Fluconazole Resistance and Virulence in In Vitro Induced-Fluconazole Resistant Strains and in Clinical Fluconazole Resistant Strain of Cryptococcus deuterogattii
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Media
2.2. Antifungal Susceptibility Testing
2.3. RNA Isolation, Reverse Transcription, and Real-Time Quantitative PCR Analysis
2.4. DNA Extraction and ERG11 Sequencing
2.5. Survival Curve of Galleria mellonella Infected with Cryptococcus Strains
2.6. Statistical Analysis
3. Results
3.1. No Mutations Are Detected in the ERG 11 Gene in Any of the Induced FCZ-Resistant Strains
3.2. ERG11, MDR1, AFR1 and AFR2 mRNA Expression
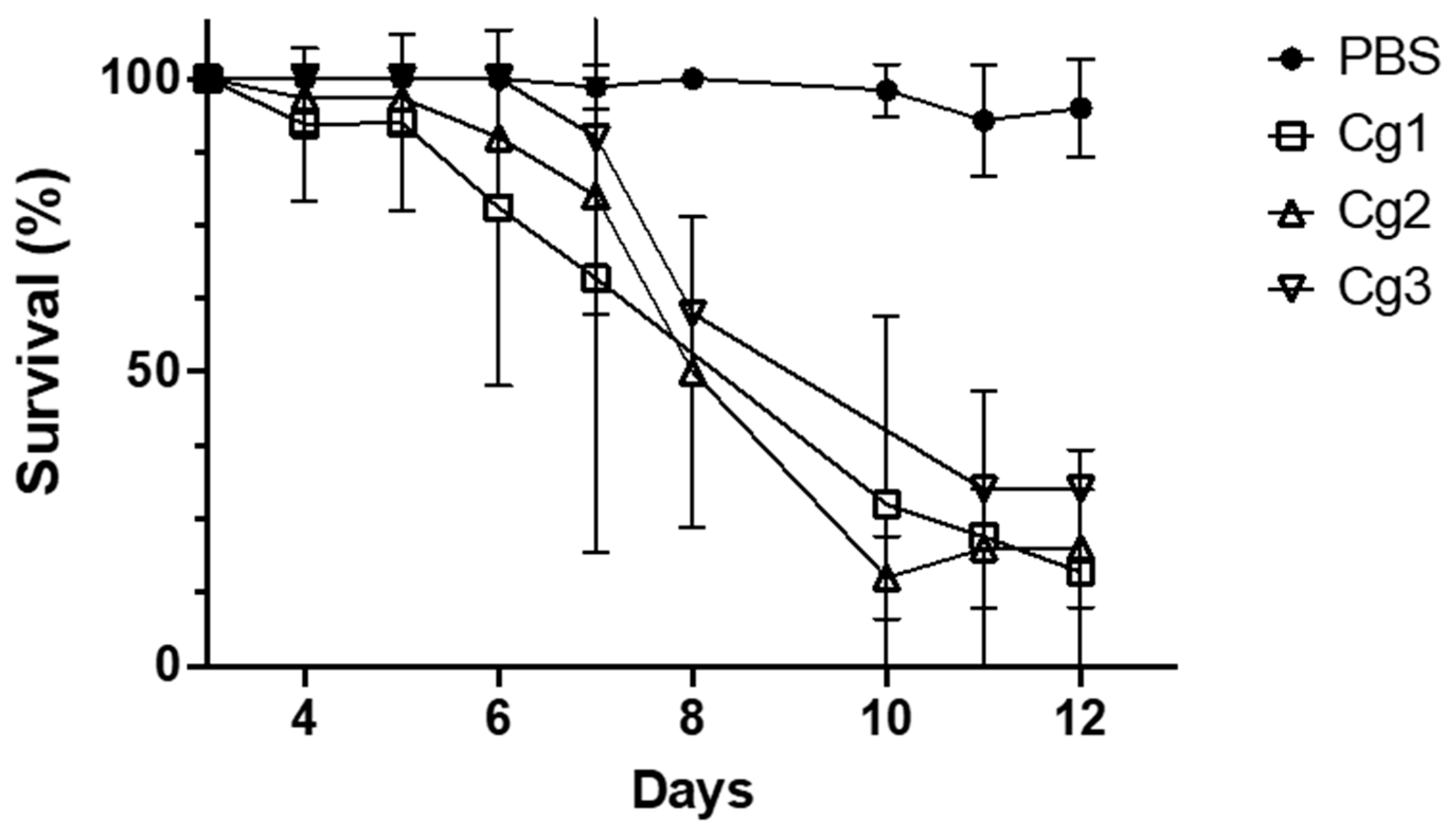
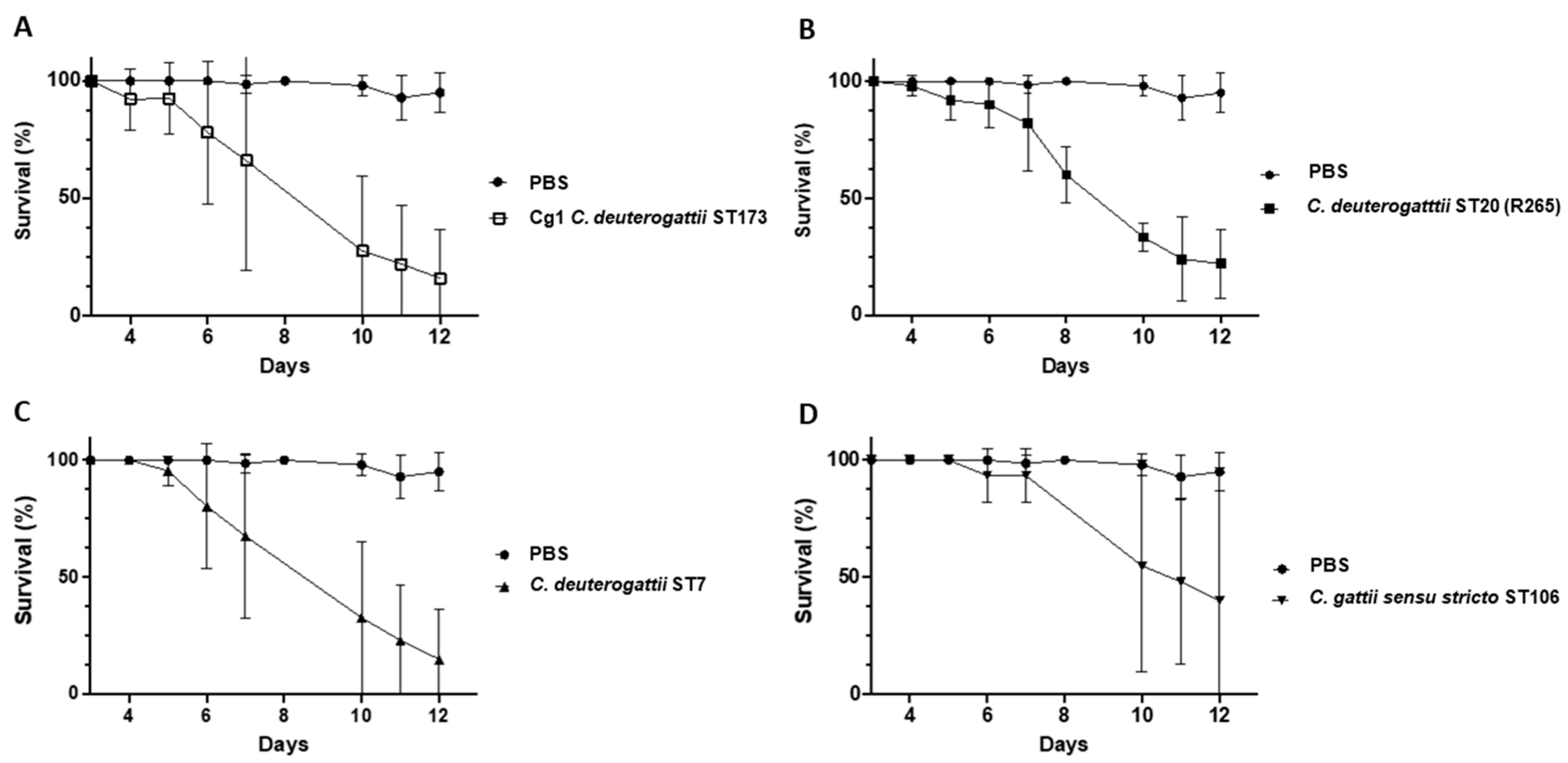
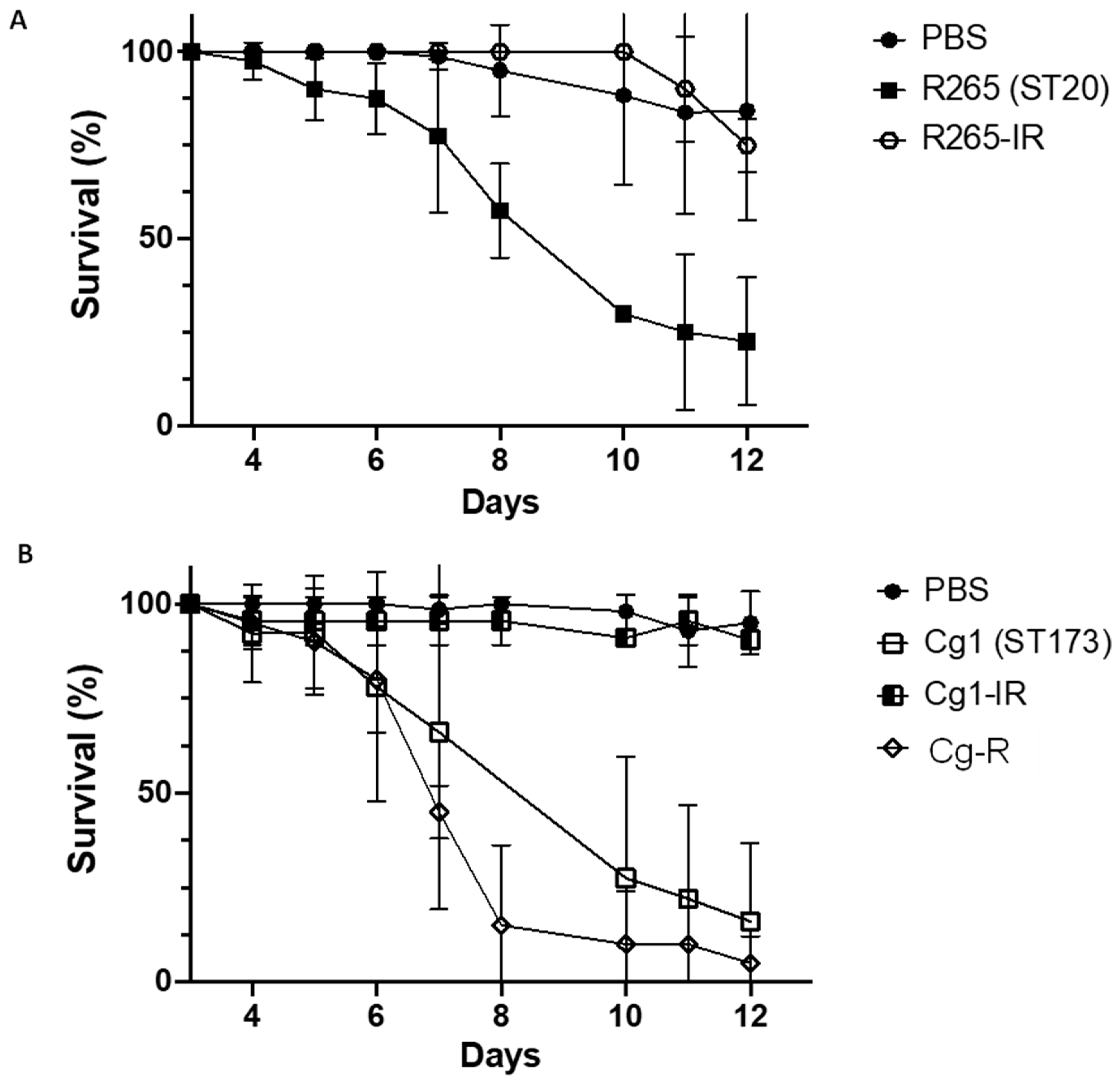
3.3. Virulence Studies on G. melonella Larvae
3.3.1. Virulence Study of Ivorian C. deuterogattii Strains
3.3.2. Study of the Relationship between Virulence and Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B.; Chiller, T.M.; Boulware, D.R. The global burden of HIV-associated cryptococcal infection in adults in 2020: A modelling analysis. Lancet Infect. Dis. 2022, 22, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Bicanic, T.; Muzoora, C.; Brouwer, A.E.; Meintjes, G.; Longley, N.; Taseera, K.; Rebe, K.; Loyse, A.; Jarvis, J.; Bekker, L.G.; et al. Independent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: Analysis of a combined cohort of 262 patients. Clin. Infect. Dis. 2009, 49, 702–709. [Google Scholar] [CrossRef]
- Lessells, R.J.; Mutevedzi, P.C.; Heller, T.; Newell, M.L. Poor long-term outcomes for cryptococcal meningitis in rural South Africa. S. Afr. Med. J. 2011, 101, 251–252. [Google Scholar] [CrossRef]
- May, R.C.; Stone, N.R.H.; Wiesner, D.L.; Bicanic, T.; Nielsen, K. Cryptococcus: From environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2016, 14, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Bicanic, T.; Harrison, T.S. Cryptococcal meningitis. Br. Med. Bull. 2005, 72, 99–118. [Google Scholar] [CrossRef]
- Lortholary, O.; Poizat, G.; Zeller, V.; Neuville, S.; Boibieux, A.; Alvarez, M.; Dellamonica, P.; Botterel, F.; Dromer, F.; Chêne, G. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS 2006, 20, 2183–2191. [Google Scholar] [CrossRef]
- Dromer, F.; Mathoulin-Pélissier, S.; Launay, O.; Lortholary, O.; French Cryptococcosis Study Group. Determinants of disease presentation and outcome during cryptococcosis: The CryptoA/D study. PLoS Med. 2007, 4, e21. [Google Scholar] [CrossRef]
- Hagen, F.; Jensen, R.H.; Meis, J.F.; Arendrup, M.C. Molecular epidemiology and in vitro antifungal susceptibility testing of 108 clinical Cryptococcus neoformans sensu lato and Cryptococcus gattii sensu lato isolates from Denmark. Mycoses 2016, 59, 576–584. [Google Scholar] [CrossRef]
- Hagen, F.; Lumbsch, H.T.; Arsic Arsenijevic, V.; Badali, H.; Bertout, S.; Billmyre, R.B.; Bragulat, M.R.; Cabañes, F.J.; Carbia, M.; Chakrabarti, A.; et al. Importance of Resolving Fungal Nomenclature: The Case of Multiple Pathogenic Species in the Cryptococcus Genus. mSphere 2017, 2, e00238-17. [Google Scholar] [CrossRef]
- Francisco, E.C.; de Jong, A.W.; Hagen, F. Cryptococcosis and Cryptococcus. Mycopathologia 2021, 186, 729–731. [Google Scholar] [CrossRef]
- Colom, M.F.; Hagen, F.; Gonzalez, A.; Mellado, A.; Morera, N.; Linares, C.; García, D.F.; Peñataro, J.S.; Boekhout, T.; Sánchez, M. Ceratonia siliqua (carob) trees as natural habitat and source of infection by Cryptococcus gattii in the Mediterranean environment. Med. Mycol. 2012, 50, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Meyer, W.; Sorrell, T.C. Cryptococcus gattii infections. Clin. Microbiol. Rev. 2014, 27, 980–1024. [Google Scholar] [CrossRef]
- Beardsley, J.; Sorrell, T.C.; Chen, S.C. Central Nervous System Cryptococcal Infections in Non-HIV Infected Patients. J. Fungi 2019, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Assogba, K.; Belo, M.; Wateba, M.I.; Gnonlonfoun, D.D.; Ossou-Nguiet, P.M.; Tsanga, B.B.; Ndiaye, M.; Grunitzky, E.K. Neuromeningeal cryptococcosis in sub-Saharan Africa: Killer disease with sparse data. J. Neurosci. Rural Pract. 2015, 6, 221–224. [Google Scholar] [CrossRef]
- Jarvis, J.; Dromer, F.; Harrison, T.; Lortholary, O. Managing cryptococcosis in the immunocompromised host. Curr. Opin. Infect. Dis. 2008, 21, 596–603. [Google Scholar] [CrossRef]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.H.; et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef] [PubMed]
- Trilles, L.; Fernández-Torres, B.; dos Santos Lazéra, M.; Wanke, B.; Guarro, J. In vitro antifungal susceptibility of Cryptococcus gattii. J. Clin. Microbiol. 2004, 42, 4815–4817. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rodríguez, J.M.; Alvarado-Ramírez, E.; Murciano, F.; Sellart, M. MICs and minimum fungicidal concentrations of posaconazole, voriconazole and fluconazole for Cryptococcus neoformans and Cryptococcus gattii. J. Antimicrob. Chemother. 2008, 62, 205–206. [Google Scholar] [CrossRef]
- Bicanic, T.; Harrison, T.; Niepieklo, A.; Dyakopu, N.; Meintjes, G. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: The role of fluconazole resistance and immune reconstitution. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2006, 43, 1069–1073. [Google Scholar] [CrossRef]
- Diaz, J.H. The Disease Ecology, Epidemiology, Clinical Manifestations, and Management of Emerging Cryptococcus gattii Complex Infections. Wilderness Environ. Med. 2020, 31, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Florio, A.R.; Ferrari, S.; De Carolis, E.; Torelli, R.; Fadda, G.; Sanguinetti, M.; Sanglard, D.; Posteraro, B. Genome-wide expression profiling of the response to short-term exposure to fluconazole in Cryptococcus neoformans serotype A. BMC Microbiol. 2011, 11, 97. [Google Scholar] [CrossRef]
- Cheong, J.W.S.; McCormack, J. Fluconazole resistance in cryptococcal disease: Emerging or intrinsic? Med. Mycol. 2013, 51, 261–269. [Google Scholar] [CrossRef]
- Naicker, S.D.; Mpembe, R.S.; Maphanga, T.G.; Zulu, T.G.; Desanto, D.; Wadula, J.; Mvelase, N.; Maluleka, C.; Reddy, K.; Dawood, H.; et al. Decreasing fluconazole susceptibility of clinical South African Cryptococcus neoformans isolates over a decade. PLoS Negl. Trop. Dis. 2020, 14, e0008137. [Google Scholar] [CrossRef]
- Bongomin, F.; Oladele, R.O.; Gago, S.; Moore, C.B.; Richardson, M.D. A systematic review of fluconazole resistance in clinical isolates of Cryptococcus species. Mycoses 2018, 61, 290–297. [Google Scholar] [CrossRef]
- Franco-Paredes, C.; Womack, T.; Bohlmeyer, T.; Sellers, B.; Hays, A.; Patel, K.; Lizarazo, J.; Lockhart, S.R.; Siddiqui, W.; Marr, K.A. Management of Cryptococcus gattii meningoencephalitis. Lancet Infect. Dis. 2015, 15, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Gago, S.; Serrano, C.; Alastruey-Izquierdo, A.; Cuesta, I.; Martín-Mazuelos, E.; Aller, A.I.; Gomez-Lopez, A.; Mellado, E. Molecular identification, antifungal resistance and virulence of Cryptococcus neoformans and Cryptococcus deneoformans isolated in Seville, Spain. Mycoses 2017, 60, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Mukaremera, L.; McDonald, T.R.; Nielsen, J.N.; Molenaar, C.J.; Akampurira, A.; Schutz, C.; Taseera, K.; Muzoora, C.; Meintjes, G.; Meya, D.B.; et al. The Mouse Inhalation Model of Cryptococcus neoformans Infection Recapitulates Strain Virulence in Humans and Shows that Closely Related Strains Can Possess Differential Virulence. Infect. Immun. 2019, 87, e00046–e00119. [Google Scholar] [CrossRef]
- Trevijano-Contador, N.; Zaragoza, O. Immune Response of Galleria mellonella against Human Fungal Pathogens. J. Fungi 2018, 5, 3. [Google Scholar] [CrossRef]
- Kassi, F.K.; Drakulovski, P.; Bellet, V.; Roger, F.; Chabrol, A.; Krasteva, D.; Doumbia, A.; Landman, R.; Kakou, A.; Reynes, J.; et al. Cryptococcus genetic diversity and mixed infections in Ivorian HIV patients: A follow up study. PLoS Negl. Trop. Dis. 2019, 13, e0007812. [Google Scholar] [CrossRef]
- Bertout, S.; Roger, F.; Drakulovski, P.; Martin, A.S.; Gouveia, T.; Kassi, F.; Menan, H.; Krasteva, D.; Delaporte, E.; Bellet, V. African ST173 Cryptococcus deuterogattii strains are commonly less susceptible to fluconazole: An unclear mechanism of resistance. J. Glob. Antimicrob. Resist. 2020, 21, 262–269. [Google Scholar] [CrossRef]
- Clinical Laboratory Standards Institute (CLSI). Epidemiological Cutoff Values for Antifungal Susceptibility Testing. Approved Standard. M59, 2nd ed.; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Espinel-Ingroff, A.; Aller, A.I.; Canton, E.; Castañón-Olivares, L.R.; Chowdhary, A.; Cordoba, S. Cryptococcus neoformans-Cryptococcus gattii species complex: An international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for fluconazole, itraconazole, posaconazole, and voriconazole. Antimicrob. Agents Chemother. 2012, 56, 5898–5906. [Google Scholar] [CrossRef]
- Hagen, F.; Colom, M.F.; Swinne, D.; Tintelnot, K.; Iatta, R.; Montagna, M.T.; Torres-Rodriguez, J.M.; Cogliati, M.; Velegraki, A.; Burggraaf, A.; et al. Autochthonous and Dormant Cryptococcus gattii Infections in Europe. Emerg. Infect. Dis. 2012, 18, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.A.; Trevijano-Contador, N.; Scorzoni, L.; Mesa-Arango, A.C.; de Oliveira, H.C.; Werther, K.; de Freitas Raso, T.; Mendes-Giannini, M.J.; Zaragoza, O.; Fusco-Almeida, A.M. Impact of Resistance to Fluconazole on Virulence and Morphological Aspects of Cryptococcus neoformans and Cryptococcus gattii Isolates. Front. Microbiol. 2016, 16, 153. [Google Scholar]
- Yang, M.L.; Uhrig, J.; Vu, K.; Singapuri, A.; Dennis, M.; Gelli, A.; Thompson, G.R., 3rd. Fluconazole Susceptibility in Cryptococcus gattii Is Dependent on the ABC Transporter Pdr11. Antimicrob. Agents Chemother. 2015, 60, 1202–1207. [Google Scholar] [CrossRef]
- Chang, M.; Sionov, E.; Khanal Lamichhane, A.; Kwon-Chung, K.J.; Chang, Y.C. Roles of Three Cryptococcus neoformans and Cryptococcus gattii Efflux Pump-Coding Genes in Response to Drug Treatment. Antimicrob. Agents Chemother. 2018, 62, e01751-17. [Google Scholar] [CrossRef]
- Oliveira, N.K.; Bhattacharya, S.; Gambhir, R.; Joshi, M.; Fries, B.C. Novel ABC Transporter Associated with Fluconazole Resistance in Aging of Cryptococcus neoformans. J. Fungi 2022, 8, 677. [Google Scholar] [CrossRef]
- Winski, C.J.; Qian, Y.; Mobashery, S.; Santiago-Tirado, F.H. An Atypical ABC Transporter Is Involved in Antifungal Resistance and Host Interactions in the Pathogenic Fungus Cryptococcus neoformans. mBio 2022, 13, e0153922. [Google Scholar] [CrossRef] [PubMed]
- Velez, N.; Alvarado, M.; Parra-Giraldo, C.M.; Sánchez-Quitian, Z.A.; Escandón, P.; Castañeda, E. Genotypic Diversity Is Independent of Pathogenicity in Colombian Strains of Cryptococcus neoformans and Cryptococcus gattii in Galleria mellonella. J. Fungi 2018, 4, 82. [Google Scholar] [CrossRef]
- Firacative, C.; Duan, S.; Meyer, W. Galleria mellonella Model Identifies Highly Virulent Strains among All Major Molecular Types of Cryptococcus gattii. PLoS ONE 2014, 9, e105076. [Google Scholar] [CrossRef] [PubMed]
- Kaocharoen, S.; Ngamskulrungroj, P.; Firacative, C.; Trilles, L.; Piyabongkarn, D.; Banlunara, W.; Poonwan, N.; Chaiprasert, A.; Meyer, W.; Chindamporn, A. Molecular Epidemiology Reveals Genetic Diversity amongst Isolates of the Cryptococcus neoformans/C. gattii Species Complex in Thailand. PLoS Negl. Trop. Dis. 2013, 7, e2297. [Google Scholar] [CrossRef] [PubMed]
- Souto, A.C.P.; Bonfietti, L.X.; Ferreira-Paim, K.; Trilles, L.; Martins, M.; Ribeiro-Alves, M.; Pham, C.D.; Martins, L.; dos Santos, W.; Chang, M.; et al. Population Genetic Analysis Reveals a High Genetic Diversity in the Brazilian Cryptococcus gattii VGII Population and Shifts the Global Origin from the Amazon Rainforest to the Semi-arid Desert in the Northeast of Brazil. PLoS Negl. Trop. Dis. 2016, 10, e0004885. [Google Scholar] [CrossRef]
- Beceiro, A.; Tomas, M.; Bou, G. Antimicrobial Resistance and Virulence: A Successful or Deleterious Association in the Bacterial World? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef] [PubMed]
- Say Coskun, U.S. Investigation of the relationship between virulence factors and antibiotic resistance of Enterococci isolates. Cell. Mol. Biol. Noisy Gd. Fr. 2019, 65, 14–17. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Kontoyiannis, D.P. Resistance to echinocandins comes at a cost: The impact of FKS1 hotspot mutations on Candida albicans fitness and virulence. Virulence 2012, 3, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.R.; Holanda, R.A.; Frases, S.; Bravim, M.; Araujo Gde, S.; Santos, P.C.; Costa, M.C.; Ribeiro, M.J.; Ferreira, G.F.; Baltazar, L.M.; et al. Fluconazole alters the polysaccharide capsule of Cryptococcus gattii and leads to distinct behaviors in murine Cryptococcosis. PLoS ONE 2014, 9, e112669. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertout, S.; Laroche, L.; Roger, F.; Krasteva, D.; Drakulovski, P.; Bellet, V. Fluconazole Resistance and Virulence in In Vitro Induced-Fluconazole Resistant Strains and in Clinical Fluconazole Resistant Strain of Cryptococcus deuterogattii. Pathogens 2023, 12, 758. https://doi.org/10.3390/pathogens12060758
Bertout S, Laroche L, Roger F, Krasteva D, Drakulovski P, Bellet V. Fluconazole Resistance and Virulence in In Vitro Induced-Fluconazole Resistant Strains and in Clinical Fluconazole Resistant Strain of Cryptococcus deuterogattii. Pathogens. 2023; 12(6):758. https://doi.org/10.3390/pathogens12060758
Chicago/Turabian StyleBertout, Sébastien, Laetitia Laroche, Frédéric Roger, Donika Krasteva, Pascal Drakulovski, and Virginie Bellet. 2023. "Fluconazole Resistance and Virulence in In Vitro Induced-Fluconazole Resistant Strains and in Clinical Fluconazole Resistant Strain of Cryptococcus deuterogattii" Pathogens 12, no. 6: 758. https://doi.org/10.3390/pathogens12060758
APA StyleBertout, S., Laroche, L., Roger, F., Krasteva, D., Drakulovski, P., & Bellet, V. (2023). Fluconazole Resistance and Virulence in In Vitro Induced-Fluconazole Resistant Strains and in Clinical Fluconazole Resistant Strain of Cryptococcus deuterogattii. Pathogens, 12(6), 758. https://doi.org/10.3390/pathogens12060758







