Systematic Review and Meta-Analysis of the Efficacy and Effectiveness of Pneumococcal Vaccines in Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Abstraction and Cleaning
2.4. Data Analysis
3. Results
3.1. Literature Search
3.2. Vaccine-Type Invasive Pneumococcal Disease
3.2.1. PCV13
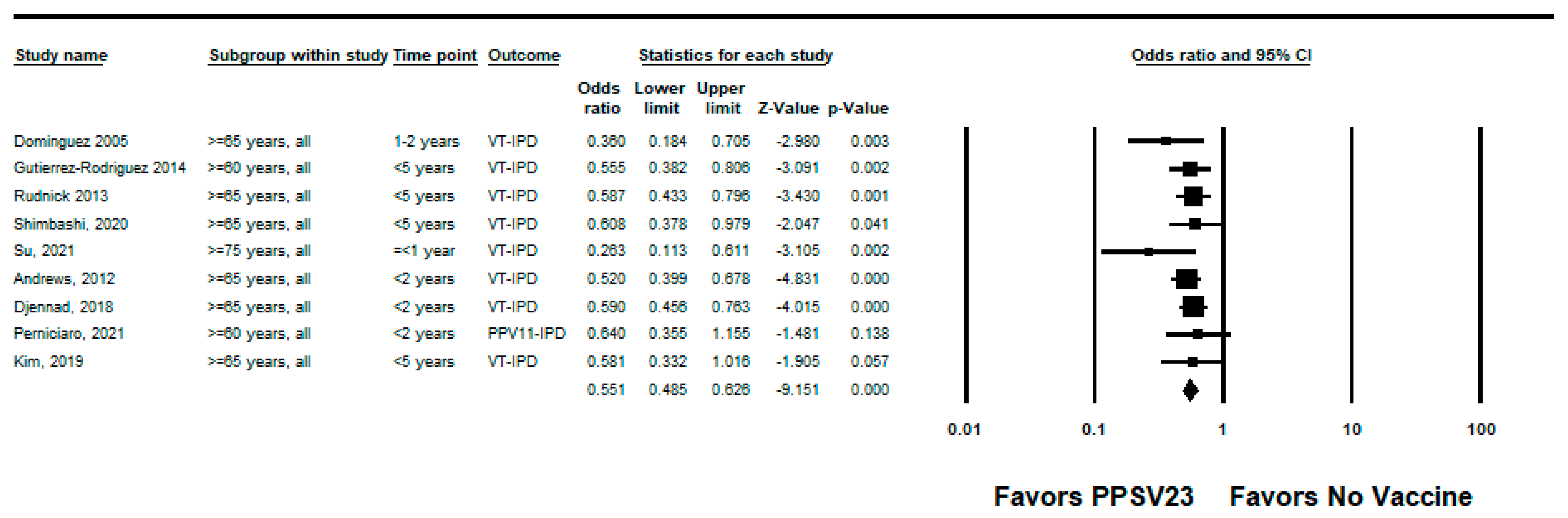
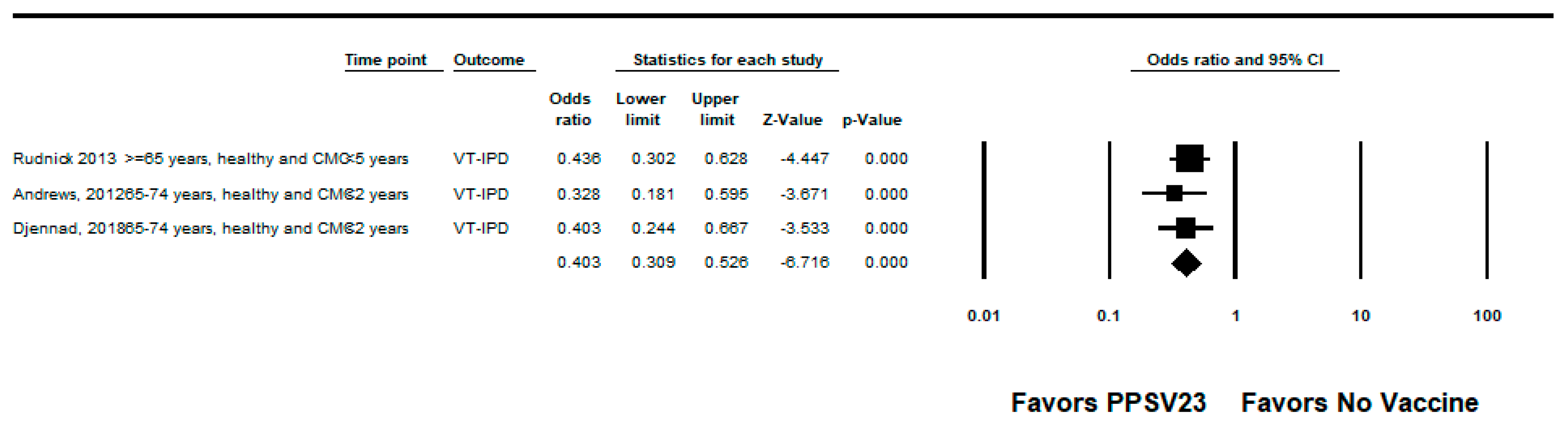
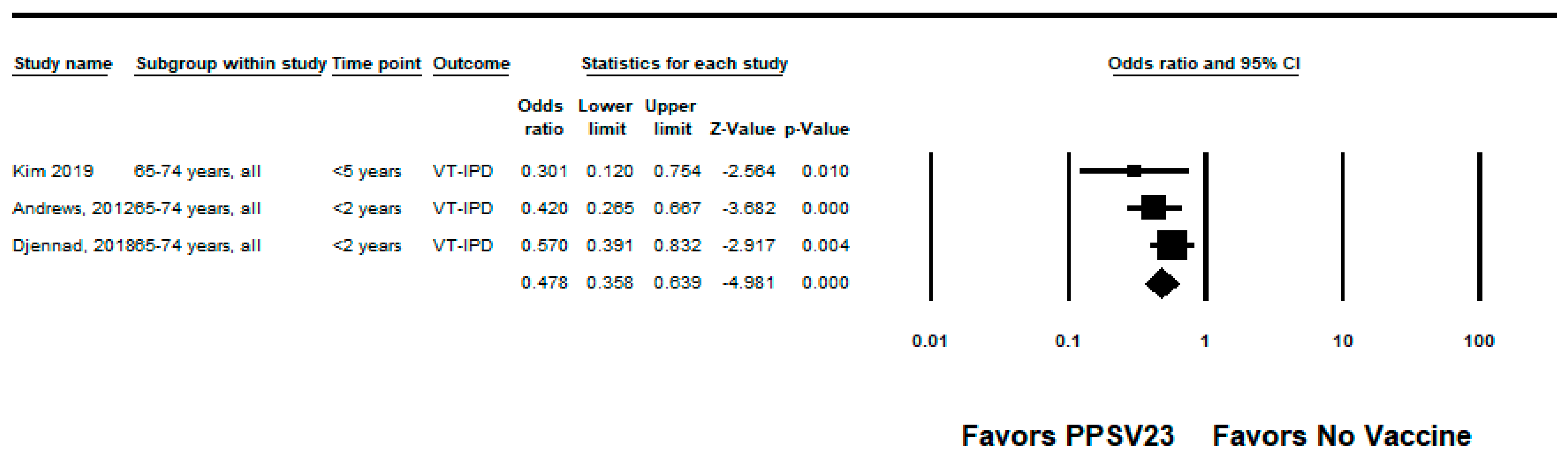
3.2.2. PPSV23
3.3. Vaccine-Type Pneumococcal Pneumonia
3.3.1. PCV13
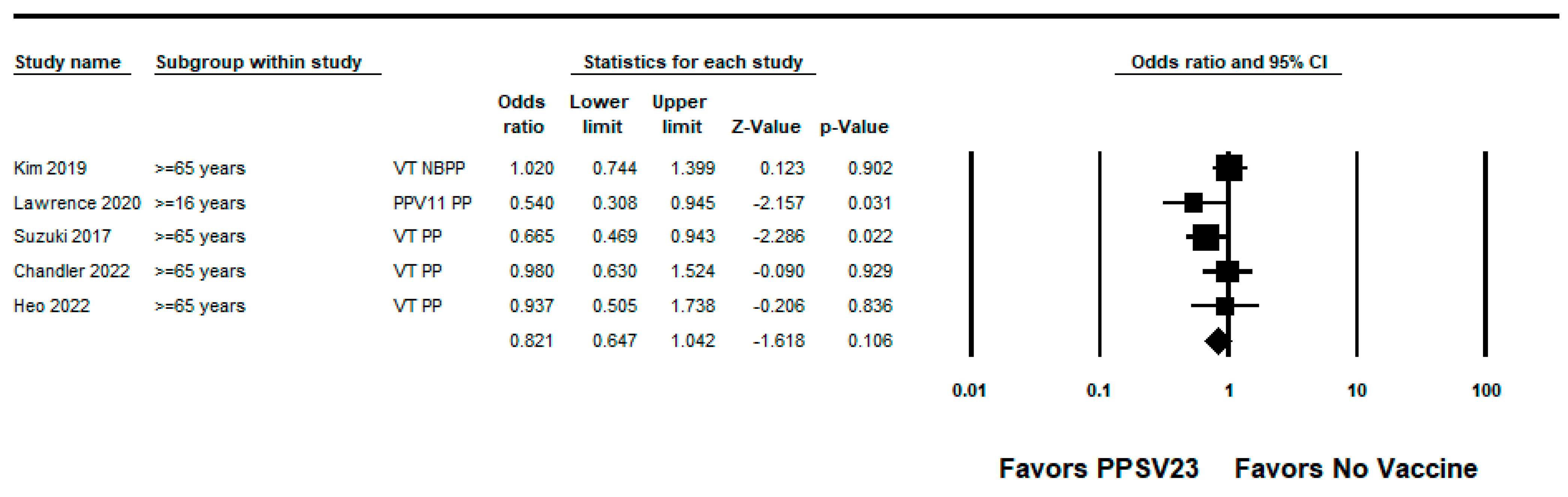
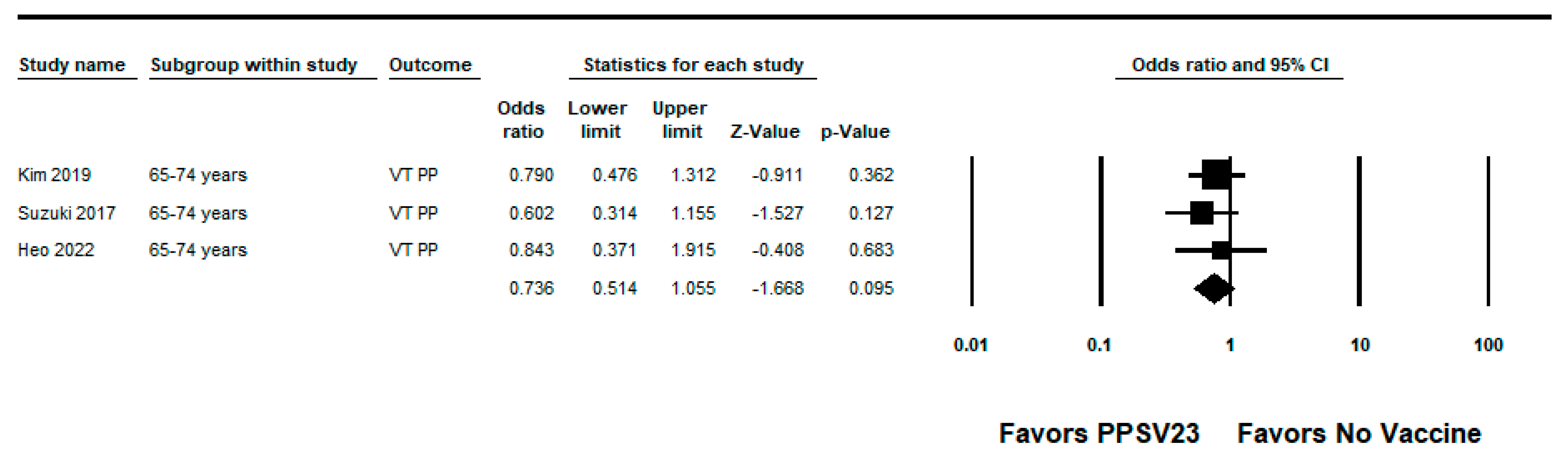
3.3.2. PPSV23
3.4. Certainty of Evidence
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Database | Strategy |
|---|---|
| Medline (OVID) | 1. streptococcus pneumoniae/ 2. (pneumococcal or pneumococci or “streptococcus pneumonie” or “streptococcus pneumoniae” or “s pneumoniae”).tw,kf. 3. 1 or 2 4. vaccination/or immunization/or Vaccines, Conjugate/ 5. (vaccine? or vaccination? or immunisation or immunization).tw,kf. 6. 4 or 5 7. Pneumococcal Vaccines/ 8. (heptavalent or “7 valent” or “7valent” or “pcv 7” or pcv7* or “10 valent” or “10valent” or pcv10 or “pcv 10” or “13valent” or “13 valent” or pcv13 or “pcv 13” or ppv23 or “ppv 23” or “23 valent” or “23valent”).tw,kf. 9. 7 or 8 10. (3 and 6) 11. 10 or 9 12. comparative effectiveness research/or treatment outcome/or Vaccine Potency/ 13. (efficacy or effectiveness or effects or “immune response” or impact or “treatment outcome”).tw,kf. 14. 12 or 13 15. adult/or aged/or “aged, 80 and over”/or frail elderly/ 16. (adult? or elderly or elderlies).tw,kf. 17. 15 or 16 18. 11 and 14 and 17 19. (201904* OR 201905* OR 201906* OR 201907* OR 201908* OR 201909* OR 201910* OR 201911* OR 201912* OR 2020* OR 2021*).ed,ep,yr,dp,dt. 20. 17 and 18 |
| Embase (OVID) | 1. Streptococcus pneumoniae/ 2. (pneumococcal or pneumococci or “streptococcus pneumonie” or “streptococcus pneumoniae” or “s pneumoniae”).tw,kw. 3. 1 or 2 4. vaccination/or immunization/or vaccine/ 5. (vaccine? or vaccination? or immunisation or immunization).tw,kw. 6. 4 or 5 7. Pneumococcus vaccine/ 8. (heptavalent or “7 valent” or “7valent” or “pcv 7” or pcv7* or “10 valent” or “10valent” or pcv10 or “pcv 10” or “13valent” or “13 valent” or pcv13 or “pcv 13” or ppv23 or “ppv 23” or “23 valent” or “23valent”).tw,kw. 9. 7 or 8 10. 3 and 6 11. 9 OR 10 12. comparative effectiveness/or treatment outcome/ 13. (efficacy or effectiveness or effects or “immune response” or impact or “treatment outcome”).tw,kw. 14. 12 or 13 15. aged/or adult/or aged hospital patient/or frail elderly/or institutionalized elderly/or very elderly/ 16. (adult? or elderly or elderlies).tw,kw. 17. 15 or 16 18. 11 and 14 and 17 19. (201904* OR 201905* OR 201906* OR 201907* OR 201908* OR 201909* OR 201910* OR 201911* OR 201912* OR 2020* OR 2021*).dc 20. limit 19 to (conference abstracts or embase) |
| Cochrane Library | ( ( (pneumococcal or pneumococci or “streptococcus pneumonie” or “streptococcus pneumoniae” or “s pneumoniae”):ti,ab AND ([mh Vaccines] OR [mh Immunization] OR (vaccine# or vaccination# or immunisation or immunization):ti,ab) ) OR (MH “Pneumococcal Vaccine”) OR TI (heptavalent or “7 valent” or “7valent” or “pcv 7” or pcv7* or “10 valent” or “10valent” or pcv10 or “pcv 10” or “13valent” or “13 valent” or pcv13 or “pcv 13” or ppv23 or “ppv 23” or “23 valent” or “23valent”) OR AB (heptavalent or “7 valent” or “7valent” or “pcv 7” or pcv7* or “10 valent” or “10valent” or pcv10 or “pcv 10” or “13valent” or “13 valent” or pcv13 or “pcv 13” or ppv23 or “ppv 23” or “23 valent” or “23valent”) ) AND [mh “Treatment Outcomes”] OR (efficacy or effectiveness or effects or “immune response” or impact or “treatment outcome”):ti,ab AND [mh Adult] OR [mh Aged+] OR (adult# or elderly or elderlies):ti,ab |
| CINAHL (EbscoHost) | ( (TI (pneumococcal or pneumococci or “streptococcus pneumonie” or “streptococcus pneumoniae” or “s pneumoniae”) OR AB (pneumococcal or pneumococci or “streptococcus pneumonie” or “streptococcus pneumoniae” or “s pneumoniae”)) AND ((MH “Vaccines”) OR (MH “Immunization”) OR TI (vaccine# or vaccination# or immunisation or immunization) OR AB (vaccine# or vaccination# or immunisation or immunization)) OR (MH “Pneumococcal Vaccine”) OR TI (heptavalent or “7 valent” or “7valent” or “pcv 7” or pcv7* or “10 valent” or “10valent” or pcv10 or “pcv 10” or “13valent” or “13 valent” or pcv13 or “pcv 13” or ppv23 or “ppv 23” or “23 valent” or “23valent”) OR AB (heptavalent or “7 valent” or “7valent” or “pcv 7” or pcv7* or “10 valent” or “10valent” or pcv10 or “pcv 10” or “13valent” or “13 valent” or pcv13 or “pcv 13” or ppv23 or “ppv 23” or “23 valent” or “23valent”) ) AND (MH “Treatment Outcomes”) OR TI (efficacy or effectiveness or effects or “immune response” or impact or “treatment outcome”) OR AB (efficacy or effectiveness or effects or “immune response” or impact or “treatment outcome”) AND ((MH “Adult”) OR (MH “Aged+”) OR TI (adult# or elderly or elderlies) OR AB (adult# or elderly or elderlies)) Limiters-Published Date: 20190401-20210218; Exclude MEDLINE records |
| Scopus (for WOS) | (TITLE-ABS-KEY(“heptavalent” or “7 valent” or “7valent” or “pcv 7” or pcv7* or “10 valent” or “10valent” or “pcv10” or “pcv 10” or “13valent” or “13 valent” or “pcv13” or “pcv 13” or “ppv23” or “ppv 23” or “23 valent” or “23valent”) OR (TITLE-ABS-KEY(“vaccine$” or “vaccination$” or “immunisation” or “immunization”) AND TITLE-ABS-KEY(“pneumococcal” or “pneumococci” or “streptococcus pneumonie” or “streptococcus pneumoniae” or “s pneumoniae”))) AND TITLE-ABS-KEY(“adult$” or “elderly” or “elderlies”) AND TITLE-ABS-KEY(“efficacy” or “effectiveness” or “effects” or “immune response” or “impact” or “treatment outcome”) Limit April 2019- |
| Epistemonikos | Search 1: (pneumococcal OR pneumococci OR “streptococcus pneumonie” OR “streptococcus pneumoniae” OR “s pneumoniae”) AND (vaccine* OR vaccination* OR immunisation OR immunization) AND (efficacy OR effectiveness OR effects OR “immune response” OR impact OR “treatment outcome”) AND (adult* OR elderly OR elderlies) –limited to 2019–2021 Search 2: (heptavalent OR “7 valent” OR “7valent” OR “pcv 7” OR pcv7* OR “10 valent” OR “10valent” OR pcv10 OR “pcv 10” OR “13valent” OR “13 valent” OR pcv13 OR “pcv 13” OR ppv23 OR “ppv 23” OR “23 valent” OR “23valent”) AND (efficacy OR effectiveness OR effects OR “immune response” OR impact OR “treatment outcome”) AND (adult* OR elderly OR elderlies) –limited to 2019–2021 |
References
- Horácio, A.N.; Silva-Costa, C.; Lopes, J.P.; Ramirez, M.; Melo-Cristino, J.; Portuguese Group for the Study of Streptococcal Infections; Vaz, T.; Gião, M.; Ferreira, R.; Fonseca, A.B.; et al. Serotype 3 Remains the Leading Cause of Invasive Pneumococcal Disease in Adults in Portugal (2012–2014) Despite Continued Reductions in Other 13-Valent Conjugate Vaccine Serotypes. Front. Microbiol. 2016, 7, 1616. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.R.; Link-Gelles, R.; Schaffner, W.; Lynfield, R.; Lexau, C.; Bennett, N.M.; Petit, S.; Zansky, S.M.; Harrison, L.H.; Reingold, A.; et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: Analysis of multisite, population-based surveillance. Lancet Infect. Dis. 2015, 15, 301–309. [Google Scholar] [CrossRef]
- Slotved, H.-C.; Dalby, T.; Harboe, Z.B.; Valentiner-Branth, P.; de Casadevante, V.F.; Espenhain, L.; Fuursted, K.; Konradsen, H.B. The incidence of invasive pneumococcal serotype 3 disease in the Danish population is not reduced by PCV-13 vaccination. Heliyon 2016, 2, e00198. [Google Scholar] [CrossRef]
- Gierke, R.; Farley, M.M.; Schaffner, W.; Thomas, A.; Reingold, A.; Harrison, L.; Holtzman, C.; Burzlaff, K.; Petit, S.; Barnes, M.; et al. 1299. Epidemiology of Invasive Pneumococcal Disease (IPD) in the United States 2011–2019. Open Forum Infect. Dis. 2021, 8 (Suppl. 1), S737–S738. [Google Scholar] [CrossRef]
- Isturiz, R.; Grant, L.; Gray, S.; Alexander-Parrish, R.; Jiang, Q.; Jodar, L.; Peyrani, P.; Ford, K.D.; Pride, M.W.; Self, W.H.; et al. Expanded Analysis of 20 Pneumococcal Serotypes Associated With Radiographically Confirmed Community-acquired Pneumonia in Hospitalized US Adults. Clin. Infect. Dis. 2021, 73, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Gierke, R.; Matanock, A.; Shang, N.; Farley, M.M.; Schaffner, W.; Thomas, A.; Reingold, A.; Harrison, L.; Schleiss, K.; Burzlaff, K.; et al. 1474. Impact of 13-valent Pneumococcal Conjugate Vaccine (PCV13) on Non-bacteremic Pneumococcal Pneumonia (NBPP) among Adults in the United States, 2013–2017. Open Forum Infect. Dis. 2020, 7 (Suppl. 1), S738–S739. [Google Scholar] [CrossRef]
- Jain, S.; Self, W.H.; Wunderink, R.G. Community-Acquired Pneumonia Requiring Hospitalization. N. Engl. J. Med. 2015, 373, 2382. [Google Scholar] [CrossRef]
- Simonsen, L.; Taylor, R.J.; Schuck-Paim, C.; Lustig, R.; Haber, M.; Klugman, K.P. Effect of 13-valent pneumococcal conjugate vaccine on admissions to hospital 2 years after its introduction in the USA: A time series analysis. Lancet Respir. Med. 2014, 2, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 2012, 61, 816–819. [Google Scholar]
- Tomczyk, S.; Bennett, N.M.; Stoecker, C.; Gierke, R.; Moore, M.R.; Whitney, C.G.; Hadler, S.; Pilishvili, T. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 822–825. [Google Scholar]
- Bonten, M.J.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; van Werkhoven, C.H.; van Deursen, A.M.M.; Sanders, E.A.M.; Verheij, T.J.M.; et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N. Engl. J. Med. 2015, 372, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Matanock, A.; Lee, G.; Gierke, R.; Kobayashi, M.; Leidner, A.; Pilishvili, T. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged ≥65 Years: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. VAXNEUVANCE 2021. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/vaxneuvance (accessed on 30 June 2022).
- Food and Drug Administration. PREVNAR 20 2021. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/prevnar-20 (accessed on 30 June 2022).
- Berild, J.D.; Winje, B.A.; Vestrheim, D.F.; Slotved, H.-C.; Valentiner-Branth, P.; Roth, A.; Storsäter, J. A Systematic Review of Studies Published between 2016 and 2019 on the Effectiveness and Efficacy of Pneumococcal Vaccination on Pneumonia and Invasive Pneumococcal Disease in an Elderly Population. Pathogens 2020, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Veritas Health Innovation. Covidence Systematic Review Software; Veritas Health Innovation: Melbourne, Australia, 2021. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis; The Ottawa Hospital Research Institute: Ottawa, Canada, 2000. [Google Scholar]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis Version 3; Biostat: Englewood, NJ, USA, 2013. [Google Scholar]
- Rudnick, W.; Liu, Z.; Shigayeva, A.; Low, D.E.; Green, K.; Plevneshi, A.; Devlin, R.; Downey, J.; Katz, K.; Kitai, I.; et al. Pneumococcal vaccination programs and the burden of invasive pneumococcal disease in Ontario, Canada, 1995–2011. Vaccine 2013, 31, 5863–5871. [Google Scholar] [CrossRef]
- Suzuki, M.; Dhoubhadel, B.G.; Ishifuji, T.; Yasunami, M.; Yaegashi, M.; Asoh, N.; Ishida, M.; Hamaguchi, S.; Aoshima, M.; Ariyoshi, K.; et al. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: A multicentre, prospective, test-negative design study. Lancet Infect. Dis. 2017, 17, 313–321. [Google Scholar] [CrossRef]
- Andrews, N.J.; Waight, P.A.; George, R.C.; Slack, M.P.; Miller, E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine 2012, 30, 6802–6808. [Google Scholar] [CrossRef]
- Djennad, A.; Ramsay, M.E.; Pebody, R.; Fry, N.K.; Sheppard, C.; Ladhani, S.N.; Andrews, N.J. Effectiveness of 23-Valent Polysaccharide Pneumococcal Vaccine and Changes in Invasive Pneumococcal Disease Incidence from 2000 to 2017 in Those Aged 65 and Over in England and Wales. Eclinicalmedicine 2018, 6, 42–50. [Google Scholar] [CrossRef]
- Gutierrez Rodriguez, M.A.; Ordobas Gavin, M.A.; Garcia-Comas, L.; Sanz Moreno, J.C.; Cordoba Deorador, E.; Lasheras Carbajo, M.D.; Taveira Jiménez, J.A.; Martín Martínez, F.; Iniesta Fornies, D.; Arce Arnaez, A. Effectiveness of 23-valent pneumococcal polysaccharide vaccine in adults aged 60 years and over in the Region of Madrid, Spain, 2008–2011. Eurosurveillance 2014, 19, 20922. [Google Scholar] [CrossRef]
- Ochoa-Gondar, O.; Vila-Corcoles, A.; Rodriguez-Blanco, T.; Gomez-Bertomeu, F.; Figuerola-Massana, E.; Raga-Luria, X.; Hospital-Guardiola, I. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against community-acquired pneumonia in the general population aged >/= 60 years: 3 years of follow-up in the CAPAMIS study. Clin. Infect. Dis. 2014, 58, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.Y.; Bin Seo, Y.; Choi, W.S.; Kim, E.J.; Jeong, H.W.; Lee, J.; Yoon, J.G.; Noh, J.Y.; Cheong, H.J.; Kim, W.J.; et al. Effectiveness of Pneumococcal Vaccination Against Pneumococcal Pneumonia Hospitalization in Older Adults: A Prospective, Test-Negative Study. J. Infect. Dis. 2022, 225, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chun, B.C.; Song, J.Y.; Kim, H.Y.; Bae, I.G.; Kim, D.M.; Choi, Y.H.; Jun, Y.H.; Choi, W.S.; Kang, S.H.; et al. Direct effectiveness of pneumococcal polysaccharide vaccine against invasive pneumococcal disease and non-bacteremic pneumococcal pneumonia in elderly population in the era of pneumococcal conjugate vaccine: A case-control study. Vaccine 2019, 37, 2797–2804. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.; Hsiao, A.; Hansen, J.; Yee, A.; Chao, C.; Suaya, J.A.; Alexander-Parrish, R.; Isturiz, R.E.; McLaughlin, J.M.; Gessner, B.D.; et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in older adults. Open Forum Infect. Dis. 2019, 6 (Suppl. 2), S953–S954. [Google Scholar] [CrossRef]
- McLaughlin, J.M.; Jiang, Q.; Isturiz, R.E.; Sings, H.L.; Swerdlow, D.L.; Gessner, B.D.; Carrico, R.M.; Peyrani, P.; Wiemken, T.L.; Mattingly, W.A.; et al. Effectiveness of 13-Valent Pneumococcal Conjugate Vaccine Against Hospitalization for Community-Acquired Pneumonia in Older US Adults: A Test-Negative Design. Clin. Infect. Dis. 2018, 67, 1498–1506. [Google Scholar] [CrossRef]
- Pilishvili, T.; Almendares, O.; Xing, W.; Farley, M.M.; Schaffner, W.; Thomas, A.; Reingold, A.; Harrison, L.; Holtzman, C.; Rowlands, J.; et al. Effectiveness of Pneumococcal Vaccines against Invasive Pneumococcal Disease (IPD) among Adults >=65 Years Old in the United States. In Proceedings of the 11th Symposium for Pneumococcus and Pneumococcal Diseases, Melbourne, Australia, 15–19 April 2018. [Google Scholar]
- Pilishvili, T.; Almendares, O.M.; Nanduri, S.; Warnock, R.; Wu, X.; McKean, S.; Kelman, J.; Farley, M.M.; Schaffner, W.; Thomas, A.; et al. Evaluation of pneumococcal vaccine effectiveness against invasive pneumococcal disease among us medicare beneficiaries >=65 years old. Open Forum Infect. Dis. 2018, 5 (Suppl. 1), S10–S11. [Google Scholar] [CrossRef]
- Prato, R.; Fortunato, F.; Cappelli, M.G.; Chironna, M.; Martinelli, D. Effectiveness of the 13-valent pneumococcal conjugate vaccine against adult pneumonia in Italy: A case–control study in a 2-year prospective cohort. BMJ Open 2018, 8, e019034. [Google Scholar] [CrossRef]
- Chandler, T.; Furmanek, S.; Carrico, R.; Balcom, D.; Arnold, F.; Ramirez, J. 23-Valent Pneumococcal Polysaccharide Vaccination Does Not Prevent Community-Acquired Pneumonia Hospitalizations Due to Vaccine-Type Streptococcus pneumoniae. Microorganisms 2022, 10, 560. [Google Scholar] [CrossRef]
- Dominguez, A.; Salleras, L.; Fedson, D.S.; Izquierdo, C.; Ruiz, L.; Ciruela, P. Effectiveness of Pneumococcal Vaccination for Elderly People in Catalonia, Spain: A Case-control Study. Clin. Infect. Dis. 2005, 40, 1250–1257. [Google Scholar] [CrossRef]
- Lawrence, H.; Pick, H.; Baskaran, V.; Daniel, P.; Rodrigo, C.; Ashton, D.; Edwards-Pritchard, R.C.; Sheppard, C.; Eletu, S.D.; Litt, D.; et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against vaccine serotype pneumococcal pneumonia in adults: A case-control test-negative design study. PLOS Med. 2020, 17, e1003326. [Google Scholar] [CrossRef]
- Perniciaro, S.; van der Linden, M. Pneumococcal vaccine uptake and vaccine effectiveness in older adults with invasive pneumococcal disease in Germany: A retrospective cohort study. Lancet Reg. Health-Eur. 2021, 7, 100126. [Google Scholar] [CrossRef] [PubMed]
- Shimbashi, R.; Suzuki, M.; Chang, B.; Watanabe, H.; Tanabe, Y.; Kuronuma, K.; Oshima, K.; Maruyama, T.; Takeda, H.; Kasahara, K.; et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in adults, Japan, 2013–2017. Emerg. Infect. Dis. 2020, 26, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Su, W.J.; Chuang, P.H.; Chang, L.Y.; Lo, H.Y.; Chiang, C.S.; Wang, E.T.; Yang, C.-H. Application of the screening and indirect cohort methods to evaluate the effectiveness of pneumococcal vaccination program in adults 75 years and older in Taiwan. BMC Infect. Dis. 2021, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Falkenhorst, G.; Remschmidt, C.; Harder, T.; Hummers-Pradier, E.; Wichmann, O.; Bogdan, C. Effectiveness of the 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23) against Pneumococcal Disease in the Elderly: Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169368. [Google Scholar] [CrossRef]
- Schiffner-Rohe, J.; Witt, A.; Hemmerling, J.; von Eiff, C.; Leverkus, F.-W. Efficacy of PPV23 in Preventing Pneumococcal Pneumonia in Adults at Increased Risk—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0146338. [Google Scholar] [CrossRef]
- Htar, M.T.T.; Stuurman, A.L.; Ferreira, G.; Alicino, C.; Bollaerts, K.; Paganino, C.; Reinert, R.R.; Schmitt, H.-J.; Trucchi, C.; Vestraeten, T.; et al. Effectiveness of pneumococcal vaccines in preventing pneumonia in adults, a systematic review and meta-analyses of observational studies. PLoS ONE 2017, 12, e0177985. [Google Scholar]
- Sings, H.L.; Gessner, B.D.; Wasserman, M.D.; Jodar, L. Pneumococcal Conjugate Vaccine Impact on Serotype 3: A Review of Surveillance Data. Infect. Dis. Ther. 2021, 10, 521–539. [Google Scholar] [CrossRef]
- Choi, E.H.; Zhang, F.; Lu, Y.J.; Malley, R. Capsular Polysaccharide (CPS) Release by Serotype 3 Pneumococcal Strains Reduces the Protective Effect of Anti-Type 3 CPS Antibodies. Clin. Vaccine Immunol. 2016, 23, 162–167. [Google Scholar] [CrossRef]

| Author and Publication Year | Country | Population | Study Design | Study Period | Outcome | Factors Adjusted for in VE Estimate, if Reported | VE (95% CI) 2 | Certainty of Evidence |
|---|---|---|---|---|---|---|---|---|
| PCV13 | ||||||||
| Bonten, 2015 [11] 1 | The Netherlands | Dutch pneumococcal vaccine-naïve, community-dwelling adults ≥65 years | Randomized Controlled Trial (CAPITA) | 2008–2013 | PCV13-type IPD | Not applicable | 75% (41, 91) | High 3 |
| Non-invasive non-bacteremic PCV13-type CAP | Not applicable | 45% (14, 65) | ||||||
| Heo, 2022 [27] | South Korea | Hospitalized adults ≥65 years | Test-negative | 2015–2017 | PCV13-type community-acquired pneumonia | Age, sex, risk group based on underlying conditions, disease severity according to the CURB-65 score (confusion, urea level, respiratory rate, blood pressure, age ≥ 65), influenza vaccination status, and 23-valent pneumococcal polysaccharide vaccination status | 41% (−104, 83) | High |
| Lewis, 2019 [29] 1 | US | Kaiser Permanente Northern California members with no record of prior receipt of PPSV23, adults ≥65 years | Cohort study | 2014–2018 | PCV13-type IPD | Not reported | 68% (38, 84) | High |
| McLaughlin, 2018 [30] 1 | US | Inpatient adults ≥65 years | Test-negative | 2013–2016 | Non-bacteremic PCV13-type pneumococcal pneumonia | Seasonality, age, gender, race, ethnicity, place of residence, risk level, BMI category, pneumonia severity index, healthcare facility exposure in last 3 months, weekly exposure to child <5, influenza vaccination in previous year, PPSV23 vaccination within 5 years | 68% (−6, 90) | High |
| Pilishvili, 2018 [31] | US | Active Bacterial Core Surveillance (ABCs) IPD cases, adults ≥65 years | Case-control; controls identified using ReferenceUSAGov | 2015–2017 | PCV13-type + 6C IPD | Presence of chronic and immunocompromising conditions | 59% (11, 81) | High |
| Pilishvili, 2018 [32] | US | Active Bacterial Core Surveillance (ABCs) IPD cases, adults ≥65 years | Case-control; controls identified as Medicare beneficiaries with no record of IPD or pneumonia | 2015–2016 | PCV13-type + 6C IPD | Gender, presence of chronic and immunocompromising conditions | 47% (4, 71) | High |
| Prato, 2018 [33] | Italy | Inpatient and outpatient adults ≥65 years | Test-negative | 2013–2015 | PCV13-type pneumococcal pneumonia | Not reported | 38% (−132, 89) | Low |
| PPSV23 | ||||||||
| Andrews, 2012 [23] | UK | Adults ≥65 years | Indirect cohort | 2003–2010 | PPSV23-type IPD | Age and year of illness (implied by group matching) | 48% (32, 60) | Medium |
| Chandler, 2022 [34] | US | Inpatient adults | Test-negative | 2014–2017 | PPSV23-type pneumococcal pneumonia | Age, diabetes, COPD, congestive heart failure, hyperlipidemia | 2% (−50, 38) | High |
| Djennad, 2018 [24] | UK | Adults ≥65 years | Indirect cohort | 2012–2016 | PPSV23-type IPD | Age, clinical risk group, gender, year of notification, ethnicity | 41% (23, 54) | High |
| Dominguez, 2005 [35] | Spain | Adults ≥65 years | Case-control; hospital controls | 2001–2002 | PPSV23-type IPD | Length of hospital stay, hospital period, COPD, use of corticosteroids, death | 64% (31, 82) | Medium |
| Gutiérrez-Rodriguez, 2014 [25] | Spain | Adults ≥60 years | Indirect cohort | 2008–2011 | PPSV23-type IPD | Age, sex, year of symptom onset and presence of high-risk medical conditions | 44% (19, 62) | High |
| Heo, 2022 [27] | South Korea | Hospitalized adults ≥65 years | Test-negative | 2015–2017 | PPSV23-type pneumococcal pneumonia | Age, sex, risk group based on underlying conditions, disease severity according to the CURB-65 score (confusion, urea level, respiratory rate, blood pressure, age ≥65), influenza vaccination status, and PCV13 vaccination status | 6% (−74, 50) | High |
| Kim, 2019 [28] | South Korea | Adults ≥65 years | Case-control | 2013–2015 | PPSV23-type IPD | Chronic kidney disease, diabetes mellitus, smoking, and recent influenza vaccine exposure | 42% (−2, 67) | High |
| PPSV23-type non bacteremic pneumococcal pneumonia | Immunocompromised status, chronic heart disease, chronic pulmonary disease, chronic alcohol consumption, smoking, long-term care facility residence, and hospital center | −2% (−40, 26) | ||||||
| Lawrence, 2020 [36] | England | Adults ≥16 years | Case-control | 2013–2018 | PPSV23-type pneumococcal pneumonia (PPSV23 non-PCV13 serotypes) | Age, sex, receipt of seasonal flu vaccination, and presence or absence of certain risk factors | 46% (5, 69) | Medium |
| Perniciaro, 2021 [37] | Germany | Adults ≥60 years (German National Reference Center for Streptococci (GNRCS)) | Indirect cohort | 2018–2019 | PPSV23-type IPD (PPSV23 non-PCV13 serotypes) | Age and gender | 35% (−14, 65) | High |
| Rudnick, 2013 [21] | Canada | Adults ≥65 years | Indirect cohort | 1995–2011 | PPSV23-type IPD | Year of illness, gender | 41% (21, 56) | Medium |
| Shimbashi, 2020 [38] | Japan | Adults ≥20 years | Indirect cohort | 2013–2017 | PPSV23-type IPD | Sex, age, prefecture, year, season, BMI group, underlying conditions, and smoking history with clustering by public health center | 39.3% (−2.9, 64.2) | Medium |
| Adults ≥65 years | 39.4% (−6.1, 65.3) | |||||||
| Su, 2021 [39] | Taiwan | Adults ≥75 years | Indirect cohort | 2008–2016 | PPSV23-type IPD | 74% (39, 89) | High | |
| Screening method | Age and gender | 43.4% (34.4, 51.2) | ||||||
| Suzuki, 2017 [22] | Japan | Adults ≥65 years | Test-negative | 2011–2014 | PPSV23-type pneumococcal pneumonia | Study site, sex, age, underlying disorder, smoking status, pre-hospital antibiotic treatment, and year of hospital visit | 34% (6, 53) | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrar, J.L.; Childs, L.; Ouattara, M.; Akhter, F.; Britton, A.; Pilishvili, T.; Kobayashi, M. Systematic Review and Meta-Analysis of the Efficacy and Effectiveness of Pneumococcal Vaccines in Adults. Pathogens 2023, 12, 732. https://doi.org/10.3390/pathogens12050732
Farrar JL, Childs L, Ouattara M, Akhter F, Britton A, Pilishvili T, Kobayashi M. Systematic Review and Meta-Analysis of the Efficacy and Effectiveness of Pneumococcal Vaccines in Adults. Pathogens. 2023; 12(5):732. https://doi.org/10.3390/pathogens12050732
Chicago/Turabian StyleFarrar, Jennifer L., Lana Childs, Mahamoudou Ouattara, Fahmina Akhter, Amadea Britton, Tamara Pilishvili, and Miwako Kobayashi. 2023. "Systematic Review and Meta-Analysis of the Efficacy and Effectiveness of Pneumococcal Vaccines in Adults" Pathogens 12, no. 5: 732. https://doi.org/10.3390/pathogens12050732
APA StyleFarrar, J. L., Childs, L., Ouattara, M., Akhter, F., Britton, A., Pilishvili, T., & Kobayashi, M. (2023). Systematic Review and Meta-Analysis of the Efficacy and Effectiveness of Pneumococcal Vaccines in Adults. Pathogens, 12(5), 732. https://doi.org/10.3390/pathogens12050732





