Serological Prevalence of Crimean–Congo Hemorrhagic Fever Virus Infection in Small Ruminants and Cattle in The Gambia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sample Population
2.2. Serological Analysis
2.3. Statistical Analysis
3. Results
3.1. CCHFV Seroprevalence in Sheep
3.2. CCHFV Seroprevalence in Goats
3.3. CCHFV Seropositivity in the Administrative Regions and Agroecological Zones
3.4. CCHFV Seroprevalence in Cattle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bente, D.A.; Forrester, N.L.; Watts, D.M.; McAuley, A.J.; Whitehouse, C.A.; Bray, M. Crimean-Congo hemorrhagic fever: History, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antivir. Res. 2013, 100, 159–189. [Google Scholar] [CrossRef]
- Spengler, J.R.; Bente, D.A.; Bray, M.; Burt, F.; Hewson, R.; Korukluoglu, G.; Mirazimi, A.; Weber, F.; Papa, A. Second International Conference on Crimean-Congo Hemorrhagic Fever. Antivir. Res. 2018, 150, 137–147. [Google Scholar] [CrossRef]
- Nasirian, H. Crimean-Congo hemorrhagic fever (CCHF) seroprevalence: A systematic review and meta-analysis. Acta Trop. 2019, 196, 102–120. [Google Scholar] [CrossRef] [PubMed]
- Garrison, A.R.; Alkhovsky, S.V.; Avšič-Županc, T.; Bente, D.A.; Bergeron, É.; Burt, F.; Di Paola, N.; Ergünay, K.; Hewson, R.; Kuhn, J.H.; et al. ICTV Virus Taxonomy Profile: Nairoviridae. J. Gen. Virol. 2020, 101, 798–799. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, C.A. Crimean-Congo hemorrhagic fever. Antivir. Res. 2004, 64, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Zivcec, M.; Scholte, F.E.; Spiropoulou, C.F.; Spengler, J.R.; Bergeron, E. Molecular Insights into Crimean-Congo Hemorrhagic Fever Virus. Viruses 2016, 8, 106. [Google Scholar] [CrossRef]
- Mhamadi, M.; Badji, A.; Dieng, I.; Gaye, A.; Ndiaye, E.H.; Ndiaye, M.; Mhamadi, M.; Touré, C.T.; Mbaye, M.R.; Barry, M.A.; et al. Crimean-Congo Hemorrhagic Fever Virus Survey in Humans, Ticks, and Livestock in Agnam (Northeastern Senegal) from February 2021 to March 2022. Trop. Med. Infect. Dis. 2022, 7, 324. [Google Scholar] [CrossRef]
- Gargili, A.; Estrada-Pena, A.; Spengler, J.R.; Lukashev, A.; Nuttall, P.A.; Bente, D.A. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: A review of published field and laboratory studies. Antivir. Res. 2017, 144, 93–119. [Google Scholar] [CrossRef]
- Ksiazek, T.G. Crimean Congo hemorrhagic fever in animals (2022). Merck Vet. Man. 2022. Available online: https://www.msdvetmanual.com/generalized-conditions/crimean-congo-hemorrhagic-fever/crimean-congo-hemorrhagic-fever-in-animals (accessed on 18 May 2023).
- Hartlaub, J.; Daodu, O.B.; Sadeghi, B.; Keller, M.; Olopade, J.; Oluwayelu, D.; Groschup, M.H. Cross-Reaction or Co-Infection? Serological Discrimination of Antibodies Directed against Dugbe and Crimean-Congo Hemorrhagic Fever Orthonairovirus in Nigerian Cattle. Viruses 2021, 13, 1398. [Google Scholar] [CrossRef]
- Schulz, A.; Barry, Y.; Stoek, F.; Ba, A.; Schulz, J.; Haki, M.L.; Sas, M.A.; Doumbia, B.A.; Kirkland, P.; Bah, M.Y.; et al. Crimean-Congo hemorrhagic fever virus antibody prevalence in Mauritanian livestock (cattle, goats, sheep and camels) is stratified by the animal’s age. PLoS Negl. Trop. Dis. 2021, 15, e0009228. [Google Scholar] [CrossRef]
- Akuffo, R.; Brandful, J.A.; Zayed, A.; Adjei, A.; Watany, N.; Fahmy, N.T.; Hughes, R.; Doman, B.; Voegborlo, S.V.; Aziati, D.; et al. Crimean-Congo hemorrhagic fever virus in livestock ticks and animal handler seroprevalence at an abattoir in Ghana. BMC Infect. Dis. 2016, 16, 324. [Google Scholar] [CrossRef] [PubMed]
- Oluwayelu, D.; Afrough, B.; Adebiyi, A.; Varghese, A.; Eun-Sil, P.; Fukushi, S.; Yoshikawa, T.; Saijo, M.; Neumann, E.; Morikawa, S.; et al. Prevalence of Antibodies to Crimean-Congo Hemorrhagic Fever Virus in Ruminants, Nigeria, 2015. Emerg. Infect. Dis. 2020, 26, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Dzikwi-Emennaa, A.A.; Meseko, C.; Emennaa, P.; Adeyinka, A.J.; Adamu, A.M.; Adegboye, O.A. Detection of Crimean-Congo Hemorrhagic Fever Virus Antibodies in Cattle in Plateau State, Nigeria. Viruses 2022, 14, 2618. [Google Scholar] [CrossRef]
- Hoogstraal, H. The Epidemiology of Tick-Borne Crimean-Congo Hemorrhagic Fever in Asia, Europe and Africa. J. Med. Entomol. 1979, 15, 307–417. [Google Scholar] [CrossRef]
- Chunikhin, S.; Chumakov, M.; Butenko, A.; Smirnova, S.; Taufflieb, R.; Camicas, J.-L.; Robin, Y.; Cornet, J.; Shabon, Z. Results from Investigating Human and Domestic and Wild Animal Blood Sera in the Sénégal Republic (Western Africa) for Antibodies to Crimean Hemorrhagic Fever Virus. Mater. 16. Nauchn. Sess. Inst. Polio. Virus. Entsefalitov 1969, 2, 158–160. Available online: https://www.documentation.ird.fr/hor/fdi:07498 (accessed on 18 May 2023).
- Mangombi, J.B.; Roqueplo, C.; Sambou, M.; Dahmani, M.; Mediannikov, O.; Comtet, L.; Davoust, B. Seroprevalence of Crimean-Congo Hemorrhagic Fever in Domesticated Animals in Northwestern Senegal. Vector Borne Zoonotic Dis. 2020, 20, 797–799. [Google Scholar] [CrossRef]
- Faburay, B.; Jongejan, F.; Taoufik, A.; Ceesay, A.; Geysen, D. Genetic diversity of Ehrlichia ruminantium in Amblyomma variegatum ticks and small ruminants in The Gambia determined by restriction fragment profile analysis. Vet. Microbiol. 2007, 126, 189–199. [Google Scholar] [CrossRef]
- Sas, M.A.; Comtet, L.; Donnet, F.; Mertens, M.; Vatansever, Z.; Tordo, N.; Pourquier, P.; Groschup, M.H. A novel double-antigen sandwich ELISA for the species-independent detection of Crimean-Congo hemorrhagic fever virus-specific antibodies. Antivir. Res. 2018, 151, 24–26. [Google Scholar] [CrossRef]
- Belobo, J.T.E.; Kenmoe, S.; Kengne-Nde, C.; Emoh, C.P.D.; Bowo-Ngandji, A.; Tchatchouang, S.; Sowe Wobessi, J.N.; Mbongue Mikangue, C.A.; Tazokong, H.R.; Kingue Bebey, S.R.; et al. Worldwide epidemiology of Crimean-Congo Hemorrhagic Fever Virus in humans, ticks and other animal species, a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2021, 15, e0009299. [Google Scholar] [CrossRef]
- Temur, A.I.; Kuhn, J.H.; Pecor, D.B.; Apanaskevich, D.A.; Keshtkar-Jahromi, M. Epidemiology of Crimean-Congo Hemorrhagic Fever (CCHF) in Africa-Underestimated for Decades. Am. J. Trop. Med. Hyg. 2021, 104, 1978–1990. [Google Scholar] [CrossRef]
- Zohaib, A.; Saqib, M.; Athar, M.A.; Hussain, M.H.; Sial, A.U.; Tayyab, M.H.; Batool, M.; Sadia, H.; Taj, Z.; Tahir, U.; et al. Crimean-Congo Hemorrhagic Fever Virus in Humans and Livestock, Pakistan, 2015–2017. Emerg. Infect. Dis. 2020, 26, 773–777. [Google Scholar] [CrossRef]
- Gueye, A.; Mbengue, M.; Diouf, A.; Vassiliadès, G. Prophylaxis of cowdriosis and observation of pathologies in ovine in the region of Niayes in Senegal. Rev. Elev. Med. Vet. Pays Trop. 1989, 42, 497–503. [Google Scholar]
- Rocha, J.F.; Martínez, R.; López-Villalobos, N.; Morris, S.T. Tick burden in Bos taurus cattle and its relationship with heat stress in three agroecological zones in the tropics of Colombia. Parasites Vectors 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Adjogoua, E.V.; Coulibaly-Guindo, N.; Diaha-Kouame, C.A.; Diane, M.K.; Kouassi, R.; Coulibaly, J.T.; Dosso, M. Geographical Distribution of Ticks Ixodidae in Cote d’Ivoire: Potential Reservoir of the Crimean-Congo Hemorrhagic Fever Virus. Vector Borne Zoonotic Dis. 2021, 21, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Appannanavar, S.B.; Mishra, B. An update on crimean congo hemorrhagic Fever. J. Glob. Infect. Dis. 2011, 3, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.C.; Bah, M.; Faye, J.; Kora, S.; Cassama, M. A comparison of field tick infestation on N’Dama, Zebu and N’Dama x Zebu crossbred cattle. Vet. Parasitol. 1993, 47, 139–148. [Google Scholar] [CrossRef]
- Mattioli, R.C.; Janneh, L.; Corr, N.; Faye, J.A.; Pandey, V.S.; Verhulst, A. Seasonal prevalence of ticks and tick-transmitted haemoparasites in traditionally managed N’Dama cattle with reference to strategic tick control in the Gambia. Med. Vet. Entomol. 1997, 11, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Maiga, O.; Sas, M.A.; Rosenke, K.; Kamissoko, B.; Mertens, M.; Sogoba, N.; Traore, A.; Sangare, M.; Niang, M.; Schwan, T.G.; et al. Serosurvey of Crimean-Congo Hemorrhagic Fever Virus in Cattle, Mali, West Africa. Am. J. Trop. Med. Hyg. 2017, 96, 1341–1345. [Google Scholar] [CrossRef]
- Mariner, J.C.; Morrill, J.; Ksiazek, T.G. Antibodies to hemorrhagic fever viruses in domestic livestock in Niger: Rift Valley fever and Crimean-Congo hemorrhagic fever. Am. J. Trop. Med. Hyg. 1995, 53, 217–221. [Google Scholar] [CrossRef]
- Simo Tchetgna, H.; Yousseu, F.S.; Cosset, F.L.; de Freitas, N.B.; Kamgang, B.; McCall, P.J.; Ndip, R.N.; Legros, V.; Wondji, C.S. Molecular and serological evidence of Crimean-Congo hemorrhagic fever orthonairovirus prevalence in livestock and ticks in Cameroon. Front. Cell. Infect. Microbiol. 2023, 13, 1132495. [Google Scholar] [CrossRef]
- Altaliby, M.A.S.; Esmaeel, S.A.; Hussain, K.J. Seroprevalence of Crimean-Congo Haemorrhagic Fever in sheep and goats in Iraq. Bulg. J. Vet. Med. 2021, 26, 1–6. [Google Scholar] [CrossRef]
- Zouaghi, K.; Bouattour, A.; Aounallah, H.; Surtees, R.; Krause, E.; Michel, J.; Mamlouk, A.; Nitsche, A.; M’ghirbi, Y. First Serological Evidence of Crimean-Congo Hemorrhagic Fever Virus and Rift Valley Fever Virus in Ruminants in Tunisia. Pathogens 2021, 10, 769. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Nijhof, A.M.; Sauter-Louis, C.; Schauer, B.; Staubach, C.; Conraths, F.J. Distribution of ticks infesting ruminants and risk factors associated with high tick prevalence in livestock farms in the semi-arid and arid agro-ecological zones of Pakistan. Parasites Vectors 2017, 10, 190. [Google Scholar] [CrossRef]
- Ceesay, S.J.; Casals-Pascual, C.; Nwakanma, D.C.; Walther, M.; Gomez-Escobar, N.; Fulford, A.J.; Takem, E.N.; Nogaro, S.; Bojang, K.A.; Corrah, T.; et al. Continued decline of malaria in The Gambia with implications for elimination. PLoS ONE 2010, 5, e12242. [Google Scholar] [CrossRef] [PubMed]
- Faburay, B.; Geysen, D.; Ceesay, A.; Marcelino, I.; Alves, P.M.; Taoufik, A.; Postigo, M.; Bell-Sakyi, L.; Jongejan, F. Immunisation of sheep against heartwater in The Gambia using inactivated and attenuated Ehrlichia ruminantium vaccines. Vaccine 2007, 25, 7939–7947. [Google Scholar] [CrossRef] [PubMed]
- Nabeth, P.; Thior, M.; Faye, O.; Simon, F. Human Crimean-Congo hemorrhagic fever, senegal. Emerg. Infect. Dis. 2004, 10, 1881. [Google Scholar] [CrossRef]
- Nabeth, P.; Cheikh, D.O.; Lo, B.; Faye, O.; Vall, I.O.M.; Niang, M.; Wague, B.; Diop, D.; Diallo, M.; Diallo, B. Crimean-Congo hemorrhagic fever, mauritania. Emerg. Infect. Dis. 2004, 10, 2143. [Google Scholar] [CrossRef]

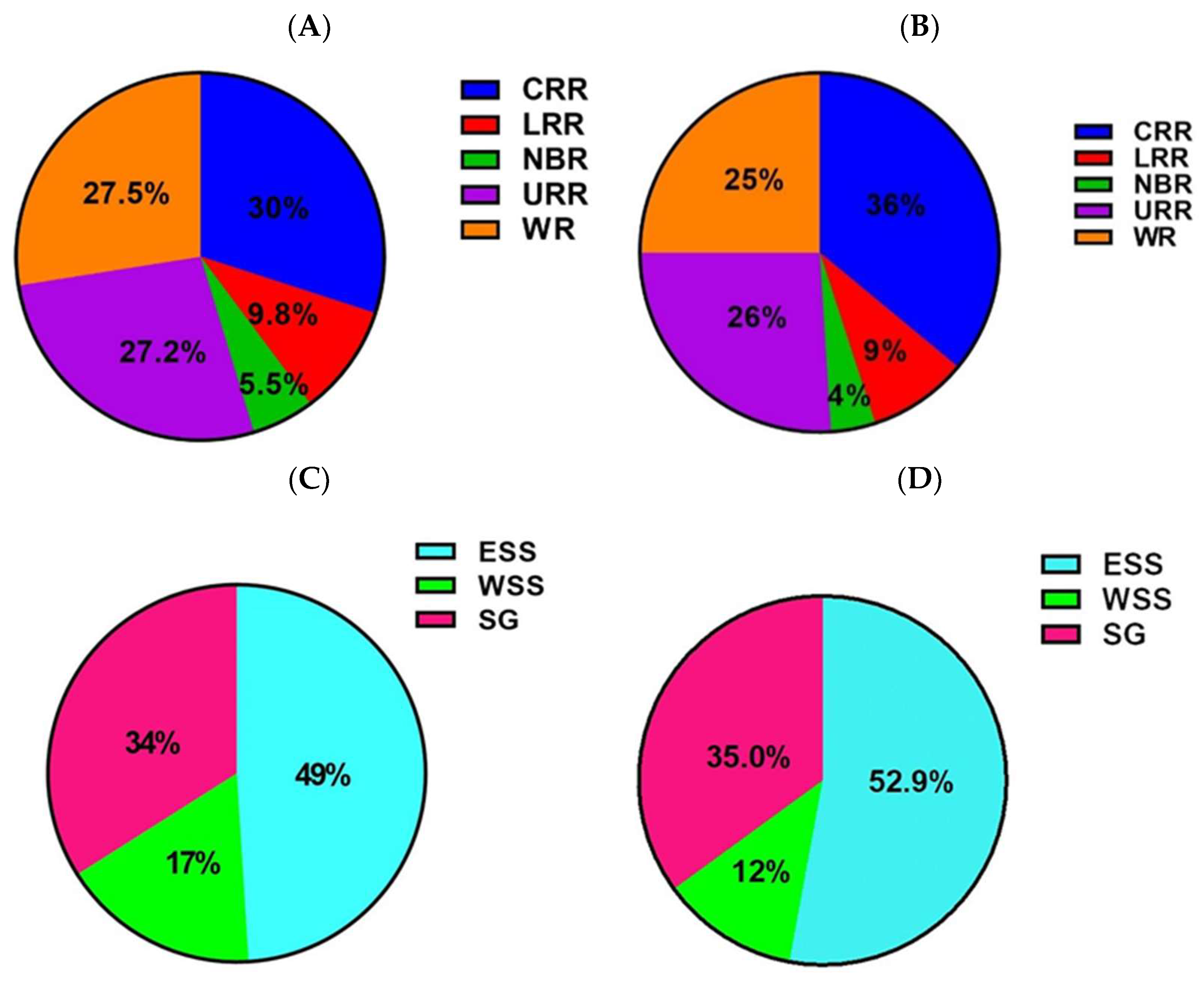

| Variable | * n/N | Seroprevalence % (95% CI) | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Overall | 89/470 | 18.9 (15.5, 22.8) | ||
| Site | ||||
| Abuko abattoir | 37/125 | 29.6 (21.8, 38.4) | REF | |
| Basse livestock market | 8/34 | 23.5 (10.7, 41.2) | 0.73 (0.30, 1.76) | 0.487 |
| Brikama livestock market | 1/34 | 2.9 (0.1, 15.3) | 0.07 (0.01, 0.54) | 0.011 |
| Brikamaba livestock market | 6/53 | 11.3 (4.2, 23.0) | 0.30 (0.11, 0.77) | 0.012 |
| Farafenni livestock market | 1/21 | 4.8 (0.1, 23.8) | 0.11 (0.01, 0.91) | 0.041 |
| Keneba WALIC station | 11/129 | 8.5 (4.3, 14.7) | 0.22 (0.10, 0.45) | <0.001 |
| Soma livestock market | 1/11 | 9.1 (0.2, 41.3) | 0.23 (0.03, 1.92) | 0.178 |
| Sololo WALIC station | 24/63 | 38.1 (26.1, 51.2) | 1.46 (0.77, 2.76) | 0.241 |
| Region | ||||
| Central River | 30/116 | 25.9 (18.2, 34.8) | REF | |
| Lower River | 12/140 | 8.6 (4.5, 14.5) | 0.26 (0.13, 0.55) | <0.001 |
| North Bank | 1/21 | 4.8 (0.1, 23.8) | 0.14 (0.02, 1.11) | 0.063 |
| Upper River | 8/34 | 23.5 (10.7, 41.2) | 0.88 (0.36, 2.15) | 0.783 |
| Western | 38/159 | 23.9 (17.5, 31.3) | 0.90 (0.51, 1.56) | 0.710 |
| Agroecological zone | ||||
| Eastern Sudano Sahelian | 32/97 | 32.9 (23.8, 43.3) | REF | |
| Western Sudano Sahelian | 19/214 | 8.9 (5.4, 13.5) | 0.19 (0.10, 0.37) | <0.001 |
| Sudano Guinean | 38/159 | 23.9 (17.5, 31.3) | 0.63 (0.36, 1.11) | 0.115 |
| Variable | * n/N | Seroprevalence % (95% CI) | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Overall | 49/544 | 9.0 (6.7, 11.7) | ||
| Site | ||||
| Abuko abattoir | 10/51 | 19.6 (9.8, 33.1) | REF | |
| Basse livestock market | 10/79 | 12.6 (6.2, 22.0) | 0.59 (0.22, 1.55) | 0.287 |
| Brikama livestock market | 7/91 | 7.7 (3.1, 15.2) | 0.34 (0.12, 0.96) | 0.042 |
| Brikamaba livestock market | 2/38 | 5.3 (0.6, 17.7) | 0.22 (0.04, 1.10) | 0.067 |
| Farafenni livestock market | 1/56 | 1.8 (0.1, 9.5) | 0.07 (0.01, 0.61) | 0.015 |
| Keneba WALIC station | 7/152 | 4.6 (1.8, 9.2) | 0.19 (0.07, 0.55) | 0.002 |
| Soma livestock market | 2/45 | 4.4 (0.5, 15.1) | 0.19 (0.03, 0.92) | 0.039 |
| Sololo WALIC station | 10/32 | 31.2 (16.1, 50.0) | 1.86 (0.67, 5.15) | 0.231 |
| Region | ||||
| Central River | 12/70 | 17.1 (9.1, 28.0) | REF | |
| Lower River | 9/197 | 4.6 (2.1, 8.5) | 0.23 (0.09, 0.57) | 0.002 |
| North Bank | 1/56 | 1.8 (0.1, 9.5) | 0.08 (0.01, 0.69) | 0.021 |
| Upper River | 10/79 | 12.7 (6.2, 22.0) | 0.70 (0.28, 1.73) | 0.443 |
| Western | 17/142 | 11.9 (7.1, 18.5) | 0.65 (0.29, 1.46) | 0.305 |
| AEZ | ||||
| Eastern Sudano Sahelian | 20/111 | 18.0 (11.3, 26.4) | REF | |
| Western Sudano-Sahelian | 12/291 | 4.1 (2.1, 7.1) | 0.19 (0.09, 0.41) | <0.001 |
| Sudano-Guinean | 17/142 | 11.9 (7.1, 18.5) | 0.61 (0.30, 1.24) | 0.179 |
| Variable | * n/N | Seroprevalence % (95% CI) | Unadjusted Odds Ratio (95% CI) | p-Value | Adjusted Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Cattle seroprevalence | 239/399 | 59.9 (54.9, 64.7) | ||||
| Site | ||||||
| Bajana | 24/30 | 80.0 (61.4, 92.3) | REF | |||
| Bateling | 12/40 | 30.0 (16.6, 46.5) | 0.10 (0.03, 0.32) | <0.001 | 0.10 (0.03, 0.32) | <0.001 |
| Dumbuto | 28/65 | 43.1 (30.8, 55.9) | 0.18 (0.07, 0.52) | 0.001 | 0.20 (0.07, 0.57) | 0.003 |
| Jali | 13/30 | 43.3 (25.5, 62.6) | 0.19 (0.06, 0.60) | 0.005 | 0.19 (0.06, 0.64) | 0.007 |
| Jiffarong | 24/30 | 80.0 (61.4, 92.3) | 1.00 (0.28, 3.54) | 1.000 | 1.26 (0.34, 4.62) | 0.727 |
| Kuli Kunda | 22/30 | 73.3 (54.1, 87.7) | 0.68 (0.21, 2.29) | 0.543 | 0.74 (0.21, 2.54) | 0.635 |
| Niorro Jataba | 36/49 | 73.5 (58.9, 85.1) | 0.69 (0.23, 2.07) | 0.511 | 0.86 (0.27, 2.65) | 0.794 |
| Sankandi | 15/45 | 33.3 (20.0, 48.9) | 0.12 (0.04, 0.37) | <0.001 | 0.12 (0.03, 0.36) | <0.001 |
| Tankular | 23/30 | 76.7 (57.7, 90.1) | 0.82 (0.23, 2.81) | 0.754 | 1.02 (0.28, 3.63) | 0.971 |
| Wudeba | 42/50 | 84.0 (70.9, 92.8) | 1.31 (0.40, 4.23) | 0.649 | 1.72 (0.51, 5.75) | 0.377 |
| Age group | ||||||
| ≤4 years | 109/193 | 56.5 (49.1, 63.6) | 0.76 (0.50, 1.13) | 0.177 | 0.78 (0.49, 1.23) | 0.291 |
| >4 years | 130/206 | 63.1 (56.1, 69.7) | REF | |||
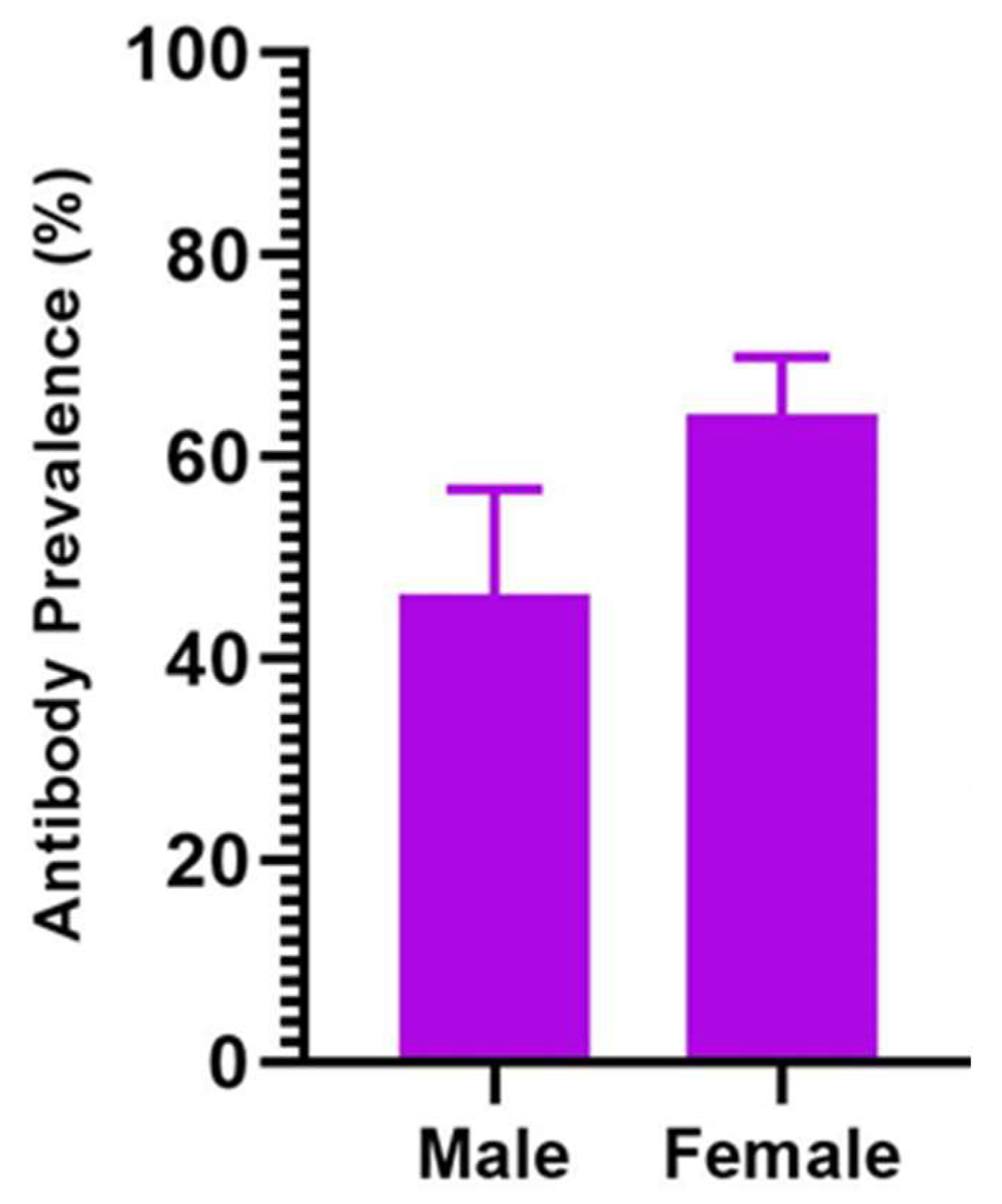
| Sex | ||||||
| Male | 45/97 | 46.4 (36.2, 56.8) | 0.48 (0.30, 0.76) | 0.002 | 0.31 (0.18, 0.54) | <0.001 |
| Female | 194/302 | 64.2 (58.5, 69.6) | REF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matthews, J.; Secka, A.; McVey, D.S.; Dodd, K.A.; Faburay, B. Serological Prevalence of Crimean–Congo Hemorrhagic Fever Virus Infection in Small Ruminants and Cattle in The Gambia. Pathogens 2023, 12, 749. https://doi.org/10.3390/pathogens12060749
Matthews J, Secka A, McVey DS, Dodd KA, Faburay B. Serological Prevalence of Crimean–Congo Hemorrhagic Fever Virus Infection in Small Ruminants and Cattle in The Gambia. Pathogens. 2023; 12(6):749. https://doi.org/10.3390/pathogens12060749
Chicago/Turabian StyleMatthews, Jerusha, Arss Secka, D. Scott McVey, Kimberly A. Dodd, and Bonto Faburay. 2023. "Serological Prevalence of Crimean–Congo Hemorrhagic Fever Virus Infection in Small Ruminants and Cattle in The Gambia" Pathogens 12, no. 6: 749. https://doi.org/10.3390/pathogens12060749
APA StyleMatthews, J., Secka, A., McVey, D. S., Dodd, K. A., & Faburay, B. (2023). Serological Prevalence of Crimean–Congo Hemorrhagic Fever Virus Infection in Small Ruminants and Cattle in The Gambia. Pathogens, 12(6), 749. https://doi.org/10.3390/pathogens12060749








