Pathogenicity and Pathological Characteristics of African Swine Fever Virus Strains from Pig Farms in South Korea from 2022 to January 2023
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus
2.2. Experimental Design
2.3. Clinical Presentation
2.4. Sample Collection
2.5. Viral Load Analysis and Detection of Anti-ASFV Antibody
2.6. Histopathology
2.7. Statistical Analysis
3. Results
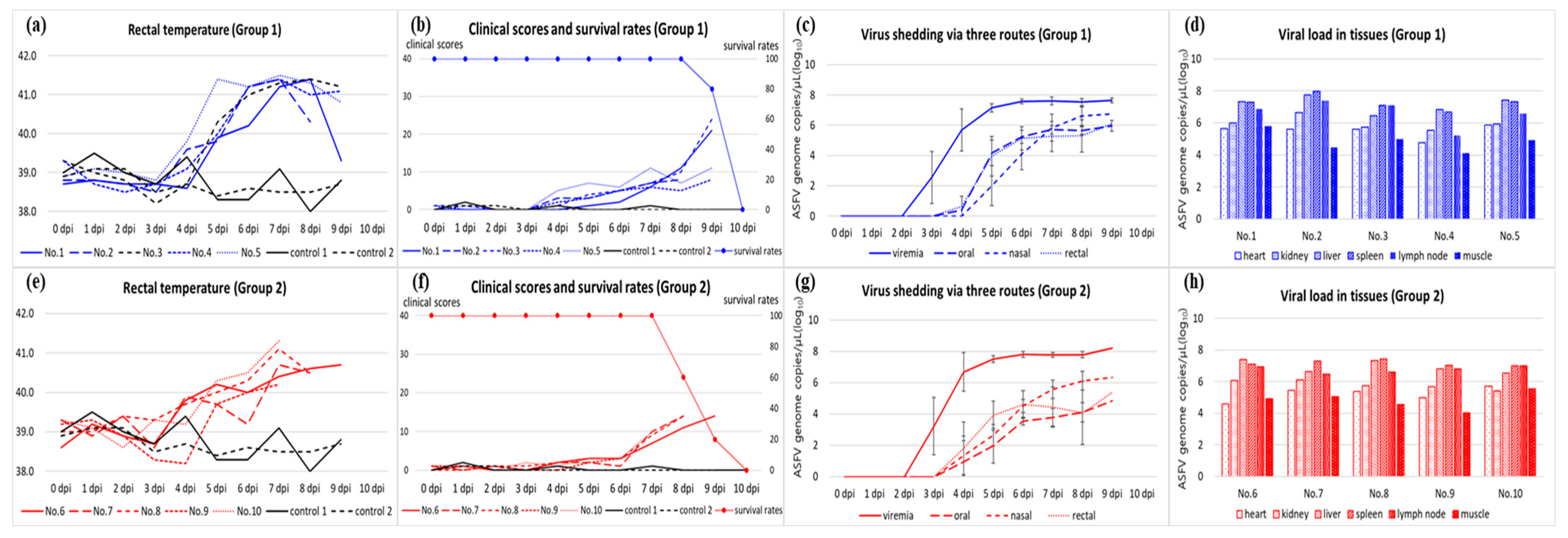
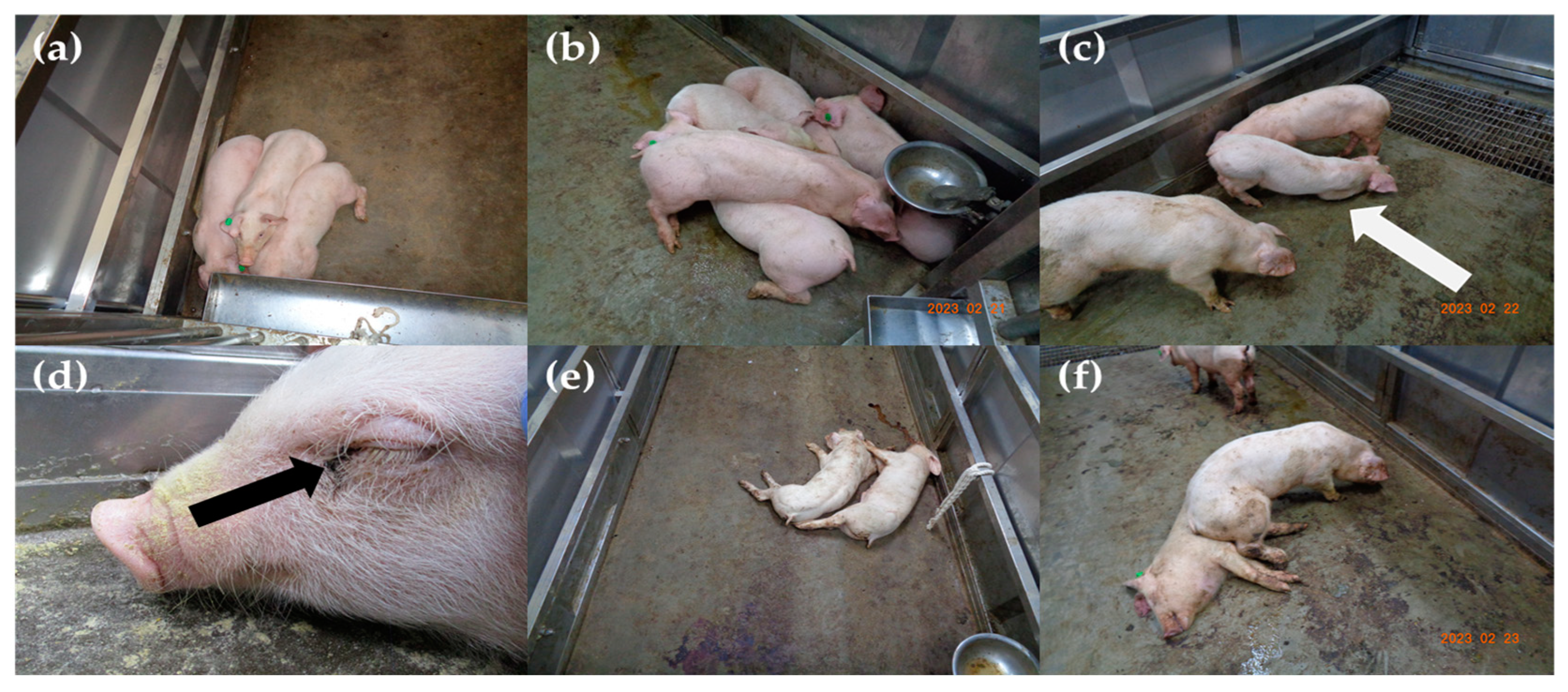
3.1. Disease Progression and Observed Clinical Signs
3.2. Viral Load in Blood, Virus Shedding via Oral, Nasal, and Rectal Routes, and Antibody Detection
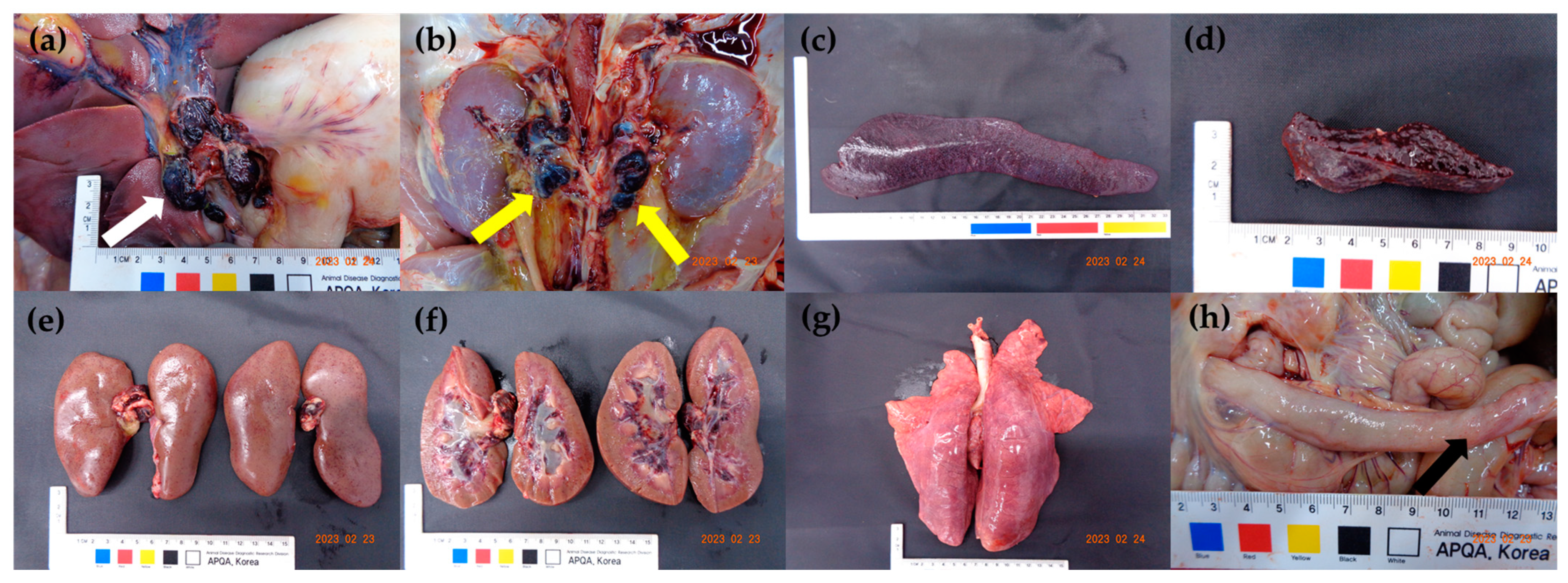
3.3. Viral Load in the Tissues and Post-Mortem Lesions
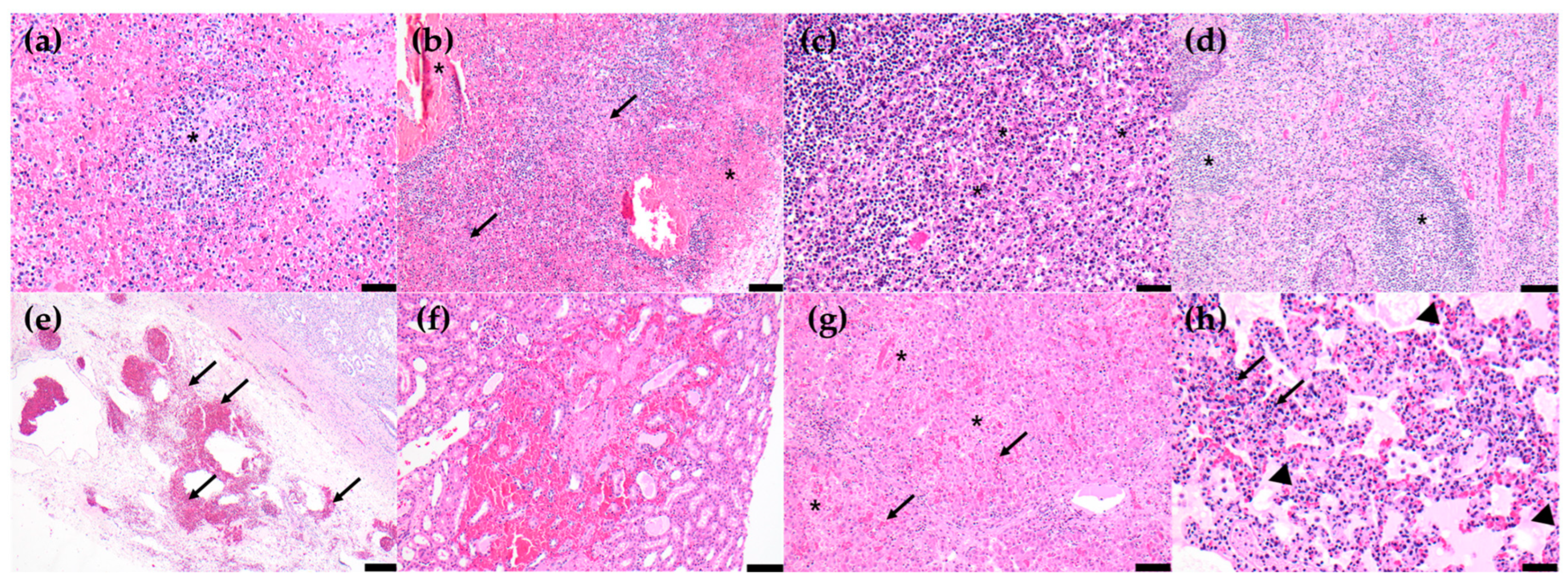
3.4. Histopathological Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muñoz-Pérez, C.; Jurado, C.; Sánchez-Vizcaíno, J.M. African swine fever vaccine: Turning a dream into reality. Transbound. Emerg. Dis. 2021, 68, 2657–2668. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health (WOAH). Technical Disease Card of ASF. Available online: https://www.woah.org/app/uploads/2021/03/oie-african-swine-fever-technical-disease-card.pdf (accessed on 1 April 2023).
- Urbano, A.C.; Ferreira, F. African swine fever control and prevention: An update on vaccine development. Emerg. Microbes Infect. 2022, 11, 2021–2033. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, B.; Kang, H.E. Control measures to African swine fever outbreak: Active response in South Korea, preparation for the future, and cooperation. J. Vet. Sci. 2021, 22, e13. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Chapman, D.A.G.; Netherton, C.L.; Upton, C. African swine fever virus replication and genomics. Virus Res. 2013, 173, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Quembo, C.J.; Jori, F.; Vosloo, W.; Heath, L. Genetic characterization of African swine fever virus isolates from soft ticks at the wildlife/domestic interface in Mozambique and identification of a novel genotype. Transbound. Emerg. Dis. 2017, 65, 420–431. [Google Scholar] [CrossRef]
- Sanna, G.; Dei Giudici, S.; Bacciu, D.; Angioi, P.P.; Giammarioli, M.; De Mia, G.M.; Oggiano, A. Improved strategy for molecular characterization of African swine fever viruses from Sardinia, based on analysis of p30, CD2V and I73R/I329L variable region. Transbound. Emerg. Dis. 2017, 64, 1280–1286. [Google Scholar] [CrossRef]
- Gallardo, C.; Mwaengo, D.M.; Macharia, J.M.; Arias, M.; Taracha, E.A.; Soler, A.; Okoth, E.; Martín, E.; Kasiti, J.; Bishop, R.P. Enhanced discrimination of African swine fever virus isolates through nucleotide sequencing of the p54, p72, and pB602L (CVR) genes. Virus Genes 2009, 38, 85–95. [Google Scholar] [CrossRef]
- Malogolovkin, A.; Burmakina, G.; Titov, I.; Sereda, A.; Gogin, A.; Baryshnikova, E.; Kolbasov, D. Comparative analysis of African swine fever virus genotypes and serogroups. Emerg. Infect. Dis. 2015, 21, 312–315. [Google Scholar] [CrossRef]
- Gallardo, C.; Casado, N.; Soler, A.; Djadjovski, I.; Krivko, L.; Madueño, E.; Nieto, R.; Perez, C.; Simon, A.; Ivanova, E.; et al. A multi gene-approach genotyping method identifies 24 genetic clusters within the genotype II European African swine fever viruses circulating from 2007 to 2022. Front. Vet. Sci. 2023, 10, 1112850. [Google Scholar] [CrossRef]
- Mazur-Panasiuk, N.; Walczak, M.; Juszkiewicz, M.; Woźniakowski, G. The spillover of African swine fever in Western Poland revealed its estimated origin on the basis of O174L, K145R, MGF 505-5R and IGR I73R/I329L genomic sequences. Viruses 2020, 12, 1094. [Google Scholar] [CrossRef]
- Kim, H.J.; Cho, K.H.; Lee, S.K.; Kim, D.Y.; Nah, J.J.; Kim, H.J.; Kim, H.J.; Hwang, J.Y.; Sohn, H.J.; Choi, J.G.; et al. Outbreak of African swine fever in South Korea, 2019. Transbound. Emerg. Dis. 2020, 67, 473–475. [Google Scholar] [CrossRef]
- Cho, K.H.; Kim, H.J.; Jang, M.K.; Ryu, J.H.; Yoo, D.S.; Kang, H.E.; Park, J.Y. Detection of African swine fever at an abattoir in South Korea, 2020. Vet. Sci. 2022, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Kim, D.Y.; Jang, M.K.; Hong, S.K.; Ryu, J.H.; Kang, H.E.; Park, J.Y. Genetic characterization of African swine fever virus from pig farms in South Korea during outbreaks in 2019–2021. Viruses 2022, 4, 2621. [Google Scholar] [CrossRef] [PubMed]
- Zani, L.; Forth, J.H.; Forth, L.; Nurmoja, I.; Leidenberger, S.; Henke, J.; Carlson, J.; Breidenstein, C.; Viltrop, A.; Höper, D.; et al. Deletion at 5′-end of Estonian ASFV strains associated with an attenuated phenotype. Sci. Rep. 2018, 8, 6510. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Soler, A.; Rodze, I.; Nieto, R.; Cano-Gómez, C.; Fernandez-Pinero, J.; Arias, M. Attenuated and non-haemadsorbing (non-HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transbound. Emerg. Dis. 2019, 66, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Nurmoja, I.; Soler, A.; Delicado, V.; Simón, A.; Martin, E.; Perez, C.; Nieto, R.; Arias, M. Evolution in Europe of African swine fever genotype II viruses from highly to moderately virulent. Vet. Microbiol. 2018, 219, 70–79. [Google Scholar]
- Sun, E.; Zhang, Z.; Wang, Z.; He, X.; Zhang, X.; Wang, L.; Wang, W.; Huang, L.; Xi, F.; Huangfu, H.; et al. Emergence and prevalence of naturally occurring lower virulent African swine fever viruses in domestic pigs in China in 2020. Sci. China Life Sci. 2020, 64, 752–765. [Google Scholar] [CrossRef]
- Sun, E.; Huang, L.; Zhang, X.; Zhang, J.; Shen, D.; Zhang, Z.; Wang, Z.; Huo, H.; Wang, W.; Huangfu, H.; et al. Genotype I African swine fever viruses emerged in domestic pigs in China and caused chronic infection. Emerg. Microbes Infect. 2021, 10, 2183–2193. [Google Scholar] [CrossRef]
- Cho, K.H.; Hong, S.K.; Jang, M.K.; Ryu, J.H.; Kim, H.J.; Lee, Y.R.; Roh, I.S.; Sohn, H.J.; Kang, H.E.; Park, J.Y. Comparison of the virulence of Korean African swine fever isolates from pig farms during 2019–2021. Viruses 2022, 14, 2512. [Google Scholar] [CrossRef]
- Cho, K.H.; Yoo, D.S.; Hong, S.K.; Kim, D.Y.; Jang, M.K.; Kang, H.E.; Kim, Y.H. Genetic profile of African swine fever viruses circulating at pig farms during the outbreaks between 2022 and April 2023. Viruses 2023, 15, 1552. [Google Scholar] [CrossRef]
- King, K.; Chapman, D.; Argilaguet, J.M.; Fishbourne, E.; Hutet, E.; Cariolet, R.; Hutchings, G.; Oura, C.A.; Netherton, C.L.; Moffat, K.; et al. Protection of European domestic pigs from virulent African isolates of African swine fever virus by experiment immunization. Vaccine 2011, 29, 4593–4600. [Google Scholar] [CrossRef]
- King, D.P.; Reid, S.M.; Hutchings, G.H.; Grierson, S.S.; Wilkinson, P.J.; Dixon, L.K.; Bastos, A.D.S.; Drew, T.W. Development of a TaqMan® PCR assay with internal amplification control for the detection of African swine fever virus. J. Virol. Methods 2003, 107, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Cardiel, I.; Ballester, M.; Solanes, D.; Nofrarías, M.; López-Soria, S.; Argilaguet, J.M.; Lacasta, A.; Accensi, F.; Rodríguez, F.; Segalés, J. Standardization of pathological investigations in the framework of experimental ASFV infections. Virus Res. 2013, 173, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Guinat, C.; Reis, A.L.; Netherton, C.L.; Goatley, L.; Pfeiffer, D.U.; Dixon, L. Dynamics of African swine fever virus shedding and excretion in domestic pigs infected by intramuscular inoculation and contact transmission. Vet. Res. 2014, 45, 93. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Masujin, K.; Kameyama, K.I.; Yamazoe, R.; Kubo, T.; Iwata, K.; Tamura, A.; Hibi, H.; Shiratori, T.; Koizumi, S.; et al. Experimental infection of pigs with different doses of the African swine fever virus Armenia 07 strain by intramuscular injection and direct contact. J. Vet. Med. Sci. 2021, 82, 1835–1845. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, R.; Zhang, X.; Li, F.; Wang, J.; Zhang, J.; Liu, X.; Wang, L.; Zhang, J.; Wu, X.; et al. Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerg. Microbes. Infect. 2019, 8, 438–447. [Google Scholar] [CrossRef]
- Lee, H.S.; Bui, V.N.; Dao, D.T.; Bui, N.A.; Le, T.D.; Kieu, M.A.; Nguyen, Q.H.; Tran, L.H.; Roh, J.H.; So, K.M.; et al. Pathogenicity of an African swine fever virus strain isolated in Vietnam and alternative diagnostic specimens for early detection of viral infection. Porc. Health Manag. 2021, 7, 36. [Google Scholar] [CrossRef]
- McDowell, C.D.; Bold, D.; Trujillo, J.D.; Meekins, D.A.; Keating, C.; Cool, K.; Kwon, T.; Madden, D.W.; Artiaga, B.L.; Balaraman, V.; et al. Experimental infection of domestic pigs with African swine fever virus isolated in 2019 in Mongolia. Viruses 2022, 14, 2698. [Google Scholar] [CrossRef]
- Walczak, M.; Żmudzki, J.; Mazur-Panasiuk, N.; Juszkiewicz, M.; Woźniakowski, G. Analysis of the clinical course of experimental infection with highly pathogenic African swine fever strain, isolated from an outbreak in Poland. Aspects related to the disease Suspicion at the farm level. Pathogens 2020, 9, 237. [Google Scholar] [CrossRef]
- Olesen, A.S.; Lohse, L.; Boklund, A.; Halasa, T.; Gallardo, C.; Pejsak, Z.; Belsham, G.J.; Rasmussen, T.B.; Bøtner, A. Transmission of African swine fever virus from infected pigs by direct contact and aerosol routes. Vet. Microbiol. 2017, 211, 92–102. [Google Scholar] [CrossRef]
- Ramirez-Medina, E.; O’Donnell, V.; Silva, E.; Espinoza, N.; Velazquez-Salinas, L.; Moran, K.; Daite, D.A.; Barrette, R.; Faburay, B.; Holland, R.; et al. Experimental infection of domestic pigs with an African swine fever virus field strain isolated in 2021 from the Dominican Republic. Viruses 2022, 14, 1090. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.; Szczotka-Bochniarz, A.; Żmudzki, J.; Judzkiewicz, M.; Szymankiewicz, K.; Niemczuk, K.; Pérez-Núñez, D.; Liu, L.; Revilla, Y. Non-invasive sampling in the aspect of African swine fever detection-A risk to accurate diagnosis. Viruses 2022, 14, 1756. [Google Scholar] [CrossRef] [PubMed]
- Salguero, F.J.; Ruiz-Villamor, E.; Bautista, M.J.; Sánchez-Cordón, P.J.; Carrasco, L.; Gómez-Villamandos, J.C. Changes in macrophages in spleen and lymph nodes during acute African swine fever: Expression of cytokines. Vet. Immunol. Immunopathol. 2002, 90, 11–22. [Google Scholar] [CrossRef]
- Carrasco, L.; Bautista, M.J.; Gómez-Villamandos, J.C.; Martin de las Mulas, J.; Chacón, M.d.L.; Wilkinson, P.J.; Sierra, M.A. Development of microscopic lesions in splenic cords of pigs infected with African swine fever virus. Vet. Res. 1997, 28, 93–99. [Google Scholar]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Gómez-Villamandos, J.C.; Carrasco, L. An update on the epidemiology and pathology of African swine fever. J. Comp. Pathol. 2015, 152, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, L.; Chacón, M.d.L.; Martín de las Mulas, J.; Gómez-Villamandos, J.C.; Sierra, M.A.; Villeda, C.J.; Wilkinson, P.J. Ultrastructural changes related to the lymph node hemorrhages in acute African swine fever. Res. Vet. Sci. 1997, 62, 199–204. [Google Scholar] [CrossRef]
- Hervás, J.; Gómez-Villamandos, J.C.; Méndez, A.; Carrasco, L.; Sierra, M.A. The lesional changes and pathogenesis in the kidney in African swine fever. Vet. Res. Commun. 1996, 20, 285–299. [Google Scholar] [CrossRef]
- Gómez-Villamandos, J.C.; Carrasco, L.; Bautista, M.J.; Sierra, M.A.; Quezada, M.; Hervas, J.; Chacón, M.d.L.; Ruiz-Villamor, E.; Salguero, F.J.; Sánchez-Cordón, P.J.; et al. African swine fever and classical swine fever: A review of the pathogenesis. Dtsch. Tierarztl. Wochenschr. 2003, 110, 165–169. [Google Scholar]
- Salguero, F.J.; Sánchez-Cordón, P.J.; Núñez, A.; Fernández de Marco, M.; Gómez-Villamandos, J.C. Proinflammatory cytokines induce lymphocyte apoptosis in acute African swine fever infection. J. Comp. Pathol. 2005, 132, 289–302. [Google Scholar] [CrossRef]
- Salguero, F.J.; Sánchez-Cordón, P.J.; Sierra, M.A.; Jover, A.; Núñez, A.; Gómez-Villamandos, J.C. Apoptosis of thymocytes in experimental African swine fever virus infection. Histol. Histopathol. 2004, 19, 77–84. [Google Scholar]
- Izzati, U.Z.; Inanaga, M.; Hoa, N.T.; Nueangphuet, P.; Myint, O.; Truong, Q.L.; Lan, N.T.; Norimine, J.; Hirai, T.; Yamaguchi, R. Pathological investigation and viral antigen distribution of emerging African swine fever in Vietnam. Transbound. Emerg. Dis. 2021, 68, 2039–2050. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.Y.; Ho, J.H.P.; Tam, K.W.S.; Kamali, M.; Zhang, Y.; Lau, C.C.Y.; Li, S.H.; Wilson, M.T.; Guo, Z.; Li, R.; et al. Investigation of the first African swine fever outbreak in a domestic pig farm in Hong Kong. Transbound. Emerg. Dis. 2023, 15, 1720474. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, E.; Huang, L.; Ding, L.; Zhu, Y.; Zhang, J.; Shen, D.; Zhang, X.; Zhang, Z.; Ren, T.; et al. Highly lethal genotype I and II recombinant African swine fever viruses detected in pigs. Nat. Commun. 2023, 14, 3096. [Google Scholar] [CrossRef] [PubMed]
| Date of the Outbreak | Province | City/County | Number of Pigs (Production Type) | Classification | Clinical Signs | Presence of Hemorrhagic Gross Lesions at Necropsy | |
|---|---|---|---|---|---|---|---|
| 1 | 26 May 2022 | Gangwon | Hongcheon | 1175 (farrow-to-finish) | Notification | Death | Yes |
| 2 | 18 August 2022 | Gangwon | Yanggu | 5614 (farrow-to-finish) | Notification | Death | Yes |
| 3 | 18 September 2022 | Gangwon | Chuncheon | 8243 (farrow-to-finish) | Notification | Anorexia, fever, abortion, death | Yes |
| 4 | 19 September 2022 | Gangwon | Chuncheon | 6824 (farrow-to-finish) | Active surveillance (Quarantine zone) | None | No necropsy |
| 5 | 28 September 2022 | Gyeonggi | Gimpo | 5203 (farrow-to-finish) | Notification | Anorexia, abortion | No necropsy |
| 6 | 28 September 2022 | Gyeonggi | Paju | 1133 (farrow-to-finish) | Notification | Anorexia, abortion, death | Yes |
| 7 | 9 November 2022 | Gangwon | Cheorwon | 5499 (farrow-to-finish) | Notification | Anorexia, death | Yes |
| 8 | 5 January 2023 | Gyeonggi | Pocheon | 8444 (breeding) | Active surveillance (Slaughterhouse) | None | No |
| 9 | 11 January 2023 | Gangwon | Cheorwon | 1976 (fattening) | Active surveillance | None | No necropsy |
| 10 | 22 January 2023 | Gyeonggi | Gimpo | 2009 (farrow-to-finish) | Notification | Anorexia, fever, death | Yes |
| Group (Number of Pigs) | Inoculated ASFV Strain (Date of Outbreak) | Total Survival Days | Days to Onset of Fever | Clinical Signs | Viremia | Virus Shedding | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oral Route | Nasal Route | Rectal Route | |||||||||||
| Days to Onset | Max Score | Days to Onset | Max Titer 1 | Days to Onset | Max Titer 1 | Days to Onset | Max Titer 1 | Days to Onset | Max Titer 1 | ||||
| 1 (n = 5) | Korea/Pig/Hongcheon/2022 (26 May 2022) | 9.8 ± 0.4 | 5.4 ± 0.5 | 6.6 ± 0.9 | 14.4 ± 7.6 | 3.2 ± 0.4 | 6.9 ± 0.2 | 4.6 ± 0.5 | 5.1 ± 0.3 | 5.2 ± 0.4 | 6.0 ± 0.2 | 4.8 ± 0.4 | 5.4 ± 0.4 |
| 2 (n = 5) | Korea/Pig/Pocheon/2023 (5 January 2023) | 8.8 ± 0.8 | 5.6 ± 0.9 | 7.0 ± 0 | 11.8 ± 3.2 | 3.2 ± 0.4 | 7.1 ± 0.2 | 4.8 ± 0.8 | 3.2 ± 0.7 | 4.4 ± 0.5 | 4.8 ± 0.8 | 4.4 ± 0.5 | 4.4 ± 0.7 |
| Clinical Signs and Gross Lesions | Group 1 | Group 2 | Total Frequency | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||||
| Clinical signs | Total days survival | 10 | 9 | 10 | 10 | 10 | 10 | 9 | 9 | 8 | 8 | - | ||
| Fever (>40.0 °C) | + | + | + | + | + | + | + | + | + | + | 10/10 | (100.0%) | ||
| Anorexia | + | + | + | + | + | + | + | + | + | + | 10/10 | (100.0%) | ||
| Depression | + | + | + | + | + | + | + | + | + | + | 10/10 | (100.0%) | ||
| Recumbency | + | + | + | + | + | + | + | + | + | + | 10/10 | (100.0%) | ||
| Labored breathing and/or cough | + | + | + | + | + | – | + | + | – | + | 7/10 | (70.0%) | ||
| Joint swelling | + | – | + | – | + | + | + | + | – | – | 6/10 | (60.0%) | ||
| Ocular discharge | – | + | + | + | + | – | – | – | – | – | 4/10 | (40.0%) | ||
| Diarrhea | – | – | – | + | + | – | – | – | – | + | 3/10 | (30.0%) | ||
| Melena | – | – | + | – | + | – | + | – | – | – | 3/10 | (30.0%) | ||
| Epistaxis | – | – | + | – | – | – | – | – | – | – | 1/10 | (10.0%) | ||
| Skin hemorrhage | – | – | – | – | – | – | – | – | – | – | 0/10 | (0%) | ||
| Hematuria | – | – | – | – | – | – | – | – | – | – | 0/10 | (0%) | ||
| Vomit | – | – | – | – | – | – | – | – | – | – | 0/10 | (0%) | ||
| Gross lesions | Lymph nodes | Enlargement | + | + | + | + | + | + | + | + | + | + | 10/10 | (100.0%) |
| Hemorrhage | + | + | + | + | + | + | + | + | + | + | 10/10 | (100.0%) | ||
| Spleen | Enlargement | + | + | + | + | + | + | + | + | + | + | 10/10 | (100.0%) | |
| Infarction | + | + | + | + | + | + | + | + | + | + | 10/10 | (100.0%) | ||
| Kidneys | Petechiae | + | + | + | + | + | + | + | + | – | – | 8/10 | (80.0%) | |
| Hemorrhage in pelvis | + | + | + | + | + | + | + | + | – | – | 8/10 | (80.0%) | ||
| Lung | Interstitial pneumonia | + | + | – | + | + | + | + | – | + | – | 7/10 | (70.0%) | |
| Intestine | Petechiae on ileal serosa | + | + | – | + | + | + | – | + | + | – | 7/10 | (70.0%) | |
| Tonsils | Erythema | + | + | – | + | – | + | + | + | + | – | 7/10 | (70.0%) | |
| Hindlimb | Hemorrhage | – | – | – | – | – | – | – | + | – | – | 1/10 | (10.0%) | |
| Group | Spleen | Lymph Nodes | Tonsils | Kidneys | Liver | Lung | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Submandibular | Tracheobronchial | Gastrohepatic | Renal | Mesenteric | ||||||
| 1 | 3.00 ± 0 | 2.80 ± 0.45 | 2.60 ± 0.55 | 2.80 ± 0.45 | 3.00 ± 0 | 2.60 ± 0.55 | 2.80 ± 0.45 | 1.40 ± 0.55 | 1.20 ± 0.45 | 0.75 ± 0.50 |
| 2 | 3.00 ± 0 | 2.40 ± 0.89 | 3.00 ± 0 | 2.80 ± 0.45 | 3.00 ± 0 | 2.80 ± 0.45 | 2.40 ± 0.89 | 1.40 ± 0.55 | 1.40 ± 0.55 | 0.60 ± 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-H.; Hong, S.-K.; Kim, D.-Y.; Jang, M.-K.; Kim, J.-H.; Lee, H.; Kim, E.-M.; Park, J.-H.; Suh, T.-Y.; Choi, J.-G.; et al. Pathogenicity and Pathological Characteristics of African Swine Fever Virus Strains from Pig Farms in South Korea from 2022 to January 2023. Pathogens 2023, 12, 1158. https://doi.org/10.3390/pathogens12091158
Cho K-H, Hong S-K, Kim D-Y, Jang M-K, Kim J-H, Lee H, Kim E-M, Park J-H, Suh T-Y, Choi J-G, et al. Pathogenicity and Pathological Characteristics of African Swine Fever Virus Strains from Pig Farms in South Korea from 2022 to January 2023. Pathogens. 2023; 12(9):1158. https://doi.org/10.3390/pathogens12091158
Chicago/Turabian StyleCho, Ki-Hyun, Seong-Keun Hong, Da-Young Kim, Min-Kyung Jang, Jong-Ho Kim, Hyunkyoung Lee, Eun-Mi Kim, Ji-Hoon Park, Tae-Young Suh, Jun-Gu Choi, and et al. 2023. "Pathogenicity and Pathological Characteristics of African Swine Fever Virus Strains from Pig Farms in South Korea from 2022 to January 2023" Pathogens 12, no. 9: 1158. https://doi.org/10.3390/pathogens12091158
APA StyleCho, K.-H., Hong, S.-K., Kim, D.-Y., Jang, M.-K., Kim, J.-H., Lee, H., Kim, E.-M., Park, J.-H., Suh, T.-Y., Choi, J.-G., Yoo, D.-S., Kang, H.-E., & Kim, Y.-H. (2023). Pathogenicity and Pathological Characteristics of African Swine Fever Virus Strains from Pig Farms in South Korea from 2022 to January 2023. Pathogens, 12(9), 1158. https://doi.org/10.3390/pathogens12091158






