Moosa, R.; Samsunder, N.; Abdool Karim, S.S.; et al. Plasma Biomarkers of Risk of Tuberculosis Recurrence in HIV Co-Infected Patients from South Africa. Front. Immunol. 2021, 12, 631094. [Google Scholar] [CrossRef] [PubMed]
- Sigal, G.B.; Segal, M.R.; Mathew, A.; Jarlsberg, L.; Wang, M.; Barbero, S.; Small, N.; Haynesworth, K.; Davis, J.L.; Weiner, M.; et al. Biomarkers of Tuberculosis Severity and Treatment Effect: A Directed Screen of 70 Host Markers in a Randomized Clinical Trial. EBioMedicine 2017, 25, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Singh, P. Diagnosis of TB From Conventional to Modern Molecular Protocols. Front. Biosci. 2019, 11, 38–60. [Google Scholar] [CrossRef]
- Al-Zamel, F.A. Detection and Diagnosis of Mycobacterium tuberculosis. Expert Rev. Anti-Infect. Ther. 2009, 7, 1099–1108. [Google Scholar] [CrossRef]
- Heemskerk, D.; Caws, M.; Marais, B.; Farrar, J. Tuberculosis in Adults and Children. In SpringerBriefs in Public Health; Springer International Publishing: Cham, Germany, 2015; Volume 2, ISBN 978-3-319-19131-7. [Google Scholar]
- Pai, M.; Nicol, M.P.; Boehme, C.C. Tuberculosis Diagnostics: State of the Art and Future Directions. Microbiol. Spectr. 2016, 4, 16. [Google Scholar] [CrossRef]
- Saeed, M.; Hussain, S.; Riaz, S.; Rasheed, F.; Ahmad, M.; Iram, S.; Arif, M.; Rahmani, T.H.; Anwar, A.I. GeneXpert Technology for the Diagnosis of HIV-Associated Tuberculosis: Is Scale-up Worth It? Open Life Sci. 2020, 15, 458–465. [Google Scholar] [CrossRef]
- Esmail, A.; Tomasicchio, M.; Meldau, R.; Makambwa, E.; Dheda, K. Comparison of Xpert MTB/RIF (G4) and Xpert Ultra, Including Trace Readouts, for the Diagnosis of Pulmonary Tuberculosis in a TB and HIV Endemic Setting. Int. J. Infect. Dis. 2020, 95, 246–252. [Google Scholar] [CrossRef]
- Holmes, K.K.; Bertozzi, S.; Bloom, B.R.; Jha, P. (Eds.) Disease Control Priorities, Third Edition (Volume 6): Major Infectious Diseases; The World Bank: Washington, DC, USA, 2017; ISBN 978-1-4648-0524-0. [Google Scholar]
- Horne, D.J.; Kohli, M.; Zifodya, J.S.; Schiller, I.; Dendukuri, N.; Tollefson, D.; Schumacher, S.G.; Ochodo, E.A.; Pai, M.; Steingart, K.R. Xpert MTB/RIF and Xpert MTB/RIF Ultra for Pulmonary Tuberculosis and Rifampicin Resistance in Adults. Cochrane Database Syst. Rev. 2019, 6, CD009593. [Google Scholar] [CrossRef]
- Walzl, G.; Ronacher, K.; Hanekom, W.; Scriba, T.J.; Zumla, A. Immunological Biomarkers of Tuberculosis. Nat. Rev. Immunol. 2011, 11, 343–354. [Google Scholar] [CrossRef]
- Rambaran, S.; Naidoo, K.; Lewis, L.; Hassan-Moosa, R.; Govender, D.; Samsunder, N.; Scriba, T.J.; Padayatchi, N.; Sivro, A. Effect of Inflammatory Cytokines/Chemokines on Pulmonary Tuberculosis Culture Conversion and Disease Severity in HIV-Infected and -Uninfected Individuals from South Africa. Front. Immunol. 2021, 12, 641065. [Google Scholar] [CrossRef]
- Riou, C.; Perez Peixoto, B.; Roberts, L.; Ronacher, K.; Walzl, G.; Manca, C.; Rustomjee, R.; Mthiyane, T.; Fallows, D.; Gray, C.M.; et al. Effect of Standard Tuberculosis Treatment on Plasma Cytokine Levels in Patients with Active Pulmonary Tuberculosis. PLoS ONE 2012, 7, e36886. [Google Scholar] [CrossRef]
- Yong, Y.K.; Tan, H.Y.; Saeidi, A.; Wong, W.F.; Vignesh, R.; Velu, V.; Eri, R.; Larsson, M.; Shankar, E.M. Immune Biomarkers for Diagnosis and Treatment Monitoring of Tuberculosis: Current Developments and Future Prospects. Front. Microbiol. 2019, 10, 2789. [Google Scholar] [CrossRef]
- Muefong, C.N.; Sutherland, J.S. Neutrophils in Tuberculosis-Associated Inflammation and Lung Pathology. Front. Immunol. 2020, 11, 962. [Google Scholar] [CrossRef]
- Kumar, N.P.; Moideen, K.; Nancy, A.; Viswanathan, V.; Shruthi, B.S.; Sivakumar, S.; Natarajan, M.; Kornfeld, H.; Babu, S. Plasma Chemokines Are Biomarkers of Disease Severity, Higher Bacterial Burden and Delayed Sputum Culture Conversion in Pulmonary Tuberculosis. Sci. Rep. 2019, 9, 18217. [Google Scholar] [CrossRef]
- Morrison, H.; McShane, H. Local Pulmonary Immunological Biomarkers in Tuberculosis. Front. Immunol. 2021, 12, 640916. [Google Scholar] [CrossRef]
- Corleis, B.; Korbel, D.; Wilson, R.; Bylund, J.; Chee, R.; Schaible, U.E. Escape of Mycobacterium Tuberculosis from Oxidative Killing by Neutrophils: Tubercle Bacilli Escape Neutrophil Killing. Cell. Microbiol. 2012, 14, 1109–1121. [Google Scholar] [CrossRef]
- Kumar, N.P.; Moideen, K.; Viswanathan, V.; Shruthi, B.S.; Sivakumar, S.; Menon, P.A.; Kornfeld, H.; Babu, S. Elevated Levels of Matrix Metalloproteinases Reflect Severity and Extent of Disease in Tuberculosis-Diabetes Co-Morbidity and Are Predominantly Reversed Following Standard Anti-Tuberculosis or Metformin Treatment. BMC Infect. Dis. 2018, 18, 345. [Google Scholar] [CrossRef]
- Walker, N.F.; Karim, F.; Moosa, M.Y.S.; Moodley, S.; Mazibuko, M.; Khan, K.; Sterling, T.R.; van der Heijden, Y.F.; Grant, A.D.; Elkington, P.T.; et al. Elevated Plasma Matrix Metalloproteinase 8 Associates with Sputum Culture Positivity in Pulmonary Tuberculosis. J. Infect. Dis. 2022, 226, 928–932. [Google Scholar] [CrossRef]
- Rachow, A.; Ivanova, O.; Wallis, R.; Charalambous, S.; Jani, I.; Bhatt, N.; Kampmann, B.; Sutherland, J.; Ntinginya, N.E.; Evans, D.; et al. TB Sequel: Incidence, Pathogenesis and Risk Factors of Long-Term Medical and Social Sequelae of Pulmonary TB—A Study Protocol. BMC Pulm. Med. 2019, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis. Module 4: Treatment—Drug-Susceptible Tuberculosis Treatment; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Khosa, C.; Bhatt, N.; Massango, I.; Azam, K.; Saathoff, E.; Bakuli, A.; Riess, F.; Ivanova, O.; Hoelscher, M.; Rachow, A. Development of Chronic Lung Impairment in Mozambican TB Patients and Associated Risks. BMC Pulm. Med. 2020, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Ralph, A.P.; Ardian, M.; Wiguna, A.; Maguire, G.P.; Becker, N.G.; Drogumuller, G.; Wilks, M.J.; Waramori, G.; Tjitra, E.; Sandjaja; et al. A Simple, Valid, Numerical Score for Grading Chest x-Ray Severity in Adult Smear-Positive Pulmonary Tuberculosis. Thorax 2010, 65, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Muefong, C.N.; Owolabi, O.; Donkor, S.; Charalambous, S.; Mendy, J.; Sey, I.C.M.; Bakuli, A.; Rachow, A.; Geldmacher, C.; Sutherland, J.S. Major Neutrophil-Derived Soluble Mediators Associate with Baseline Lung Pathology and Post-Treatment Recovery in Tuberculosis Patients. Front. Immunol. 2021, 12, 740933. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Monin, L.; Torres, D.; Slight, S.; Mehra, S.; McKenna, K.C.; Fallert Junecko, B.A.; Reinhart, T.A.; Kolls, J.; Báez-Saldaña, R.; et al. S100A8/A9 Proteins Mediate Neutrophilic Inflammation and Lung Pathology during Tuberculosis. Am. J. Respir. Crit. Care Med. 2013, 188, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Kathamuthu, G.R.; Kumar, N.P.; Moideen, K.; Nair, D.; Banurekha, V.V.; Sridhar, R.; Baskaran, D.; Babu, S. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases Are Potential Biomarkers of Pulmonary and Extra-Pulmonary Tuberculosis. Front. Immunol. 2020, 11, 419. [Google Scholar] [CrossRef]
- Ugarte-Gil, C.A.; Elkington, P.; Gilman, R.H.; Coronel, J.; Tezera, L.B.; Bernabe-Ortiz, A.; Gotuzzo, E.; Friedland, J.S.; Moore, D.A.J. Induced Sputum MMP-1, -3 & -8 Concentrations during Treatment of Tuberculosis. PLoS ONE 2013, 8, e61333. [Google Scholar] [CrossRef]
- Ong, C.W.M.; Elkington, P.T.; Brilha, S.; Ugarte-Gil, C.; Tome-Esteban, M.T.; Tezera, L.B.; Pabisiak, P.J.; Moores, R.C.; Sathyamoorthy, T.; Patel, V.; et al. Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis. PLoS Pathog. 2015, 11, e1004917. [Google Scholar] [CrossRef]
- La Manna, M.P.; Orlando, V.; Paraboschi, E.M.; Tamburini, B.; Di Carlo, P.; Cascio, A.; Asselta, R.; Dieli, F.; Caccamo, N. Mycobacterium Tuberculosis Drives Expansion of Low-Density Neutrophils Equipped with Regulatory Activities. Front. Immunol. 2019, 10, 2761. [Google Scholar] [CrossRef]
- Montales, M.T.; Beebe, A.; Chaudhury, A.; Patil, N. Mycobacterium Tuberculosis Infection in a HIV-Positive Patient. Respir. Med. Case Rep. 2015, 16, 160–162. [Google Scholar] [CrossRef]
- Hamada, Y.; Getahun, H.; Tadesse, B.T.; Ford, N. HIV-Associated Tuberculosis. Int. J. STD AIDS 2021, 32, 780–790. [Google Scholar] [CrossRef]
- Albuquerque, M.D.F.M.D.; Albuquerque, S.C.D.; Campelo, A.R.L.; Cruz, M.; Souza, W.V.D.; Ximenes, R.A.A.; Souza, R.A.S.D. Radiographic Features of Pulmonary Tuberculosis in Patients Infected by HIV: Is There an Objective Indicator of Co-Infection? Rev. Soc. Bras. Med. Trop. 2001, 34, 369–372. [Google Scholar] [CrossRef]
- Haramati, L.B.; Jenny-Avital, E.R.; Alterman, D.D. Effect of HIV Status on Chest Radiographic and CT Findings in Patients with Tuberculosis. Clin. Radiol. 1997, 52, 31–35. [Google Scholar] [CrossRef]
- Walker, N.F.; Meintjes, G.; Wilkinson, R.J. HIV-1 and the Immune Response to TB. Future Virol. 2013, 8, 57–80. [Google Scholar] [CrossRef]
- Alisjahbana, B.; Sulastri, N.; Livia, R.; Apriani, L.; Verrall, A.J.; Sahiratmadja, E. Neutrophils and Lymphocytes in Relation to MMP-8 and MMP-9 Levels in Pulmonary Tuberculosis and HIV Co-Infection. J. Clin. Tuberc. Other Mycobact. Dis. 2022, 27, 100308. [Google Scholar] [CrossRef]
- Ravimohan, S.; Tamuhla, N.; Kung, S.-J.; Nfanyana, K.; Steenhoff, A.P.; Gross, R.; Weissman, D.; Bisson, G.P. Matrix Metalloproteinases in Tuberculosis-Immune Reconstitution Inflammatory Syndrome and Impaired Lung Function among Advanced HIV/TB Co-Infected Patients Initiating Antiretroviral Therapy. EBioMedicine 2016, 3, 100–107. [Google Scholar] [CrossRef]
- Walker, N.F.; Wilkinson, K.A.; Meintjes, G.; Tezera, L.B.; Goliath, R.; Peyper, J.M.; Tadokera, R.; Opondo, C.; Coussens, A.K.; Wilkinson, R.J.; et al. Matrix Degradation in Human Immunodeficiency Virus Type 1–Associated Tuberculosis and Tuberculosis Immune Reconstitution Inflammatory Syndrome: A Prospective Observational Study. Clin. Infect. Dis. 2017, 65, 121–132. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial Translocation Is a Cause of Systemic Immune Activation in Chronic HIV Infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef]
- Deeks, S.G.; Tracy, R.; Douek, D.C. Systemic Effects of Inflammation on Health during Chronic HIV Infection. Immunity 2013, 39, 633–645. [Google Scholar] [CrossRef]
- Nakiwala, J.K.; Walker, N.F.; Diedrich, C.R.; Worodria, W.; Meintjes, G.; Wilkinson, R.J.; Mayanja-Kizza, H.; Colebunders, R.; Kestens, L.; Wilkinson, K.A.; et al. Neutrophil Activation and Enhanced Release of Granule Products in HIV-TB Immune Reconstitution Inflammatory Syndrome. JAIDS J. Acquir. Immune Defic. Syndr. 2018, 77, 221–229. [Google Scholar] [CrossRef]
- González-López, A.; Aguirre, A.; López-Alonso, I.; Amado, L.; Astudillo, A.; Fernández-García, M.S.; Suárez, M.F.; Batalla-Solís, E.; Colado, E.; Albaiceta, G.M. MMP-8 Deficiency Increases TLR/RAGE Ligands S100A8 and S100A9 and Exacerbates Lung Inflammation during Endotoxemia. PLoS ONE 2012, 7, e39940. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Vlachopoulou, E.; Havulinna, A.S.; Tervahartiala, T.; Sattler, W.; Lokki, M.-L.; Nieminen, M.S.; Perola, M.; Salomaa, V.; Sinisalo, J.; et al. Genetic Variants Contributing to Circulating Matrix Metalloproteinase 8 Levels and Their Association with Cardiovascular Diseases: A Genome-Wide Analysis. Circ. Cardiovasc. Genet. 2017, 10, e001731. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.P.R.; Blankley, S.; Graham, C.M.; Bloom, C.I.; O’Garra, A. Systems Approaches to Studying the Immune Response in Tuberculosis. Curr. Opin. Immunol. 2013, 25, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Mattila, J.T.; Maiello, P.; Sun, T.; Via, L.E.; Flynn, J.L. Granzyme B-expressing Neutrophils Correlate with Bacterial Load in Granulomas fromMycobacterium tuberculosis-infected Cynomolgus Macaques. Cell Microbiol. 2015, 17, 1085–1097. [Google Scholar] [CrossRef]
- Railwah, C.; Lora, A.; Zahid, K.; Goldenberg, H.; Campos, M.; Wyman, A.; Jundi, B.; Ploszaj, M.; Rivas, M.; Dabo, A.; et al. Cigarette Smoke Induction of S100A9 Contributes to Chronic Obstructive Pulmonary Disease. Am. J. Physiol.-Lung Cell Mol. Physiol. 2020, 319, L1021–L1035. [Google Scholar] [CrossRef]
- Tiwari, D.; Martineau, A.R. Inflammation-Mediated Tissue Damage in Pulmonary Tuberculosis and Host-Directed Therapeutic Strategies. Semin. Immunol. 2023, 65, 101672. [Google Scholar] [CrossRef]
- McGarry Houghton, A. Matrix Metalloproteinases in Destructive Lung Disease. Matrix Biol. 2015, 44–46, 167–174. [Google Scholar] [CrossRef]
- Da Silva-Neto, P.V.; do Valle, V.B.; Fuzo, C.A.; Fernandes, T.M.; Toro, D.M.; Fraga-Silva, T.F.C.; Basile, P.A.; de Carvalho, J.C.S.; Pimentel, V.E.; Pérez, M.M.; et al. Matrix Metalloproteinases on Severe COVID-19 Lung Disease Pathogenesis: Cooperative Actions of MMP-8/MMP-2 Axis on Immune Response through HLA-G Shedding and Oxidative Stress. Biomolecules 2022, 12, 604. [Google Scholar] [CrossRef]
- Rosengren, S.; Tångefjord, S.; De Palo, G.; Horndahl, J.; Ingelsten, M.; Gilmour, A.; Long, M.B.; Keir, H.R.; Ostridge, K.; Chalmers, J.D. Airway Myeloperoxidase (MPO) is Associated with Increased Risk of Exacerbations in COPD. Eur. Respir. J. 2022, 60, 2017. [Google Scholar]
- Ravimohan, S.; Kornfeld, H.; Weissman, D.; Bisson, G.P. Tuberculosis and Lung Damage: From Epidemiology to Pathophysiology. Eur. Respir Rev. 2018, 27, 170077. [Google Scholar] [CrossRef]
- Madzime, M.; Rossouw, T.M.; Theron, A.J.; Anderson, R.; Steel, H.C. Interactions of HIV and Antiretroviral Therapy with Neutrophils and Platelets. Front. Immunol. 2021, 12, 634386. [Google Scholar] [CrossRef]


| Total | PLHIV | HIV Negatives | |
|---|---|---|---|
| N | 68 | 47 | 21 |
| Males, % (n/N) | 57.35 (39/68) | 53.19 (25/47) | 66.67 (14/21) |
| Median age, years (min-max) | 38.20 (19.0–60.6) | 38.43 (23.8–60.6) | 35.23 (19.0–59.2) |
| HIV- and ART a -naïve at BL b, % (n/N) | 48.94 (23/68) | 48.94 (23/47) | NA |
| TB treatment, % (n/N) | |||
| Standard | 95.59 (65/68) | 93.62 (44/47) | 100 (21/21) |
| TB-DR c | 4.41 (3/68) | 6.38 (3/47) | 0 (0/21) |
| Smear result at BL, % (n/N) | |||
| Negative | 11.74 (8/68) | 14.89 (7/47) | 4.76 (1/21) |
| 1+ | 8.82 (6/68) | 4.26 (2/47) | 19.05 (4/21) |
| 2+ | 20.59 (14/68) | 23.40 (11/47) | 14.29 (3/21) |
| 3+ | 36.76 (25/68) | 36.17 (17/47) | 38.09 (8/21) |
| Scanty | 22.06 (15/68) | 21.28 (10/47) | 23.81 (5/21) |
| Ralph score, in median (IQR d) | |||
| At BL (n = 64) | 15.00 (8.00–42.50) | 12.0 (8.00–20.00) | 20.00 (9.00–48.00) |
| At month 6 (n = 57) | 5.00 (3.00–10.00) | 5.00 (3.00–8.00) | 7.00 (3.00–10.00) |
| Spirometry, % (n/N) | |||
| Any lung impairment at BL | 75.76 (25/33) | 71.43 (15/21) | 76.92 (10/13) |
| Any lung impairment at month 6 | 61.22 (30/49) | 69.69 (23/33) | 50.0 (9/18) |
| Any lung impairment at month 12 | 60.78 (31/51) | 67.57 (25/37) | 50.0 (8/16) |
| CD4 T-cell count, median in cells/mm3 (IQR) (n = 45) | NA | 134.0 (66.50–343.00) | NA |
| Neutrophils, median in cells/mm3 (IQR) | |||
| At BL (n = 67) | 3.85 (2.78–5.33) | 3.77 (2.67–5.24 | 4.15 (3.5–4.48) |
| At month 6 (n = 61) | 1.61 (1.24–2.24) | 1.73 (1.26–2.30) | 1.53 (1.21–1.93) |
| At month 12 (n = 52) | 1.79 (1.35–2.56) | 1.63 (1.33–2.57) | 2.14 (1.51–2.42) |
| Time Point | MMP-1 | Neutrophils at Baseline | Neutrophils at Month 6 | Neutrophils at Month 12 |
|---|---|---|---|---|
| Baseline | MMP-1 | 0.18 (p = 0.27) | −0.063 (p = 0.72) | −0.32 (p = 0.06) |
| MMP-2 | −0.42 (p = 0.0096) | −0.03 (p = 0.86) | 0.07 (p = 0.69) | |
| MMP-8 | −0.04 (p = 0.83) | 0.12 (p = 0.48) | 0.14 (p = 0.42) | |
| S100A8 | 0.19 (p = 0.23) | 0.12 (p = 0.48) | −0.05 (p = 0.75) | |
| MPO | 0.12 (p = 0.46) | 0.09 (p = 0.46) | 0.10 (p = 0.55) | |
| Month 6 | MMP-1 | 0.26 (p = 0.14) | −0.071 (p = 0.7) | −0.35 (p = 0.046) |
| MMP-2 | −0.42 (p = 0.014) | −0.22 (p = 0.22) | −0.05 (p = 0.79) | |
| MMP-8 | 0.22 (p = 0.22) | −0.02 (p = 0.89) | −0.01 (p = 0.95) | |
| S100A8 | 0.03 (p = 0.85) | −0.06 (p = 0.75) | −0.46 (p = 0.009) | |
| MPO | −0.0005 (p = 0.99) | −0.02 (p = 0.91) | −0.18 (p = 0.34) | |
| Month 12 | MMP-1 | 0.46 (p = 0.04) | −0.14 (p = 0.54) | −0.21 (p = 0.36) |
| MMP-2 | −0.28 (p = 0.22) | 0.06 (p = 0.81) | −0.18 (p = 0.43) | |
| MMP-8 | −0.22 (p = 0.16) | −0.36 (p = 0.11) | −0.08 (p = 0.71) | |
| S100A8 | 0.19 (p = 0.39) | −0.11 (p = 0.65) | −0.13 (p = 0.58) | |
| MPO | 0.04 (p = 0.85) | −0.13 (p = 0.59) | −0.25 (p = 0.28) |
| Time Point | Biomarker | RS at Baseline | RS at Month 6 |
|---|---|---|---|
| Baseline | MMP-1 | −0.036 (p = 0.83) | 0.31 (p = 0.08) |
| MMP-2 | −0.24 (p = 0.15) | −0.1 (p = 0.58) | |
| MMP-8 | 0.06 (p = 0.73) | 0.18 (p = 0.32) | |
| S100A8 | 0.05 (p = 0.79) | 0.22 (p = 0.22) | |
| MPO | 0.07 (p = 0.68) | 0.12 (p = 0.50) | |
| Month 6 | MMP-1 | −0.13 (p = 0.46) | −0.01 (p = 0.96) |
| MMP-2 | −0.37 (p = 0.03) | −0.07 (p = 071) | |
| MMP-8 | 0.36 (p = 0.04) | 0.08 (p = 0.67) | |
| S100A8 | 0.03 (p = 0.89) | −0.01 (p = 0.95) | |
| MPO | 0.39 (p = 0.02) | 0.10 (p = 0.58) | |
| Month 12 | MMP-1 | −0.32 (p = 0.15) | 0.10 (p = 0.67) |
| MMP-2 | −0.062 (p = 0.79) | 0.23 (p = 0.33) | |
| MMP-8 | 0.07 (p = 0.77) | 0.52 (p = 0.02) | |
| S100A8 | 0.08 (p = 0.72) | 0.28 (p = 0.24) | |
| MPO | 0.09 (p = 0.68) | 0.35 (p = 0.13) |
| Time Point | Biomarker | MMP-1 | MMP-2 | MMP-8 | MPO |
|---|---|---|---|---|---|
| Baseline (n = 38) | MMP-1 | NA | −0.24 (p = 0.15) | ||
| MMP-8 | 0.14 (p = 0.41) | 0.09 (p = 0.57) | 0.27 (p = 0.09) | ||
| S100A8 | 0.63 (p < 0.0001) | 0.01 (p = 0.097) | 0.33 (p = 0.04) | 0.06 (p = 0.74) | |
| MPO | 0.01 (p = 0.94) | 0.39 (p = 0.02) | NA | ||
| Month 6 (n = 37) | MMP-1 | NA | −0.14 (p = 0.39) | ||
| MMP-8 | 0.07 (p = 0.66) | −0.04 (p = 0.82) | 0.71 (p < 0.0001) | ||
| S100A8 | 0.74 (p < 0.0001) | −0.06 (p = 0.73) | 0.36 (p = 0.03) | 0.38 (p = 0.02) | |
| MPO | 0.11 (p = 0.51) | 0.23 (p = 0.17) | NA | ||
| Month 12 (n = 42) | MMP-1 | NA | −0.24 (p = 0.13) | ||
| MMP-8 | 0.14 (p = 0.37) | 0.23 (p = 0.15) | 0.54 (p = 0.0003) | ||
| S100A8 | 0.42 (p = 0.0057) | 0.28 (p = 0.07) | 0.61 (p < 0.0001) | 0.69 (p < 0.0001) | |
| MPO | 0.19 (p = 0.22) | 0.31 (p = 0.04) | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitoe, N.; Chelene, I.; Ligeiro, S.; Castiano, C.; Ahmed, M.I.M.; Held, K.; Nhassengo, P.; Khosa, C.; Matavele-Chissumba, R.; Hoelscher, M.; et al. Effect of TB Treatment on Neutrophil-Derived Soluble Inflammatory Mediators in TB Patients with and without HIV Coinfection. Pathogens 2023, 12, 794. https://doi.org/10.3390/pathogens12060794
Sitoe N, Chelene I, Ligeiro S, Castiano C, Ahmed MIM, Held K, Nhassengo P, Khosa C, Matavele-Chissumba R, Hoelscher M, et al. Effect of TB Treatment on Neutrophil-Derived Soluble Inflammatory Mediators in TB Patients with and without HIV Coinfection. Pathogens. 2023; 12(6):794. https://doi.org/10.3390/pathogens12060794
Chicago/Turabian StyleSitoe, Nádia, Imelda Chelene, Sofia Ligeiro, Celso Castiano, Mohamed I. M. Ahmed, Kathrin Held, Pedroso Nhassengo, Celso Khosa, Raquel Matavele-Chissumba, Michael Hoelscher, and et al. 2023. "Effect of TB Treatment on Neutrophil-Derived Soluble Inflammatory Mediators in TB Patients with and without HIV Coinfection" Pathogens 12, no. 6: 794. https://doi.org/10.3390/pathogens12060794
APA StyleSitoe, N., Chelene, I., Ligeiro, S., Castiano, C., Ahmed, M. I. M., Held, K., Nhassengo, P., Khosa, C., Matavele-Chissumba, R., Hoelscher, M., Rachow, A., Geldmacher, C., & on behalf of the TB Sequel Consortium. (2023). Effect of TB Treatment on Neutrophil-Derived Soluble Inflammatory Mediators in TB Patients with and without HIV Coinfection. Pathogens, 12(6), 794. https://doi.org/10.3390/pathogens12060794






