Exposure of Cryptococcus neoformans to Seven Commonly Used Agricultural Azole Fungicides Induces Resistance to Fluconazole as Well as Cross-Resistance to Voriconazole, Posaconazole, Itraconazole and Isavuconazole
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Azole Antifungals
2.3. In Vitro Exposure to Antifungal Pesticides
2.4. Antifungal Susceptibility Testing
2.5. ERG11 Gene Mutation Assessment
2.6. mRNA Expression Level Quantification
3. Results
3.1. Mean Growth in Regard to the Pesticide Concentration Range
3.2. Increase in MIC of FCZ after Exposure to Pesticide
3.3. Increase in MIC of Other Azoles
3.4. Theorical Field Surface where Colonies with Elevated FCZ MIC May Appear
3.5. Assessment of ERG11 Point Mutations
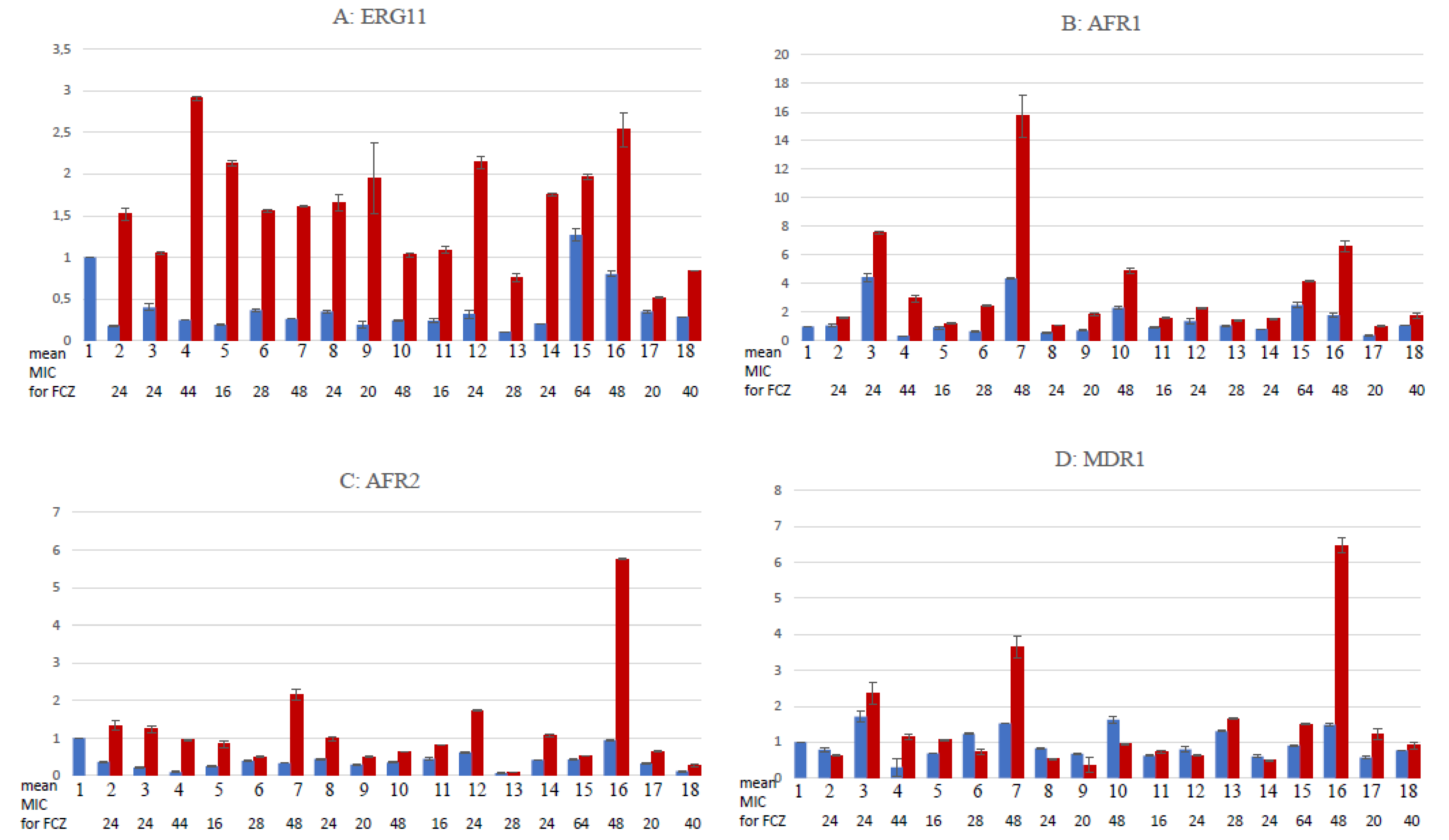
3.6. Gene Expression Quantification
4. Discussion
4.1. Gradation of Risk of Generating Strains with High MIC of FCZ by Pesticide
4.2. Cross-Resistance to Other Medical Azoles
4.3. Estimation of Field Areas at Risk of Generating High-MIC Fungi
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Naughton, S.X.; Terry, A.V., Jr. Neurotoxicity in acute and repeated organophosphate exposure. Toxicology 2018, 408, 101–112. [Google Scholar] [CrossRef]
- Karalexi, M.A.; Tagkas, C.F.; Markozannes, G.; Tseretopoulou, X.; Hernández, A.F.; Schüz, J.; Halldorsson, T.I.; Psaltopoulou, T.; Petridou, E.T.; Tzoulaki, I.; et al. Exposure to pesticides and childhood leukemia risk: A systematic review and meta-analysis. Environ. Pollut. 2021, 285, 117376. [Google Scholar] [CrossRef]
- Pascale, A.; Laborde, A. Impact of pesticide exposure in childhood. Rev. Environ. Health. 2020, 35, 221–227. [Google Scholar] [CrossRef]
- Moreau, J.; Rabdeau, J.; Badenhausser, I.; Giraudeau, M.; Sepp, T.; Crépin, M.; Gaffard, A.; Bretagnolle, V.; Monceau, K. Pesticide impacts on avian species with special reference to farmland birds: A review. Environ. Monit. Assess. 2022, 194, 790. [Google Scholar] [CrossRef]
- Marques, R.D.; Lima, M.A.P.; Marques, R.D.; Bernardes, R.C. A Spinosad-Based Formulation Reduces the Survival and Alters the Behavior of the Stingless Bee Plebeia lucii. Neotrop. Entomol. 2020, 49, 578–585. [Google Scholar] [CrossRef]
- Walker, E.K.; Brock, G.N.; Arvidson, R.S.; Johnson, R.M. Acute Toxicity of Fungicide-Insecticide-Adjuvant Combinations Applied to Almonds During Bloom on Adult Honey Bees. Environ. Toxicol. Chem. 2022, 41, 1042–1053. [Google Scholar] [CrossRef]
- Parker, J.E.; Warrilow, A.G.; Price, C.L.; Mullins, J.G.; Kelly, D.E.; Kelly, S.L. Resistance to antifungals that target CYP51. J. Chem. Biol. 2014, 7, 143–161. [Google Scholar] [CrossRef]
- Chen, P.; Liu, J.; Zeng, M.; Sang, H. Exploring the molecular mechanism of azole resistance in Aspergillus fumigatus. J. Mycol. Med. 2020, 30, 100915. [Google Scholar] [CrossRef]
- Pérez-Cantero, A.; López-Fernández, L.; Guarro, J.; Capilla, J. Azole resistance mechanisms in Aspergillus: Update and recent advances. Int. J. Antimicrob. Agents 2020, 55, 105807. [Google Scholar] [CrossRef]
- Allizond, V.; Comini, S.; Bianco, G.; Costa, C.; Boattini, M.; Mandras, N. Exposure to the agricultural fungicide tebuconazole promotes Aspergillus fumigatus cross-resistance to clinical azoles. New Microbiol. 2021, 44, 234–240. [Google Scholar] [PubMed]
- Wang, F.; Yao, S.; Cao, D.; Ju, C.; Yu, S.; Xu, S.; Fang, H.; Yu, Y. Increased triazole-resistance and cyp51A mutations in Aspergillus fumigatus after selection with a combination of the triazole fungicides difenoconazole and propiconazole. J. Hazard. Mater. 2020, 400, 123200. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Wu, R.; Dong, S.; Wang, F.; Ju, C.; Yu, S.; Xu, S.; Fang, H.; Yu, Y. Triazole resistance in Aspergillus fumigatus in crop plant soil after tebuconazole applications. Environ. Pollut. 2020, 266 Pt 1, 115124. [Google Scholar] [CrossRef]
- Cao, D.; Wang, F.; Yu, S.; Dong, S.; Wu, R.; Cui, N.; Ren, J.; Xu, T.; Wang, S.; Wang, M.; et al. Prevalence of Azole-Resistant Aspergillus fumigatus is Highly Associated with Azole Fungicide Residues in the Fields. Environ. Sci. Technol. 2021, 55, 3041–3049. [Google Scholar] [CrossRef]
- Bradley, K.; Le-Mahajan, A.; Morris, B.; Peritz, T.; Chiller, T.; Forsberg, K.; Nunnally, N.S.; Lockhart, S.R.; Gold, J.A.; Gould, J.M. Fatal Fungicide-Associated Triazole-Resistant Aspergillus fumigatus Infection, Pennsylvania, USA. Emerg. Infect. Dis. 2022, 28, 1904–1905. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; El Chazli, Y.; Babu, A.F.; Coste, A.T. Azole Resistance in Aspergillus fumigatus: A Consequence of Antifungal Use in Agriculture? Front. Microbiol. 2017, 8, 1024. [Google Scholar] [CrossRef]
- Vaezi, A.; Fakhim, H.; Javidnia, J.; Khodavaisy, S.; Abtahian, Z.; Vojoodi, M.; Nourbakhsh, F.; Badali, H. Pesticide behavior in paddy fields and development of azole-resistant Aspergillus fumigatus: Should we be concerned? J. Mycol. Med. 2018, 28, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Ahangarkani, F.; Puts, Y.; Nabili, M.; Khodavaisy, S.; Moazeni, M.; Salehi, Z.; Kargar, M.L.; Badali, H.; Meis, J.F. First azole-resistant Aspergillus fumigatus isolates with the environmental TR46/Y121F/T289A mutation in Iran. Mycoses 2020, 63, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Sirag, B.; Khidir, E.S.; Dumyati, M.; Sindi, B.; Alsinnari, M.; Faidah, H.; Ahmed, A. Cryptococcus neoformans and Other Opportunistic Cryptococcus Species in Pigeon Dropping in Saudi Arabia: Identification and Characterization by DNA Sequencing. Front. Microbiol. 2021, 12, 726203. [Google Scholar] [CrossRef]
- Serna-Espinosa, B.N.; Guzmán-Sanabria, D.; Forero-Castro, M.; Escandón, P.; Sánchez-Quitian, Z.A. Environmental Status of Cryptococcus neoformans and Cryptococcus gattii in Colombia. J. Fungi 2021, 7, 410. [Google Scholar] [CrossRef]
- Dos Santos Bentes, A.; Wanke, B.; Dos Santos Lazéra, M.; Freire, A.K.L.; da Silva Júnior, R.M.; Rocha, D.F.S.; Pinheiro, S.B.; Zelski, S.E.; Matsuura, A.B.J.; da Rocha, L.C.; et al. Cryptococcus gattii VGII isolated from native forest and river in Northern Brazil. Braz. J. Microbiol. 2019, 50, 495–500. [Google Scholar] [CrossRef]
- Jesus, M.S.; Rodrigues, W.C.; Barbosa, G.; Trilles, L.; Wanke, B.; Lazéra Mdos, S.; Silva, M.d. Cryptococcus neoformans carried by Odontomachus bauri ants. Memórias Inst. Oswaldo Cruz 2012, 107, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Schmertmann, L.J.; Irinyi, L.; Malik, R.; Powell, J.R.; Meyer, W.; Krockenberger, M.B. The mycobiome of Australian tree hollows in relation to the Cryptococcus gattii and C. neoformans species complexes. Ecol Evol. 2019, 9, 9684–9700. [Google Scholar] [CrossRef] [PubMed]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The microbial ecology of wine grape berries. Int. J. Food Microbiol. 2012, 153, 243–259. [Google Scholar] [CrossRef]
- Pasquier, E.; Kunda, J.; De Beaudrap, P.; Loyse, A.; Temfack, E.; Molloy, S.F.; Harrison, T.S.; Lortholary, O. Long-term Mortality and Disability in Cryptococcal Meningitis: A Systematic Literature Review. Clin. Infect. Dis. 2018, 66, 1122–1132. [Google Scholar] [CrossRef]
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B.; Chiller, T.M.; Boulware, D.R. The global burden of HIV-associated cryptococcal infection in adults in 2020: A modelling analysis. Lancet Infect. Dis. 2022, 22, 1748–1755. [Google Scholar] [CrossRef]
- Assogba, K.; Belo, M.; Wateba, M.I.; Gnonlonfoun, D.D.; Ossou-Inguiet, P.M.; Tsanga, B.B. Neuromeningeal cryptococcosis in sub-Saharan Africa: Killer disease with sparse data. J. Neurosci. Rural Pract. 2015, 6, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.; Smart, J.; Goeb, J.; Tschirley, D. Pesticide use in Sub-Saharan Africa: Estimates, projections, and implications in the context of food system transformation. In Proceedings of the 2018 Agricultural and Applied Economics Association Annual Meeting, Washington, DC, USA, 5–7 August 2018. [Google Scholar]
- Pesticide Management in West Africa/Gestion des pesticides en Afrique de l’Ouest. Special Issue. Towards the Harmonization of Pesticides Legislation and Registration in West and Central Africa. Numéro Spécial: Vers une Harmonisation de L’homologation et de la Législation sur les Pesticides en Afrique de l’Ouest et du Centre. Newsletter Jointly Published by FAO and ECOWAS/Bulletin D’information Publié Conjointement par la FAO et la CEDEAO. Number 8. November 2011. Available online: https://www.fao.org/3/ap291b/ap291b00.htm (accessed on 21 June 2021).
- de Bon, H.; Huat, J.; Parrot, L.; Sinzogan, A.; Martin, T.; Malézieux, E.; Vayssières, J.-F. Pesticide risks from fruit and vegetable pest management by small farmers in sub-Saharan Africa. A review. Agron. Sustain. Dev. 2014, 34, 723–736. [Google Scholar] [CrossRef]
- Bertout, S.; Drakulovski, P.; Kouanfack, C.; Krasteva, D.; Ngouana, T.; Dunyach-Rémy, C.; Dongtsa, J.; Aghokeng, A.; Delaporte, E.; Koulla-Shiro, S.; et al. Genotyping and antifungal susceptibility testing of Cryptococcus neoformans isolates from Cameroonian HIV-positive adult patients. Clin. Microbiol. Infect. 2013, 19, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Ngouana, T.K.; Dongtsa, J.; Kouanfack, C.; Tonfack, C.; Fomena, S.; Mallié, M.; Delaporte, E.; Boyom, F.-F.; Bertout, S. Cryptoccocal meningitis in Yaoundé (Cameroon) HIV infected patients: Diagnosis, frequency and Cryptococcus neoformans isolates susceptibility study to fluconazole. J. Mycol. Med. 2015, 25, 11–16. [Google Scholar] [CrossRef]
- Ngouana, T.K.; Drakulovski, P.; Krasteva, D.; Kouanfack, C.; Reynes, J.; Delaporte, E.; Boyom, F.F.; Mallié, M.; Bertout, S. Cryptococcus neoformans isolates from Yaoundé human immunodeficiency virus-infected patients exhibited intra-individual genetic diversity and variation in antifungal susceptibility profiles between isolates from the same patient. J. Med. Microbiol. 2016, 65, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Kassi, F.K.; Drakulovski, P.; Bellet, V.; Roger, F.; Chabrol, A.; Krasteva, D.; Doumbia, A.; Landman, R.; Kakou, A.; Reynes, J.; et al. Cryptococcus genetic diversity and mixed infections in Ivorian HIV patients: A follow up study. PLoS Negl. Trop Dis. 2019, 13, e0007812. [Google Scholar] [CrossRef] [PubMed]
- Bive, B.Z.; Sacheli, R.; Nani-Tuma, H.S.; Zakayi, P.K.; Ka, A.; Mambimbi, M.M.; Muendele, G.; Boreux, R.; Landu, N.; Mudogo, C.N.; et al. Clinical epidemiology and high genetic diversity amongst Cryptococcus spp. isolates infecting people living with HIV in Kinshasa, Democratic Republic of Congo. PLoS ONE 2022, 17, e0267842. [Google Scholar] [CrossRef] [PubMed]
- Naicker, S.D.; Mpembe, R.S.; Maphanga, T.G.; Zulu, T.G.; Desanto, D.; Wadula, J.; Mvelase, N.; Maluleka, C.; Reddy, K.; Dawood, H.; et al. Decreasing fluconazole susceptibility of clinical South African Cryptococcus neoformans isolates over a decade. PLoS Negl. Trop Dis. 2020, 14, e0008137. [Google Scholar] [CrossRef] [PubMed]
- Naicker, S.D.; Firacative, C.; van Schalkwyk, E.; Maphanga, T.G.; Monroy-Nieto, J.; Bowers, J.R.; Engelthaler, D.M.; Meyer, W.; Govender, N.P.; Germs-Sa, F. Molecular type distribution and fluconazole susceptibility of clinical Cryptococcus gattii isolates from South African laboratory-based surveillance, 2005–2013. PLoS Negl. Trop Dis. 2022, 16, e0010448. [Google Scholar] [CrossRef]
- Bongomin, F.; Oladele, R.O.; Gago, S.; Moore, C.B.; Richardson, M.D. A systematic review of fluconazole resistance in clinical isolates of Cryptococcus species. Mycoses 2018, 61, 290–297. [Google Scholar] [CrossRef]
- Smith, K.D.; Achan, B.; Hullsiek, K.H.; McDonald, T.R.; Okagaki, L.H.; Alhadab, A.A.; Akampurira, A.; Rhein, J.R.; Meya, D.B.; Boulware, D.R.; et al. Increased Antifungal Drug Resistance in Clinical Isolates of Cryptococcus neoformans in Uganda. Antimicrob Agents Chemother. 2015, 59, 7197–7204. [Google Scholar] [CrossRef]
- Lortholary, O. Management of cryptococcal meningitis in AIDS: The need for specific studies in developing countries. Clin. Infect. Dis. 2007, 45, 81–83. [Google Scholar] [CrossRef]
- Cryptococcus: From Human Pathogen to Model Yeast, 1st ed.; Heitman, J., Kozel, T.R., Kwon-Chung, K.J., Perfect, J.R., Eds.; ASM Press: Washington, DC, USA, 2011. [Google Scholar]
- Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast, 4th ed.; CLSI Standard; Clinical and Laboratory Standards Institute M27: Wayne, PA, USA, 2017.
- Pfaller, M.A.; Castanheira, M.; Diekema, D.J.; Messer, S.A.; Jones, R.N. Wild-type MIC distributions and epidemiologic cutoff values for fluconazole, posaconazole, and voriconazole when testing Cryptococcus neoformans as determined by the CLSI broth microdilution method. Diagn. Microbiol. Infect. Dis. 2011, 71, 252–259. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Cantón, E.; Pemán, J. Antifungal Resistance among Less Prevalent Candida Non-albicans and Other Yeasts versus Established and under Development Agents: A Literature Review. J. Fungi 2021, 7, 24. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Chowdhary, A.; Gonzalez, G.M.; Guinea, J.; Hagen, F.; Meis, J.F.; Thompson, G.R.; Turnidge, J. Multicenter study of isavuconazole MIC distributions and epidemiological cutoff values for the Cryptococcus neoformans-Cryptococcus gattii species complex using the CLSI M27-A3 broth microdilution method. Antimicrob. Agents Chemother. 2015, 59, 666–668. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Aller, A.I.; Canton, E.; Castañón-Olivares, L.R.; Chowdhary, A.; Cordoba, S.; Cuenca-Estrella, M.; Fothergill, A.; Fuller, J.; Govender, N.; et al. Cryptococcus neoformans-Cryptococcus gattii species complex: An international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for fluconazole, itraconazole, posaconazole, and voriconazole. Antimicrob. Agents Chemother. 2012, 56, 5898–5906. [Google Scholar] [CrossRef]
- Druart, C.; Millet, M.; Scheifler, R.; Delhomme, O.; Raeppel, C.; de Vaufleury, A. Snails as indicators of pesticide drift, deposit, transfer and effects in the vineyard. Sci. Total Environ. 2011, 409, 4280–4288. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Li, D.; Wu, Y.; Pan, C. Deposition and distribution of myclobutanil and tebuconazole in a semidwarf apple orchard by hand-held gun and air-assisted sprayer application. Pest Manag. Sci. 2020, 76, 4123–4130. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.W.; Carneiro, H.C.S.; Oliveira, L.V.N.; Rocha, K.M.; Freitas, G.J.C.; Costa, M.C.; Magalhães, T.F.F.; Carvalho, V.S.D.; Rocha, C.E.; Ferreira, G.F.; et al. Environmental Triazole Induces Cross-Resistance to Clinical Drugs and Affects Morphophysiology and Virulence of Cryptococcus gattii and C. neoformans. Antimicrob. Agents Chemother. 2017, 62, e01179-17. [Google Scholar] [CrossRef]
- Bertrand, P.G. Uses and Misuses of Agricultural Pesticides in Africa: Neglected Public Health Threats for Workers and Population. In Pesticides-Use and Misuse and Their Impact in the Environment; Larramendy, M., Soloneski, S., Eds.; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/chapters/65752 (accessed on 20 January 2023).
- Aller, A.I.; Martin-Mazuelos, E.; Lozano, F.; Gomez-Mateos, J.; Steele-Moore, L.; Holloway, W.J.; Gutiérrez, M.J.; Recio, F.J.; Espinel-Ingroff, A. Correlation of fluconazole MICs with clinical outcome in cryptococcal infection. Antimicrob. Agents Chemother. 2000, 44, 1544–1548. [Google Scholar] [CrossRef]
- Mwaba, P.; Mwansa, J.; Chintu, C.; Pobee, J.; Scarborough, M.; Portsmouth, S.; Zumla, A. Clinical presentation, natural history, and cumulative death rates of 230 adults with primary cryptococcal meningitis in Zambian AIDS patients treated under local conditions. Postgrad. Med. J. 2001, 77, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Schaars, C.F.; Meintjes, G.A.; Morroni, C.; Post, F.A.; Maartens, G. Outcome of AIDS-associated cryptococcal meningitis initially treated with 200 mg/day or 400 mg/day of fluconazole. BMC Infect. Dis. 2006, 6, 118. [Google Scholar] [CrossRef]
- Bicanic, T.; Meintjes, G.; Wood, R.; Hayes, M.; Rebe, K.; Bekker, L.-G.; Harrison, T. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin. Infect. Dis. 2007, 45, 76–80. [Google Scholar] [CrossRef]
- Hope, W.; Stone, N.R.H.; Johnson, A.; McEntee, L.; Farrington, N.; Santoro-Castelazo, A.; Liu, X.; Lucaci, A.; Hughes, M.; Oliver, J.D.; et al. Fluconazole Monotherapy Is a Suboptimal Option for Initial Treatment of Cryptococcal Meningitis Because of Emergence of Resistance. mBio 2019, 10, e02575-19. [Google Scholar] [CrossRef]
- Mandras, N.; Roana, J.; Tullio, V.; Allizond, V.; Banche, G.; Scalas, D.; Fucale, G.; Cuffini, A. A case of fluconazole, voriconazole-resistant Cryptococcus neoformans isolated from and immunocompetent patient. J. Chemother. 2011, 23, 379–380. [Google Scholar] [CrossRef]
- Kano, R.; Okubo, M.; Hasegawa, A.; Kamata, H. Multi-azole-resistant strains of Cryptococcus neoformans var. grubii isolated from a FLZ-resistant strain by culturing in medium containing voriconazole. Med. Mycol. 2017, 55, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Bosco-Borgeat, M.E.; Mazza, M.; Taverna, C.G.; Córdoba, S.; Murisengo, O.A.; Vivot, W.; Davel, G. Amino acid substitution in Cryptococcus neoformans lanosterol 14-α-demethylase involved in fluconazole resistance in clinical isolates. Rev. Argent. Microbiol. 2016, 48, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Sionov, E.; Khanal Lamichhane, A.; Kwon-Chung, K.J.; Chang, Y.C. Roles of Three Cryptococcus neoformans and Cryptococcus gattii Efflux Pump-Coding Genes in Response to Drug Treatment. Antimicrob. Agents Chemother. 2018, 62, e01751-17. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Altamirano, S.; Ballou, E.R.; Nielsen, K. A titanic drug resistance threat in Cryptococcus neoformans. Curr. Opin Microbiol. 2019, 52, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Bouklas, T.; Pechuan, X.; Goldman, D.L.; Edelman, B.; Bergman, A.; Fries, B.C. Old Cryptococcus neoformans cells contribute to virulence in chronic cryptococcosis. mBio 2013, 4, e00455-13. [Google Scholar] [CrossRef]
- Oliveira, N.K.; Bhattacharya, S.; Gambhir, R.; Joshi, M.; Fries, B.C. Novel ABC Transporter Associated with Fluconazole Resistance in Aging of Cryptococcus neoformans. J. Fungi 2022, 8, 677. [Google Scholar] [CrossRef]
- Winski, C.J.; Qian, Y.; Mobashery, S.; Santiago-Tirado, F.H. An Atypical ABC Transporter Is Involved in Antifungal Resistance and Host Interactions in the Pathogenic Fungus Cryptococcus neoformans. mBio 2022, 13, e0153922. [Google Scholar] [CrossRef]
- Quinn, L.; de Vos, J.; Fernandes-Whaley, M.; Roos, C.; Bouwman, H.; Kylin, H.; Pieters, R.; Berg, R.P.A.J.V.D. Pesticide Use in South Africa: One of the Largest Importers of Pesticides in Africa. In Pesticides in the Modern World-Pesticides Use and Management; Stoytcheva, M., Ed.; IntechOpen: London, UK, 2011; pp. 49–96. Available online: http://www.intechopen.com/books/pesticides-in-the-modern-world-pesticides-use-and-management/pesticide-use-in-south-africa-one-of-the-largest-importers-of-pesticides-in-africa (accessed on 3 January 2023).
- Bromilow, R.H.; Evans, A.A.; Nicholls, P.H. Factors affecting degradation rates of five triazole fungicides in two soil types: 2. Field studies. Pestic. Sci. 1999, 55, 1135–1142. [Google Scholar] [CrossRef]
- Kim, I.S.; Shim, J.H.; Suh, Y.T. Laboratory studies on formation of bound residues and degradation of propiconazole in soils. Pest Manag. Sci. 2003, 59, 324–330. [Google Scholar] [CrossRef]
- Singh, N. Mobility of four triazole fungicides in two Indian soils. Pest Manag. Sci. 2005, 61, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, I.; Friberg, H.; Steinberg, C.; Persson, P. Fungicide effects on fungal community composition in the wheat phyllosphere. PLoS ONE 2014, 9, e111786. [Google Scholar] [CrossRef]
- Beukes, I.; Rose, L.J.; Shephard, G.S.; Flett, B.C.; Viljoen, A. Mycotoxigenic Fusarium species associated with grain crops in South Africa-A review. South Afr. J. Sci. 2017, 113, 12. [Google Scholar] [CrossRef]
- Grover, N.; Nawange, S.R.; Naidu, J.; Singh, S.M.; Sharma, A. Ecological niche of Cryptococcus neoformans var. grubii and Cryptococcus gattii in decaying wood of trunk hollows of living trees in Jabalpur City of Central India. Mycopathologia 2007, 164, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhu, Y.; Zhang, Z.; Cao, Y.; Zhang, W. Variations in Fungal Community and Diversity in Doushen With Different Flavors. Front. Microbiol. 2020, 11, 447. [Google Scholar] [CrossRef]
- Sheahan, M.; Barrett, C.B.; Goldvale, C. Human health and pesticide use in Sub-Saharan Africa. Agric. Econ. 2017, 48, 27–41. [Google Scholar] [CrossRef]
- Kemoi, E.K.; Okemo, P.; Bii, C.C. Presence of Cryptococcus species in domestic chickens (Gallus gallus) droppings and the possible risk it posed to humans in Kabigeriet village, Nakuru County, Kenya. East Afr. Med. J. 2013, 90, 202–206. [Google Scholar]
- Kuroki, M.; Phichaichumpon, C.; Yasuoka, A.; Chiranairadul, P.; Chosa, T.; Sirinirund, P.; Miyazaki, T.; Kakeya, H.; Higashiyama, Y.; Miyazaki, Y.; et al. Environmental isolation of Cryptococcus neoformans from an endemic region of HIV-associated cryptococcosis in Thailand. Yeast 2004, 21, 809–812. [Google Scholar] [CrossRef]

| Antifungal | Acronym | Highest Commercial Concentrations as Advertised by Manufacturers for Use on Fields in Agriculture |
|---|---|---|
| Tebuconazole | TBZ | 250 g L Ha |
| Bromuconazole | BMZ | 200 g L Ha |
| Prothioconazole | PTZ | 200 g L Ha |
| Difenoconazole | DFZ | 125 g L Ha |
| Epoxiconazole | EPZ | 125 g L Ha |
| Penconazole | PNZ | 100 g L Ha |
| Metconazole | MTZ | 90 g L Ha |
| Mean Growth of H99 Culture Exposed to Various Amounts of Antifungals in Comparison with an Untreated Control | ||||||||
|---|---|---|---|---|---|---|---|---|
| Antifungal Molecule | 100% | 75% | 50% | 25% | 15% | 10% | 5% | 2.5% |
| DFZ | 0 | 0 | 0 | 0 | 0 | 0.1% (±0.05) | 9.3% (±2.7) | 64.8% (±11.3) |
| EPZ | 0 | 0 | 0 | 0.06% (±0.03) | 0.24% (±0.11) | 5.15% (±2.5) | 52.4% (±9.1) | |
| PNZ | 2.5% (±1.3) | 14.4% (±7.2) | 57.2% (±15.9) | 91.9% (±12.4) | ||||
| PTZ | 0 | 0 | 0 | 0.06% (±0.02) | 2.5% (±1.5) | 5.6% (±1.7) | 43.6% (±18.1) | |
| TBZ | 0 | 0 | 0 | 2% (±0.23) | 6.7% (±3.1) | 35.8% (±11.9) | 87.1% (±5.2) | |
| MTZ | 0 | 0 | 0 | 0.4% (±0.09) | 1.65% (±1.51) | 5.7% (±0.9) | 86% (±10.8) | |
| BMZ | 0 | 0 | 0 | 4.9% (±2.2) | 11.6% (±4.4) | 57.2% (±13.9) | 80.1% (±14.1) | |
| Distribution of 30 Colonies Selected by Exposure to Pesticides According to Their MIC of Fluconazole (µg/mL) | % of Colonies with Elevated MIC of FCZ | % of Colonies with a Phenotype of Resistance to FCZ | |||
|---|---|---|---|---|---|
| Antifungal Molecules | 0.5–2 | 4–8 | 16–64 | ||
| DFZ | 26 | 1 | 3 | 13.3% | 10% |
| EPZ | 22 | 6 | 2 | 26.6% | 6.6% |
| PNZ | 22 | 5 | 3 | 26.6% | 10% |
| TBZ | 25 | 4 | 1 | 16.6% | 3.3% |
| MTZ | 26 | 3 | 1 | 13.3% | 3.3% |
| BMZ | 22 | 5 | 3 | 26.6% | 10% |
| PTZ | 23 | 3 | 4 | 23.3% | 13.3% |
| Mean MIC ATF 72h | |||||
|---|---|---|---|---|---|
| Clones | FCZ | POZ | VOR | ITR | ISV |
| H99 | 0.5–2 | 0.125 | 0.06 | 0.125 | <0.03 |
| DFZ 5 | 24 | <0.03 | <0.03 | <0.03 | 0.06 |
| DFZ 6 | 24 | <0.03 | 0.06 | <0.03 | <0.03 |
| DFZ 7 | 44 | 0.25 | 0.0925 | 0.125 | 0.25 |
| EPZ 20 | 16 | <0.03 | 0.125 | 0.125 | 0.125 |
| EPZ 29 | 28 | 0.06 | <0.03 | <0.03 | <0.03 |
| PNZ 3 | 48 | <0.03 | 0.06 | 0.5 | 0.125 |
| PNZ 29 | 24 | 0.06 | <0.03 | 0.06 | <0.03 |
| PNZ 30 | 20 | 0.5 | 0.0925 | 0.1875 | 0.06 |
| TBZ 14 | 48 | 1 | 0.25 | 1 | 0.5 |
| MTZ 11 | 16 | 0.5 | 0.125 | 0.25 | <0.03 |
| BMZ 5 | 24 | 0.06 | 0.125 | 0.25 | <0.03 |
| BMZ 19 | 28 | <0.03 | <0.03 | 0.06 | <0.03 |
| BMZ 30 | 24 | <0.03 | 0.06 | 0.06 | <0.03 |
| PTZ 4 | 64 | 0.25 | 0.125 | 0.25 | 0.125 |
| PTZ 7 | 48 | 0.1875 | 0.06 | 0.125 | <0.03 |
| PTZ 8 | 20 | <0.03 | <0.03 | <0.03 | <0.03 |
| PTZ 9 | 40 | 0.5 | 0.1875 | 0.25 | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drakulovski, P.; Krasteva, D.; Bellet, V.; Randazzo, S.; Roger, F.; Pottier, C.; Bertout, S. Exposure of Cryptococcus neoformans to Seven Commonly Used Agricultural Azole Fungicides Induces Resistance to Fluconazole as Well as Cross-Resistance to Voriconazole, Posaconazole, Itraconazole and Isavuconazole. Pathogens 2023, 12, 662. https://doi.org/10.3390/pathogens12050662
Drakulovski P, Krasteva D, Bellet V, Randazzo S, Roger F, Pottier C, Bertout S. Exposure of Cryptococcus neoformans to Seven Commonly Used Agricultural Azole Fungicides Induces Resistance to Fluconazole as Well as Cross-Resistance to Voriconazole, Posaconazole, Itraconazole and Isavuconazole. Pathogens. 2023; 12(5):662. https://doi.org/10.3390/pathogens12050662
Chicago/Turabian StyleDrakulovski, Pascal, Donika Krasteva, Virginie Bellet, Sylvie Randazzo, Frédéric Roger, Cyrille Pottier, and Sébastien Bertout. 2023. "Exposure of Cryptococcus neoformans to Seven Commonly Used Agricultural Azole Fungicides Induces Resistance to Fluconazole as Well as Cross-Resistance to Voriconazole, Posaconazole, Itraconazole and Isavuconazole" Pathogens 12, no. 5: 662. https://doi.org/10.3390/pathogens12050662
APA StyleDrakulovski, P., Krasteva, D., Bellet, V., Randazzo, S., Roger, F., Pottier, C., & Bertout, S. (2023). Exposure of Cryptococcus neoformans to Seven Commonly Used Agricultural Azole Fungicides Induces Resistance to Fluconazole as Well as Cross-Resistance to Voriconazole, Posaconazole, Itraconazole and Isavuconazole. Pathogens, 12(5), 662. https://doi.org/10.3390/pathogens12050662








