Characterization of Collagen Binding Activity of Clostridium perfringens Strains Isolated from Broiler Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
| Strain | Source | netB | tpeL | Reference |
|---|---|---|---|---|
| 13 | Soil | − | − | This study |
| CP1 | Field NE | − | − | [16] |
| CP2 | Field NE | − | − | [16] |
| CP15 | Field NE | − | − | [16] |
| CP23 | Field NE | − | − | [16] |
| JGS | Field NE | − | − | [16] |
| LLY_N10 | Healthy chickens | − | − | [16] |
| LLY_N11 | Healthy chickens | − | − | [16] |
| SM101 | Food poisoning | − | − | [17] |
| C11 | Field NE | + | ± | [15] |
| tpeL15 | Field NE | + | + | [15] |
| tpeL17 | Field NE | + | + | [15] |
| tpeL18 | Field NE | + | + | [15] |
| tpeL19 | Field NE | + | + | [15] |
| C1 | Field NE | + | − | [15] |
| C2 | Field NE | + | − | [15] |
| C3 | Field NE | + | − | [15] |
| C5 | Field NE | + | − | [15] |
| C6 | Field NE | + | − | [15] |
| C7 | Field NE | + | − | [15] |
| C8 | Field NE | + | − | [15] |
| C9 | Field NE | + | − | [15] |
| C10 | Field NE | + | − | [15] |
| C12 | Field NE | + | − | [15] |
| C13 | Field NE | + | − | This study |
| C14 | Field NE | + | − | [15] |
| C16 | Field NE | + | − | [15] |
| Del1 | Field NE | + | − | [16] |
2.2. Preparation of Genomic DNA from C. perfringens
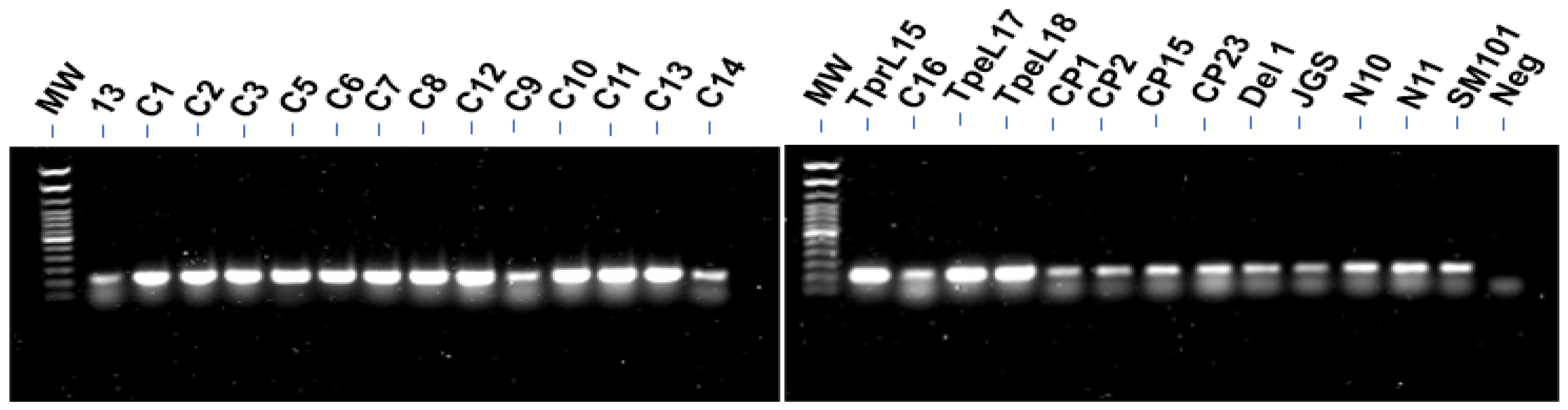
2.3. PCR for Detection of CnaA
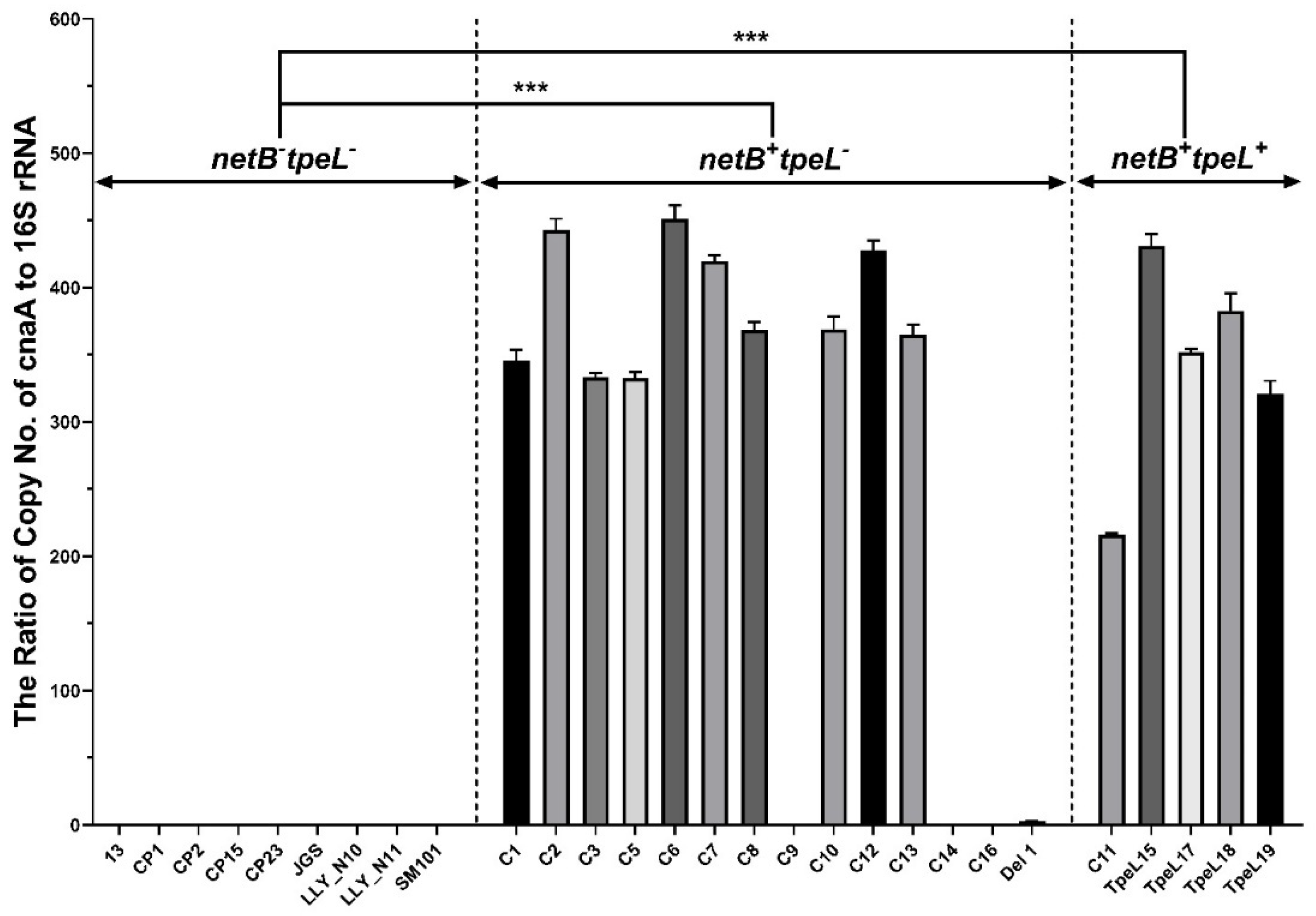
2.4. qPCR for Detection of cnaA
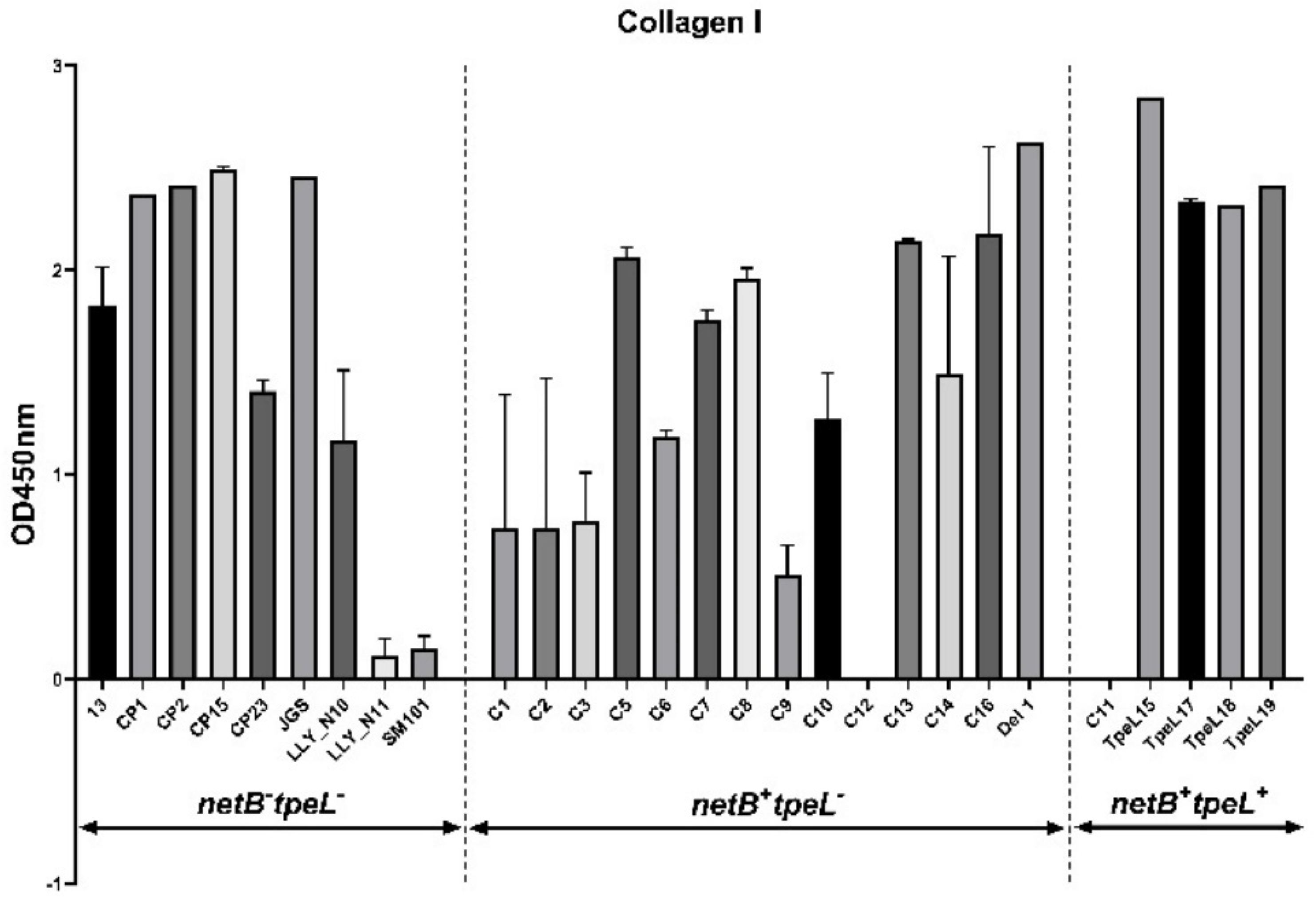
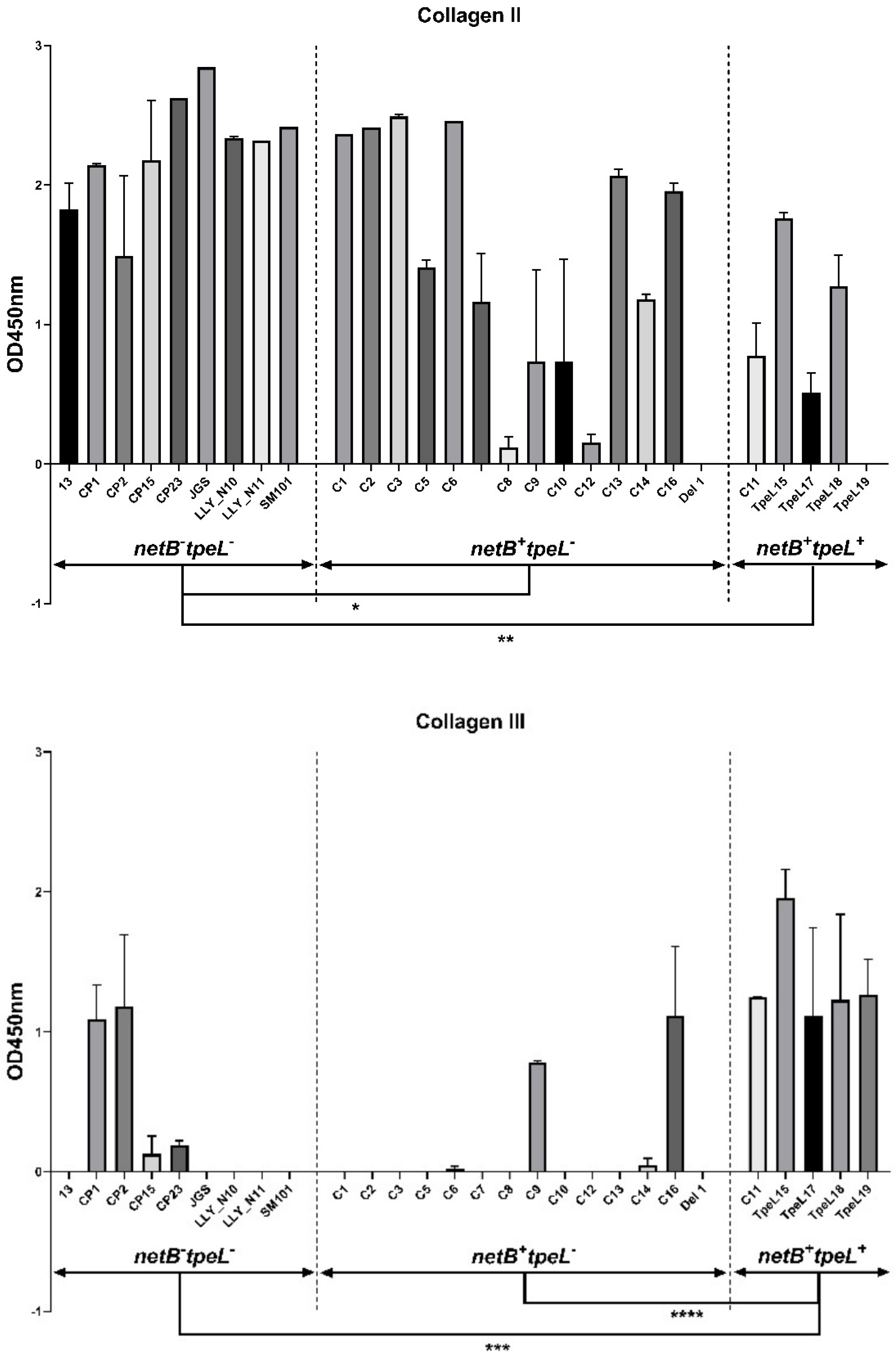
2.5. Adhesion Assay
2.6. Statistical Analysis
3. Results
3.1. PCR for Detection of CnaA
3.2. Quantitative Polymerase Chain Reaction (qPCR) for cnaA Gene Detection
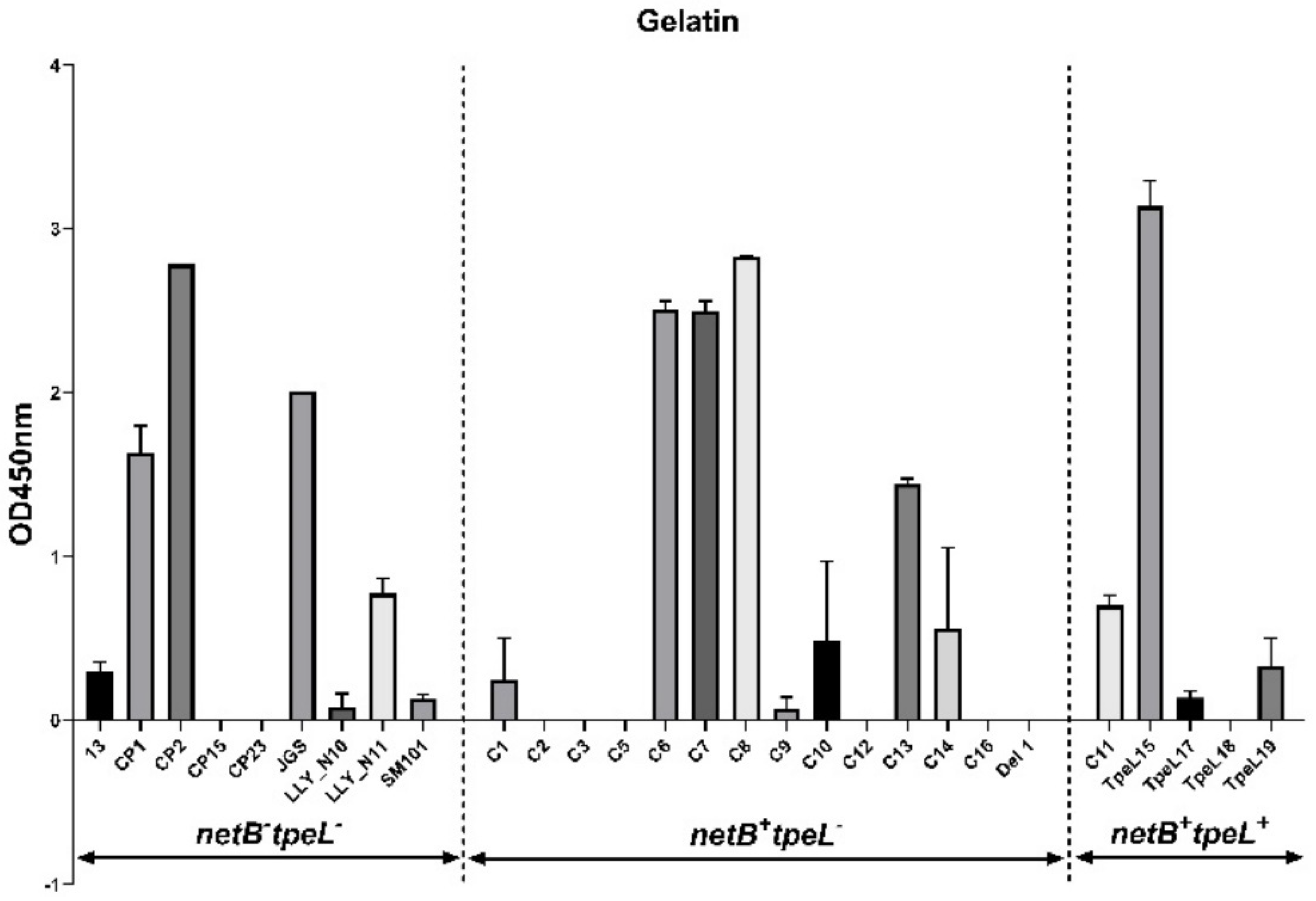
3.3. Adhesion Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rood, J.I.; Adams, V.; Lacey, J.; Lyras, D.; McClane, B.A.; Melville, S.B.; Moore, R.J.; Popoff, M.R.; Sarker, M.R.; Songer, J.G.; et al. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe 2018, 53, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Tsiouris, V. Poultry management: A useful tool for the control of necrotic enteritis in poultry. Avian Pathol. 2016, 45, 323–325. [Google Scholar] [CrossRef]
- Prescott, J.F.; Smyth, J.A.; Shojadoost, B.; Vince, A. Experimental reproduction of necrotic enteritis in chickens: A review. Avian Pathol. 2016, 45, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Keyburn, A.L.; Yan, X.X.; Bannam, T.L.; Van Immerseel, F.; Rood, J.I.; Moore, R.J. Association between avian necrotic enteritis and Clostridium perfringens strains expressing NetB toxin. Vet. Res. 2010, 41, 21. [Google Scholar] [CrossRef]
- Keyburn, A.L.; Boyce, J.D.; Vaz, P.; Bannam, T.L.; Ford, M.E.; Parker, D.; Di Rubbo, A.; Rood, J.I.; Moore, R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008, 4, e26. [Google Scholar] [CrossRef] [PubMed]
- Rood, J.I.; Keyburn, A.L.; Moore, R.J. NetB and necrotic enteritis: The hole movable story. Avian Pathol. 2016, 45, 295–301. [Google Scholar] [CrossRef]
- Shojadoost, B.; Vince, A.R.; Prescott, J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: A critical review. Vet. Res. 2012, 43, 74. [Google Scholar] [CrossRef]
- Shi, H.N.; Walker, A. Bacterial colonization and the development of intestinal defences. Can. J. Gastroenterol. 2004, 18, 493–500. [Google Scholar] [CrossRef]
- Ribet, D.; Cossart, P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 2015, 17, 173–183. [Google Scholar] [CrossRef]
- Martin, T.G.; Smyth, J.A. The ability of disease and non-disease producing strains of Clostridium perfringens from chickens to adhere to extracellular matrix molecules and Caco-2 cells. Anaerobe 2010, 16, 533–539. [Google Scholar] [CrossRef]
- Wade, B.; Keyburn, A.L.; Haring, V.; Ford, M.; Rood, J.I.; Moore, R.J. The adherent abilities of Clostridium perfringens strains are critical for the pathogenesis of avian necrotic enteritis. Vet. Microbiol. 2016, 197, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Wade, B.; Keyburn, A.L.; Seemann, T.; Rood, J.I.; Moore, R.J. Binding of Clostridium perfringens to collagen correlates with the ability to cause necrotic enteritis in chickens. Vet. Microbiol. 2015, 180, 299–303. [Google Scholar] [CrossRef]
- Lepp, D.; Gong, J.; Songer, J.G.; Boerlin, P.; Parreira, V.R.; Prescott, J.F. Identification of accessory genome regions in poultry Clostridium perfringens isolates carrying the netB plasmid. J. Bacteriol. 2013, 195, 1152–1166. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yan, X.; Lillehoj, H.S. Complete genome sequences of Clostridium perfringens Del1 strain isolated from chickens affected by necrotic enteritis. Gut Pathog. 2017, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Lillehoj, H.S.; Sun, Z.; Lee, Y.; Zhao, H.; Xianyu, Z.; Yan, X.; Wang, Y.; Lin, S.; Liu, L.; et al. Characterization of virulent netB(+)/tpeL(+) Clostridium perfringens strains from necrotic enteritis-affected broiler chicken farms. Avian Dis. 2019, 63, 461–467. [Google Scholar] [CrossRef]
- Li, C.; Lillehoj, H.S.; Gadde, U.D.; Ritter, D.; Oh, S. Characterization of Clostridium perfringens strains isolated from healthy and necrotic enteritis-afflicted broiler chickens. Avian Dis. 2017, 61, 178–185. [Google Scholar] [CrossRef]
- Liu, L.; Yan, X.; Lillehoj, H.; Sun, Z.; Zhao, H.; Xianyu, Z.; Lee, Y.; Melville, S.; Gu, C.; Wang, Y.; et al. Comparison of the pathogenicity of five Clostridium perfringens isolates using an Eimeria maxima coinfection necrotic enteritis disease model in commercial broiler chickens. Avian Dis. 2020, 64, 386–392. [Google Scholar] [CrossRef]
- Mehdizadeh Gohari, I.; Navarro, M.A.; Li, J.; Shrestha, A.; Uzal, F.; McClane, B.A. Pathogenicity and virulence of Clostridium perfringens. Virulence 2021, 12, 723–753. [Google Scholar] [CrossRef]
- Van Immerseel, F.; Lyhs, U.; Pedersen, K.; Prescott, J.F. Recent breakthroughs have unveiled the many knowledge gaps in Clostridium perfringens-associated necrotic enteritis in chickens: The first International Conference on Necrotic Enteritis in Poultry. Avian Pathol. 2016, 45, 269–270. [Google Scholar] [CrossRef]
- Jost, B.H.; Billington, S.J.; Trinh, H.T.; Songer, J.G. Association of genes encoding beta2 toxin and a collagen binding protein in Clostridium perfringens isolates of porcine origin. Vet. Microbiol. 2006, 115, 173–182. [Google Scholar] [CrossRef]
- Lepp, D.; Zhou, Y.; Ojha, S.; Mehdizadeh Gohari, I.; Carere, J.; Yang, C.; Prescott, J.F.; Gong, J. Clostridium perfringens produces an adhesive pilus required for the pathogenesis of necrotic enteritis in poultry. J. Bacteriol. 2021, 203, e00578-20. [Google Scholar] [CrossRef]
- Hitsumoto, Y.; Morita, N.; Yamazoe, R.; Tagomori, M.; Yamasaki, T.; Katayama, S. Adhesive properties of Clostridium perfringens to extracellular matrix proteins collagens and fibronectin. Anaerobe 2014, 25, 67–71. [Google Scholar] [CrossRef]
- Katayama, S.; Nozu, N.; Okuda, M.; Hirota, S.; Yamasaki, T.; Hitsumoto, Y. Characterization of two putative fibronectin-binding proteins of Clostridium perfringens. Anaerobe 2009, 15, 155–159. [Google Scholar] [CrossRef]
- Lepp, D.; Ojha, S.; Mehdizadeh Gohari, I.; Chakravarty, B.; Prescott, J.F.; Gong, J. Immunization with subunits of a novel pilus produced by virulent Clostridium perfringens strains confers partial protection against necrotic enteritis in chickens. Vet. Microbiol. 2019, 230, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Sun, Z.; Lu, M.; Lillehoj, H.; Lee, Y.; Liu, L.; Yan, X.; Yang, D.A.; Li, C. Immunization with pooled antigens for Clostridium perfringens conferred partial protection against experimental necrotic enteritis in broiler chickens. Vaccines 2022, 10, 979. [Google Scholar] [CrossRef]
- Van Damme, L.; Cox, N.; Callens, C.; Dargatz, M.; Flügel, M.; Hark, S.; Thiemann, F.; Pelzer, S.; Haesebrouck, F.; Ducatelle, R.; et al. Protein truncating avriants of colA in Clostridium perfringens Type G strains. Front. Cell. Infect. Microbiol. 2021, 11, 645248. [Google Scholar] [CrossRef] [PubMed]
- Amimoto, K.; Noro, T.; Oishi, E.; Shimizu, M. A novel toxin homologous to large clostridial cytotoxins found in culture supernatant of Clostridium perfringens type C. Microbiology 2007, 153, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; McClane, B.A. Characterization of Clostridium perfringens TpeL toxin gene carriage, production, cytotoxic contributions, and trypsin sensitivity. Infect. Immun. 2015, 83, 2369–2381. [Google Scholar] [CrossRef]
- Goo, D.; Park, I.; Nam, H.; Lee, Y.; Sawall, J.; Smith, A.; Rehberger, T.; Li, C.; Lillehoj, H. Collagen adhesin protein and necrotic enteritis B-like toxin as biomarkers for early diagnosis of necrotic enteritis in commercial broiler chickens. Poult. Sci. 2023, 102, 102647. [Google Scholar] [CrossRef]
- Li, C.; Yan, X.; Lillehoj, H.S. Complete egnome sequence of Clostridium perfringens LLY_N11, a necrotic enteritis-inducing strain isolated from a healthy chicken intestine. Genome Announc. 2017, 5, e01225-17. [Google Scholar] [CrossRef]
- Goossens, E.; Dierick, E.; Ducatelle, R.; Van Immerseel, F. Spotlight on avian pathology: Untangling contradictory disease descriptions of necrotic enteritis and necro-haemorrhagic enteritis in broilers. Avian Pathol. 2020, 49, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Madani, A.; Garakani, K.; Mofrad, M.R. Molecular mechanics of Staphylococcus aureus adhesin, CNA, and the inhibition of bacterial adhesion by stretching collagen. PLoS ONE 2017, 12, e0179601. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, G.V.; Coppolino, F.; Lentini, G.; Famà, A.; Cullotta, C.; Raffaele, I.; Motta, C.; Teti, G.; Speziale, P.; Pietrocola, G.; et al. Streptococcus pneumoniae binds collagens and C1q via the SSURE repeats of the PfbB adhesin. Mol. Microbiol. 2022, 117, 1479–1492. [Google Scholar] [CrossRef] [PubMed]

| Target Gene | Primer Sequences (5’-3’) |
|---|---|
| 16S rRNA | F-GGGGGTTTCAACACCTCC |
| R-GCAAGGGATATCAAGTGT | |
| cnaA | F-GGTGGATGGGCAACATTTAC |
| R-CCTTGCTTGGATTCACCAGT |
| GENOTYPE | netB−tpeL− | netB−tpeL+ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ISOLATE ID | 13 | CP1 | CP10 | CP15 | CP23 | JGS | N10 | N11 | SM101 | C11 | tpeL15 | tpeL17 | tpeL18 | tpeL19 |
| cnaA PCR | + | + | + | + | + | + | + | + | + | ++ | ++ | ++ | ++ | ++ |
| cnaA qPCR | − | − | − | − | − | − | − | − | − | +++ | +++ | +++ | +++ | +++ |
| GENOTYPE | netB+tpeL+ | |||||||||||||
| ISOLATE ID | C1 | C2 | C3 | C5 | C6 | C7 | C8 | C9 | CP10 | CP12 | CP13 | CP14 | CP16 | Del 1 |
| cnaA PCR | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | ++ | ++ | + | + | + |
| cnaA qPCR | +++ | +++ | +++ | +++ | +++ | +++ | +++ | − | +++ | +++ | +++ | − | − | +/− |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Lu, M.; Lillehoj, H.; Lee, Y.; Goo, D.; Yuan, B.; Yan, X.; Li, C. Characterization of Collagen Binding Activity of Clostridium perfringens Strains Isolated from Broiler Chickens. Pathogens 2023, 12, 778. https://doi.org/10.3390/pathogens12060778
Sun Z, Lu M, Lillehoj H, Lee Y, Goo D, Yuan B, Yan X, Li C. Characterization of Collagen Binding Activity of Clostridium perfringens Strains Isolated from Broiler Chickens. Pathogens. 2023; 12(6):778. https://doi.org/10.3390/pathogens12060778
Chicago/Turabian StyleSun, Zhifeng, Mingmin Lu, Hyun Lillehoj, Youngsub Lee, Doyun Goo, Baohong Yuan, Xianghe Yan, and Charles Li. 2023. "Characterization of Collagen Binding Activity of Clostridium perfringens Strains Isolated from Broiler Chickens" Pathogens 12, no. 6: 778. https://doi.org/10.3390/pathogens12060778
APA StyleSun, Z., Lu, M., Lillehoj, H., Lee, Y., Goo, D., Yuan, B., Yan, X., & Li, C. (2023). Characterization of Collagen Binding Activity of Clostridium perfringens Strains Isolated from Broiler Chickens. Pathogens, 12(6), 778. https://doi.org/10.3390/pathogens12060778








