Abstract
(1) Background: Streptococcus dysgalactiae subspecies equisimilis (SDSE) is an important β-hemolytic pathogen historically described as mainly affecting animals. Studies epidemiologically assessing the pathogenicity in the human population in Germany are rare. (2) Methods: the present study combines national surveillance data from 2010 to 2022 with a single-center clinical study conducted from 2016 to 2022, focusing on emm type, Lancefield antigen, antimicrobial resistance, patient characteristics, disease severity, and clinical infection markers. (3) Results: The nationwide reported invasive SDSE infections suggest an increasing infection burden for the German population. One particular emm type, stG62647, increased over the study period, being the dominant type in both study cohorts, suggesting a mutation-driven outbreak of a virulent clone. The patient data show that men were more affected than women, although in the single-center cohort, this trend was reversed for patients with stG62647 SDSE. Men affected by stG62647 developed predominantly fascial infections, whereas women suffering from superficial and fascial non-stG62647 SDSE infections were significantly younger than other patients. Increasing age was a general risk factor for invasive SDSE infections. (4) Conclusions: further studies are needed to further elucidate the raised questions regarding outbreak origin, underlying molecular mechanisms as well as sex-dependent pathogen adaptation.
1. Introduction
Streptococcus dysgalactiae subspecies equisimilis (SDSE) is an important β-hemolytic pathogen mainly expressing Lancefield antigens C or G, causing severe infections in humans [1]. The taxonomic and pathogenic classification of the bacterial species has undergone several changes [2,3,4], but the historic description as mainly animal pathogenic, rarely associated with zoonotic infections [5] is highly questionable [6,7]. This volatility might be a reason why its impact on human health is still underestimated by public health institutions [8], even though SDSE resembles the pathogenic potential of the major β-hemolytic human pathogen S. pyogenes able to cause severe tissue infections [9,10], sepsis [11,12], toxic shock syndrome [13,14], and in rare cases meningitis [11,15]. Furthermore, the incidence of human S. dysgalactiae infections is constantly increasing worldwide [16,17,18,19,20], in some geographical regions even overtaking S. pyogenes [21,22,23].
A potential explanation for the success of this pathogen might be its virulence factor arsenal [24], the majority of which is already well described for S. pyogenes [25], including the major virulence mediator, the M protein [26,27]. This filamentous protein, encoded by the emm gene (Encoding Mature M protein) is presented in a coiled-coil dimeric structure on the bacterial surface [28], covalently linked to the Gram-positive cell wall by the LPXTG mechanism [29]. Expressed in very high amounts, the M protein covers the bacterial cell like a protective fur [30], interacting with different host molecules [31,32,33] and in that way inhibiting phagocytosis [34,35] and mediating interaction with different cell types [36,37,38,39]. The N-terminal region of the M protein shows high variability in amino acid composition, facilitating antigenic diversity [40], that on one hand might be the reason for recurrent infections with the same bacterial species [41,42], and on the other hand, allows epidemiological tracing by nucleotide sequence-based emm typing [43,44].
To date, more than one hundred different emm types have been described for SDSE [45], of which only a small subset is frequently isolated in Germany [46,47]. Significant differences in the emm type distribution have been described for various geographical regions [18,19,20,48], but only a few studies discovered the long-term emm type shift toward one obviously very successful type, denoted stG62647. First mentioned in a multicenter study from Argentina in 2016, this emm type was already described as the most abundant in the analyzed patient cohort [48]. In 2017, a retrospective study confirmed the dominance of stG62647 also for invasive SDSE infections in Switzerland [49]. In the same year, an increase in stG62647 infections since 2013 was also described in Norway, combined with a comprehensive genome analysis approach to examine the molecular basis for the success of this particular emm type [50]. Interestingly, Oppegaard et al. [50] showed that the vast majority of stG62647 isolates from invasive infections in Bergen contained a genetic disruption in the streptococcal invasive locus (sil), induced by the insertion of a transposase into the silB-gene, potentially interfering with virulence factor regulation, offering a possible explanation for the fulminant disease development observed. Since then, the dominance of stG62647 in invasive but also non-invasive SDSE infections has been confirmed by different single- and multicenter studies in Germany [46,47], Sweden [51,52], Denmark [52], and Spain [53]. However, further research, explaining the evolutionary and pathogenic success of this particular emm type, is still lacking.
The present work combines national surveillance data with a single-center clinical study to highlight recent developments in the emm type distribution of invasive SDSE infections in Germany with a special focus on the rise of the very successful type stG62647.
2. Materials and Methods
2.1. Sample Collection
The German National Reference Center for Streptococci (GNRCS) has performed surveillance of invasive streptococcal infections in Germany since 1996. Primary microbiological laboratories are asked to send streptococcal isolates of invasive infections on a voluntary basis. The collection of corresponding patient data is addressed by a standardized questionnaire, to be filled out by the participating centers. The requested information includes primary bacterial species identification, isolation material, date of isolation, patient date of birth, patient sex, patient residence, diagnosis, and underlying diseases.
2.2. Data Preparation
All bacterial isolates sampled between 1 January 2010 and 31 December 2022, identified as SDSE were included in the study. Exclusion criteria were patient national extraction (patients without regular residence in Germany), non-invasive infection (isolate not sampled from a normally sterile anatomical site, sampling information missing, or SDSE isolate not identified as the primary source of infection), and potential clonality (isolates from the same patient with identical emm type/subtype and antibiotic sensitivity pattern).
Sample material data were consolidated into seven categories, including blood isolates, tissue isolates (tissue, biopsy, aortic valve, mitral valve, heart valve, calf muscle, knee tissue, and muscle tissue), puncture isolates (puncture, joint puncture, and knee joint puncture), swab isolates (swab and Achilles’ tendon swab), cerebrospinal fluid isolates, urine isolates, and isolates from other sources (prosthesis material, fragment knee, implant material, and secretion).
2.3. Microbiological Analyses
All bacterial isolates received at the GNRCS were cultivated on Tryptone Soya agar with sheep blood (Thermo Scientific™, Schwerte, Germany) for 18 h at 37 °C and 5% CO2. The species identification was performed by a combination of hemolysis and colony size assessment, catalase, leucine aminopeptidase [54], and pyrrolidonylarylamidase tests [55] as well as Lancefield antigen typing (Prolex™ Streptococcal Grouping Kit, Richmond Hill, ON, Canada; ImmuLex Streptococcus Group L, SSI-Diagnostica, Hillerød, Denmark), and in some cases MALDI-Biotyper® analysis and/or 16S rRNA gene sequencing (primers: 27F and 1492R). For SDSE isolates, emm typing was performed in accordance with the CDC guidelines for S. pyogenes using two different primer sets [56,57] and strepBLAST 2.0 server. The current emm nomenclature denotes the gene symbol (e.g., stG) followed by a number specifying the main type (e.g., stG62647), while the subtype, characterized by single mutation variants of the main type, is given after a dot (e.g., stG62647.0).
2.4. Antibiotic Sensitivity Pattern
Susceptibility testing for nine antimicrobial substances (penicillin, amoxicillin, cefotaxime, vancomycin, erythromycin/clarithromycin, clindamycin, chloramphenicol, tetracycline, and levofloxacin) was performed by broth microdilution (Sensititre™ NLMCP2, Thermo Scientific™, East Grinstead, UK) following the guidelines M100 (Performance Standards for Antimicrobial Susceptibility Testing) and M07 (Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically) of the Clinical and Laboratory Standards Institute (CLSI).
2.5. Single Center Microbiological Analyses
Ingolstadt Hospital is a secondary 1155-bed hospital in the center of Bavaria (southeastern Germany), providing health care for about 500,000 inhabitants in the planning region 10. The hospital boasts three intensive care units and intermediate care as well as a stroke unit. The hospital has four internal and various surgical facilities. At the microbiological laboratory of Ingolstadt Hospital, SDSE isolates were cultured in thioglycolate broth, on sheep blood agar (bioMérieux, Nürtingen, Germany), Schaedler agar containing 5% sheep blood (bioMérieux), and/or chocolate agar (bioMérieux). Urine samples were cultured on sheep blood agar covered with a 50 µg pipemidic acid disk (BioRad, Marnes-la-Coquette, France) to inhibit the growth of Gram-negative bacteria. Blood cultures were grown in aerobe and anaerobe blood culture bottles using the BactAlert blood culture system (bioMérieux). After bacterial growth was detected by the BactAlert system, blood samples from aerobe bottles were given on blood and chocolate agar and additionally on Schaedler agar in the case of bacterial growth in anaerobe blood culture bottles. Species identification was either determined using Vitek 2 MS (bioMérieux) or the Vitek 2 compact (bioMérieux) with appropriate Vitek 2 identification (ID) cards.
2.6. Clinical Chemistry
Laboratory values (concentration of leukocytes, C-reactive protein (CRP), lactate dehydrogenase (LDH), creatine phosphokinase (CPK), and aspartate aminotransferase (AST)) were regarded as surrogate markers for inflammation and tissue destruction. The values of blood samples obtained from the day of bacterial sampling were observed. If no blood had been taken on the day of bacterial sampling, data from samples obtained on the preceding or the following days were used, a maximum of three days before or after bacterial sampling. The concentrations of CRP, LDH, CPK, and AST were determined using the Alinity analyzer (Abbott, Abbott Park, IL, USA) whereas the concentration of leukocytes was measured with the UniCel DxC 600 Chemistry Analyzer (Beckman Coulter Inc., Brea, CA, USA). The laboratory of Ingolstadt Hospital is certified in accordance with the national accreditation body of the Federal Republic of Germany (Deutsche Akkreditierungsstelle; DAkkS; registration number D-ML-19856-01-00).
2.7. Clinical Cohort Description
Ingolstadt Hospital sent 123 SDSE isolates causing colonization, as well as peripheral and invasive infections from December 2016 to November 2022 to the GNRCS for emm and Lancefield antigen typing. From this single-center study cohort, 19 cases were excluded due to the lack of patients’ informed consent. The remaining 104 SDSE cases were classified into 82 invasive and 22 non-invasive infections (isolate not sampled from a normally sterile anatomical site or SDSE isolate not identified as the primary source of infection). Infections were further categorized into three groups for assessing the impact of particular emm types on the clinical course. Colonization of the skin and urinary tract, as well as infections restricted to the skin and mucous membranes, were categorized as superficial infections. Infections of soft tissue and organs including urinary tract infections with a significant concentration of bacteria (>100,000 cfu/µL) were regarded as fascial infections. Isolation of SDSE from blood culture and infections leading to systemic affections, e.g., the need for catecholamine treatment, were categorized as systemic infections. The category “Sampling (days after admission)” was defined as the time period from patient admission to the day of bacterial sampling, finally resulting in growth-dependent detection of SDSE. If SDSE had been isolated from a sample taken at the pre-hospital appointment, the number of days until sampling resulted in a negative value (patient 102). The length of hospital stay was calculated in days, as the time period from patient admission until discharge.
2.8. Statistical Analysis
Descriptive statistics for categorical variables are displayed as absolute or relative frequencies. For continuous variables, mean, median, and standard deviations are shown. Hypothesis tests for proportions were performed using a chi-square test. Mean values were compared using a t-test. In both cases, small p-values were interpreted as an indication in favor of the alternative hypothesis.
3. Results
3.1. Invasive SDSE Infections in Germany
In the study period from 2010 to 2022, the GNRCS received 1643 SDSE isolates associated with severe human infections. From this collection, 116 isolates were excluded due to not fulfilling the inclusion criteria for a national study cohort of invasive SDSE infections (Figure 1a). A total of 11 isolates were derived from patients without regular residence in Germany, 95 isolates were not sampled from normally sterile body sites, sampling information was missing, or SDSE could not be identified as the primary source of infection, and 10 isolates were identified as potential clones. The final study cohort included 1527 invasive SDSE isolates from German patients (Table S2).
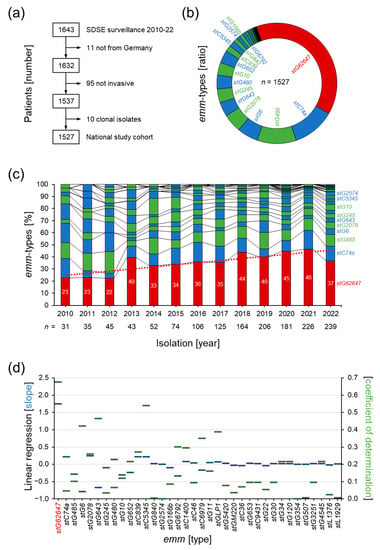
Figure 1.
Surveillance of invasive SDSE infections in Germany in the years 2010 to 2022 by the German National Reference Center for Streptococci. (a) Summary of the performed study cohort validation. (b) Distribution of emm types in the national study cohort. (c) Yearly changes in the emm type composition of the national study cohort (columns) with linear regression of the stG62647 isolate rate (dotted line). (d) Slope (blue bars) and coefficient of determination (green bars) of the linear regression of the chronological changes in emm type composition in the national study cohort.
The performed typing of the M protein gene (emm) revealed a dominance of one particular emm type denoted stG62647 (Figure 1b). A total of 39% (n = 594) of all invasive SDSE isolates in the national study cohort belonged to this type, while all other emm types showed abundance values below 12%, with stC74a (11.5%, n = 174) and stG485 (10.5%, n = 161) being the second and third most abundant types.
Chronological analysis showed a steady increase in invasive stG62647 cases over the study period, starting from 23% in 2010, doubling to 46% in 2021, but decreasing to 37% in 2022 (Figure 1b). Detailed analysis of the emm type composition of each study year clearly showed that stG62647 was the emm type with the highest linear increase, with a slope of 1.8 and a coefficient of determination of 0.67 (Figure 1c), including a sudden upsurge in 2013 to 40%. No other emm type showed a significant linear relationship during the study period, and all correlations found were spurious.
3.2. Dissection of Epidemiological Markers
The determination of the Lancefield antigen repertoire showed that stG62647, in contrast to the majority of the other emm types in the national study cohort, mainly expresses the C antigen (98.3%, n = 584) and only 1.7% (n = 10) of the stG62647 isolates were positive for the G antigen (Figure 2a). The Lancefield types A and L were not found in stG62647. Other emm types, mainly associated with Lancefield antigen C, such as stC36, stC1400, stC6979, stC9431, stG354, stG2574, stGM220, and stL1929 are much less abundant in the study cohort, with 1 to 15 isolates in twelve years. Consequently, stG62647 with 594 isolates is by far the most abundant Lancefield-C SDSE, associated with invasive infections in humans.
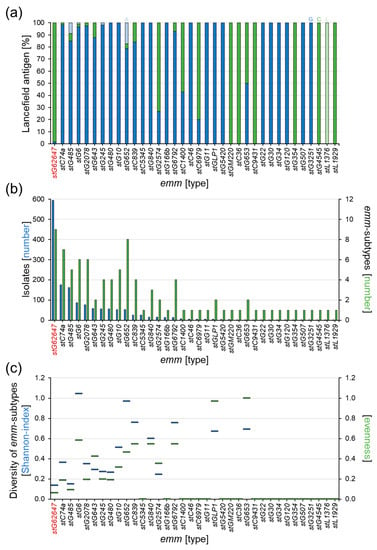
Figure 2.
Analysis of the Lancefield antigen expression and mutation rate within the different emm types of the national study cohort. (a) Ratio of Lancefield antigen expression of the 36 emm types in the national study cohort (columns), showing antigen A (light blue), antigen C (green), antigen G (blue), and antigen L (light green). (b) Number of isolates per emm type (blue columns) and number of corresponding emm subtypes (green columns) for all 36 emm types in the national study cohort. (c) Comparison of relative mutation rate within the 36 emm types by Shannon index (blue bars) and evenness (green bars).
A distinct correlation between isolate numbers and the relative mutation rate of the different emm types was not observed in the study cohort. However, even though stG62647 showed the highest number of emm subtypes in the cohort, the sample distribution among these nine different subtypes was extremely shifted toward stG62647.0, with 594 isolates (Figure 2b), leaving only twelve isolates for the remaining eight subtypes stG62647.3 (n = 1), stG62647.4 (n = 4), stG62647.5 (n = 1), stG62647.6 (n = 1), stG62647.7 (n = 1), stG62647.8 (n = 1), stG62647.11 (n = 2), and stg62647.13 (n = 1), which is clearly depicted by the resulting very low diversity indices (Figure 2c). With a Shannon index of 0.14 and an evenness of 0.06, stG62647 has the lowest intra-emm-type diversity of all emm types associated with subtypes in the national study cohort, closely followed by stG485 (H′ ≈ 0.15, J′ ≈ 0.09).
3.3. Comparison of stG62647 with Other emm Types
The geographical distribution showed no distinct accumulation of invasive SDSE infections caused by stG62647 (Figure 3a). Very high ratios of cases associated with stG62647 are the result of generally low numbers of invasive SDSE isolates, reported to the GNRCS in the corresponding federal state during the study period, as depicted for Bremen with 100% (stG62647/other = 1/0), Hamburg with 0% (stG62647/other = 0/2), and Saxony-Anhalt with 0% (stG62647/other = 0/2). The ratio of invasive stG62647 infections versus other emm types in the remaining thirteen German federal states varied between 28% in Hesse (stG62647/other = 35/90) and 57% in Berlin (stG62647/other = 4/3).
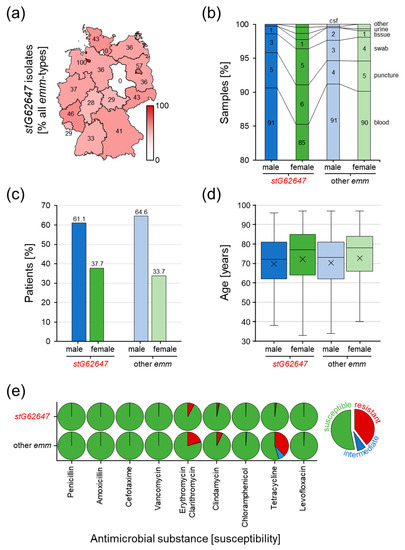
Figure 3.
Analysis of potential differences between invasive SDSE infections associated with stG62647 and other emm types in the national study cohort. (a) Geographical disaggregation of the ratio of stG62647 cases against total invasive SDSE infections (red gradation and values in the map) by the German federal state of the corresponding patient residence. (b) Differences in sample material ratio between stG62647 cases (dark colored columns) and other emm types (light colored columns), discriminating male (blue) and female (green) patients. (c) Comparison of invasive SDSE infections associated with stG62647 (dark colored columns) and all other emm types (light colored columns) of the national study cohort discriminating male (blue) and female (green) patients. (d) Analysis of invasive infections associated with stG62647 (dark colored boxes) and all other emm types (light colored boxes) of the national study cohort regarding age distribution for male (blue) and female (green) patients with the median (line) and arithmetic mean (cross). (e) Susceptibility of stG62647 isolates and isolates of all other emm types of the national study cohort to nine antimicrobial substances, discriminating susceptible (green), intermediate (blue), and resistant (red) following the guidelines of the Clinical and Laboratory Standards Institute.
The consolidation of the provided data regarding sample material clearly revealed that the vast majority of invasive SDSE isolates are sampled from blood cultures, with 90.6% for male and 85.3% for female stG62647-affected patients as well as 91.2% male and 90.1% female patients infected by other SDSE emm types (Figure 3b). The marginally lower amount of blood cultures observed for female stG62647 patients is compensated by other isolation sources, mainly swab cultures. However, no significant differences in the sampling material distribution could be observed between the different emm types.
The consideration of patient sex showed that men in general are more affected by invasive SDSE infections than women (Figure 3c). However, this trend appears to be less pronounced for stG62647, with only 61.1% of patients being male, while all other emm types show a higher ratio, with 64.6% male patients.
The combination of patient data regarding sex and age at the time of infection revealed that women are in general five years older than men when affected by an invasive SDSE infection, with a median of 77.5 years compared to 72.5 years for men (Figure 3d). Significant differences in sex-dependent age distribution between cases associated with stG62647 and other emm types could not be observed.
Determination of antimicrobial susceptibility showed differences between the compared emm types (Figure 3e). Isolates of the stG62647 emm type are more susceptible to five of the nine antimicrobial substances tested. While all analyzed SDSE isolates showed full susceptibility to penicillin, amoxicillin, cefotaxime, and vancomycin, resistance against erythromycin/clarithromycin (stG62647/other = 8%/20%), clindamycin (3%/7%), tetracycline (2%/38%), and to a minor extent levofloxacin (0.17%/0.32%) and chloramphenicol (0.00%/0.11%) was, in general, lower in stG62647 isolates compared to all other emm types.
3.4. Description of the Clinical Cohort at Ingolstadt Hospital
At Ingolstadt Hospital, 82 invasive SDSE cases were examined, of which 36 were female and 46 male (Table 1). The age of the affected patients ranged from 27 to 93 years with a median of 65. None of the included patients died as a consequence of the identified SDSE infection. Most of the bacterial isolates were identified in the blood (29.3%), swab cultures (25.6%), and sore smear (23.2%), while the remaining samples were from other isolation sources (Table S1). Based on diagnostic data and sampling material, the cases were categorized into superficial (34.1%), fascial (31.7%), and systemic infections (34.1%). The average time span between patient admission and bacterial sampling was 3.7 days, while the average patient hospitalization time was 20.8 days. The Lancefield antigen distribution of the corresponding SDSE isolates was almost uniform, with antigen C at 48.8% and antigen G at 51.2%. Sequencing of the M protein gene revealed that 38 (46.3%) isolates belonged to the emm type stG64247, all expressing Lancefield antigen C (Table S1). The remaining 44 SDSE isolates belonged to 15 additional emm types comprising one to nine isolates, respectively.

Table 1.
Invasive SDSE infections at Ingolstadt Hospital from December 2016 to November 2022.
3.5. Impact of Bacterial emm Type, Patient Sex, and Age on Clinical Courses
At Ingolstadt Hospital, SDSE isolates of 104 patients were examined, of which 82 were classified as invasive (46 male, 36 female), whereas 22 were excluded from the single-center cohort (15 male, 7 female) and categorized as non-invasive (Figure 4a).
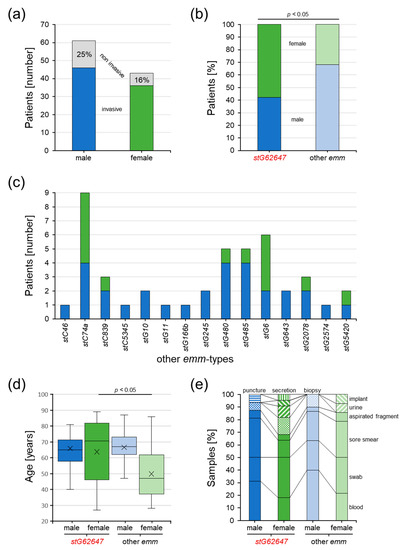
Figure 4.
Characteristics of SDSE-infected patients of the single-center cohort from Ingolstadt Hospital separated into stG62647 cases (dark colored) and cases associated with other emm types (bright colored) divided by male (blue) and female patients (green). (a) The number of invasive (colored bars) and excluded non-invasive SDSE cases (grey bars). (b) The ratio of invasive SDSE cases separated by patient sex and emm type; p-value was calculated by the chi-squared test. (c) Distribution of emm types other than stG62647 in invasive SDSE cases, separated by patient sex. (d) Distribution of patient age separated by patient sex and emm type of invasive SDSE cases with indicated median (line) and arithmetic mean (cross); p-value originated from a contrast within a two-way ANOVA model. (e) Distribution of isolation material separated by patient sex and emm type of invasive SDSE cases.
In the single-center study cohort, a significant difference in the patient sex distribution dependent on the emm type could be identified (Figure 4b). Invasive SDSE cases associated with the emm type stG62647 more frequently affected females (57.9%) than males (42.1%), whereas infections with other emm types showed the reverse trend, with 31.8% female and 68.2% male patients.
The remaining 15 emm types were not uniformly distributed between male and female patients (Figure 4c). Whereas males were affected by all 15 other emm types, invasive SDSE infections in females were associated with only seven, namely stC74a (n = 5), stG6 (n = 4), and stC839, stG480, stG485, stG2078, and stG5420 (n = 1 each).
Considering patient age, it was obvious that females affected by stG62647 SDSE isolates were significantly older (median 70.5 years) than females associated with other emm types (median 47.0 years), whereas no substantial differences could be observed in the invasive SDSE infections of male patients (Figure 4d). Interestingly, excluding the emm type assignment, male (median 66.5 years) and female (median 66.0 years) cases showed no differences in age distribution.
Taking the type of isolation material into account, invasive SDSE isolates were more frequently identified in the blood of male patients (stG62647/other emm 31.25%/40.00%) than in females (stG62647/other emm 18.18%/21.43%) (Figure 4e). However, no statistically significant correlation could be identified.
Further analyses were applied by categorizing the 82 invasive SDSE patients of the Ingolstadt Hospital single-center cohort into superficial, fascial, and systemic infections to assess the potential impact of emm type and patient sex. Male patients affected by SDSE stG62647 more frequently developed fascial infections (stG62647/other emm 56.25/23.33%), but fewer superficial (12.50/33.33%) and systemic (31.25/43.33%) characteristics than males associated with other emm types, whereas female cases showed no obvious differences in the distribution of the infection severity classification (Figure 5a). Although it was obvious that infections in males were more severe compared to female patients, the difference did not reach statistical significance.
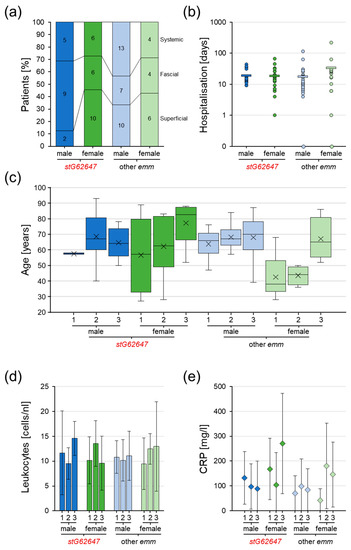
Figure 5.
Assessment of Ingolstadt Hospital invasive SDSE case infection severity classification grouped into superficial (1), fascial (2), and systemic (3), separated into stG62647 cases (dark colored) and cases associated with other emm types (bright colored), divided by male (blue) and female patients (green). (a) Distribution of infection severity separated by patient sex and emm type with total numbers (numbers inside columns). (b) Distribution of hospital stay duration separated by patient sex and emm type with single values (circles) and arithmetic mean (bar). (c) Distribution of patient age separated by infection severity, patient sex, and emm type with indicated median (line) and arithmetic mean (cross). (d) Distribution of leukocyte count in blood separated by infection severity, patient sex, and emm type indicated by arithmetic mean (columns) and standard deviation (whiskers). (e) Distribution of CRP concentration in blood separated by infection severity, patient sex, and emm type indicated by arithmetic mean (diamonds) and standard deviation (whiskers).
The duration of hospitalization showed no significant differences in the average hospital stay (Figure 5b). However, female patients affected by emm types other than stG62647 stayed on average almost twice as long in the hospital (33 days) as females affected by stG62647 (19 days) and males (other emm, 18 days; stG62647, 19 days). The variance for male patients affected by SDSE stG652647 was slightly lower (6–43 days), compared to males infected by other emm types (0–129 days) as well as females (stG62647, 1–66 days; other emm, 0–217 days).
Combining the infection severity classification with patient sex and considering the patient age distribution, it was obvious that females suffering from superficial and fascial infections of emm types other than stG62647 were distinctly younger (superficial median, 38 years; fascial median, 44 years) than female patients infected by stG62647 (superficial median, 57 years; fascial median, 63 years) as well as males in general (Figure 5c). Male patients showed no significant differences in age distribution dependent on the infection severity. However, male patients suffering from fascial infections by SDSE stG62647 were marginally older (median 67 years) than males systemically infected by this emm type (median 64 years), which is in contrast to males affected by other emm types and female patients. Besides these trends, no statistical significance could be obtained.
Considering general infection markers such as leukocyte cell count and C-reactive protein (CRP) concentration as indicators of infection severity to identify potential differences between stG62647 and other emm types dependent on patient sex, it was obvious that the average values of both clinical markers did not consistently reflect the applied infection severity classification nor the observed patient age distribution (Figure 5d,e). The huge inter-patient variance within the different groups allowed no statistically significant conclusions.
3.6. S. dysgalactiae Infections Resembling S. pyogenes and S. agalactiae
Many infections caused by SDSE have shown disease patterns and courses resembling those of S. pyogenes. Among them are erysipelas, wound infections at various body sites, and implant-associated infections, such as endo- and periprosthetic infections, as well as nosocomial infections. Interestingly, within the observation period, four women who had recently given birth suffered from SDSE-induced mastitis puerperalis (patients 2, 3, and 4: stG62647.0; patient 51: stG485.0. See Table S1), whereas no cases of S. pyogenes mastitis occurred in Ingolstadt Hospital (Figure 6).
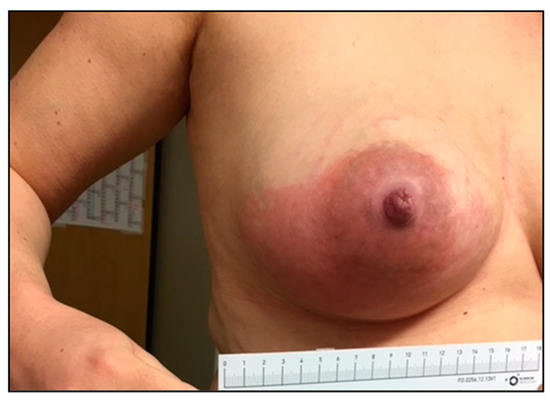
Figure 6.
Photographic documentation of an SDSE stG62647.0-induced mastitis puerperalis in a 33-year-old mother (patient 2; Table S1) after giving her first birth.
Apart from mimicking infections of S. pyogenes, SDSE also caused infections usually induced by S. agalactiae. An example was the infection of a newborn girl who developed a newborn infection but rapidly recovered after ampicillin and gentamicin application (case not included in study cohort). Interestingly, three years before, her mother had suffered from a postpartum infection caused by β-hemolytic group B streptococci after giving birth to her older daughter. This case suggests that, besides mediating infections typically caused by S. pyogenes, SDSE might also lead to infections usually associated with S. agalactiae, and even may substitute this bacterial species in predisposed patients.
4. Discussion
The incidence of invasive Streptococcus dysgalactiae ssp. equisimilis infections is rising worldwide [16,17,18,19,20,21,22,23], with one emm type, denoted stG62647, constantly increasing [46,47,48,49,50,51,52,53]. Even though a comparative genome analysis approach revealed the potential involvement of a mutated virulence regulator [50], a comprehensive explanation for the evolutionary and pathogenic success of this particular emm type is still lacking.
The presented work combines national surveillance data with a single-center clinical study, on one hand, to provide an epidemiologic overview of invasive SDSE in Germany, and, on the other hand, to highlight recent developments in the emm type distribution and related clinical aspects with a special focus on the very successful type stG62647. While the national study cohort gives insight into changes in the emm type ratio in the study years 2010 to 2022, with additional attention to microbiological, geographical, and sample characteristics, the single-center cohort intended to obtain a representative cross-section of the total SDSE burden at a single hospital site in Germany concentrating on patient characteristics, classified disease severity, and clinical infection markers.
The results of this combinatory approach suggest an increasing infection burden for the German population, indirectly indicated by the continuously increasing number of invasive SDSE isolates reported to the GNRCS from 31 in 2010 to 239 in 2022 (Figure 1c). However, it is not clear if this increase is indeed the result of extended bacterial spread, rising invasiveness, or just an increased awareness of SDSE infections within the observation period. Since epidemiological data on SDSE colonization are not available for Germany, the reason for the increased number of invasive infections remains unclear. Furthermore, epidemiologic data from the SARS-CoV-2 pandemic with the institution of non-pharmaceutical interventions, such as face masks and social distancing, suggest that carriage, transmission as well as infection initiation of SDSE are not well understood and might differ from what is known for its close relative S. pyogenes [47].
At Ingolstadt Hospital, the overall number of SDSE cases between 2010 and 2022 increased only to a very minor extent, although with great variation in the number of isolates per year, ranging from 56 cases in 2010 and 94 cases in 2012 (Figure S1). Therefore, enhanced virulence or improved attention to SDSE infections provide probable explanations for the rising numbers of invasive SDSE reported to the GNRCS. The potential change in awareness of this particular bacterial species might have been facilitated by technical innovations in microbiological routine diagnostics. Within the study period, many diagnostic laboratories in Germany shifted the streptococcal identification routine away from serotyping, only enabling the classification of isolates by Lancefield antigen groups, to species identification by MALDI-TOF MS [58]. At Ingolstadt Hospital the technique was introduced in January 2015 [59]. As the MALDI-TOF system in most cases provides the taxonomic notation of a bacterial isolate, it can be assumed that the introduction of this technique contributed to increased submission rates of S. dysgalactiae ssp. equisimilis.
In Germany, a general trend of an aging population is observed [60], which leads to a shift in the incidence of many different diseases including bacterial infections [61]. Surveillance data show invasive SDSE infections and severity primarily increasing with age (Figure 5c), mainly affecting older people [23,47,52,62]. This shift in population composition might also contribute to the observed increase in invasive SDSE infections reported to the GNRCS, potentially overlaid by a severity bias leading to increased submission rates of conspicuous cases.
The rising number of invasive SDSE infections in the national study cohort between 2010 and 2022 might be the result of multifactorial changes in bacterial virulence, clinical awareness, and population structure, potentially not reflecting a general trend. However, the change in emm type distribution toward one very successful type, stG62647, cannot be neglected (Figure 1c). The performed linear regression, even though not the ideal model to describe the observed trend in emm type distribution, clearly showed a significant increase in stG62647 in the national study cohort (Figure 1d). None of the other emm types is characterized by a comparable slope above a value of 0.3, while stG62647 on average increased by 1.8% per year. However, the corresponding coefficient of determination, although depicting the highest reliability within all emm types in the study cohort, shows a comparably low value of 0.67, clearly indicating a deviation from an ideal linear trend.
The main reason for this impaired regression fitting, besides the drop in 2022, seems to be the sudden increase in stG62647 isolates in 2013 (Figure 1c), which was initially observed in Norway by Oppegaard et al., in 2017 [50], and could now be confirmed by national surveillance data from Germany. Whether the observed rise in stG62647 in 2013 was initiated by the described mutation event in the virulence regulator needs to be clarified by a comparative genome approach including stG62647 strains isolated before 2013. However, this sudden increase within one year strengthens the hypothesis of a virulence-driven outbreak, representing a potential origin of the observed dominance of this particular emm type.
One possible reason, besides increased virulence, might be the stG62647 M protein itself. Even though not much is known about the functional repeat structure of this M type, the highly variable N-terminal region seems to be a very successful antigenic variant. Assessing the subtype distribution of all emm types in the national study cohort, it becomes obvious that stG62647 produced a comparatively low number of emm subtypes in relation to the corresponding number of reported infection events. Furthermore, the subtype distribution within this particular emm type is extremely shifted toward stG62647.0, which is clearly indicated by very low diversity indices (Figure 2c). With a Shannon index of 0.14 and an evenness of 0.06, stG62647 has the lowest intra-emm-type diversity of all emm types in the study cohort. However, whether this implies a superior antigenic structure needs to be addressed by additional immunological studies. These observations suggest a global outbreak of an stG62647.0 clone, spontaneously mutated in or before 2013, that turned out to be very successful. Interestingly, stG62647.0 occurs in two different variants, expressing mainly Lancefield antigen C, but also Lancefield antigen G (Figure 2a). Oppegaard et al. reported solely group C stG62647 isolates [50], which reflects the situation in the Ingolstadt Hospital cohort (Table S1), possibly a result of low case numbers in these single-center studies. However, a first step to address the hypothesis of a clonal origin of the observed stG62647 rise could be the tracking of the virulence-regulator mutation in the sliB-gene in stG62647 isolates expressing the Lancefield antigen G.
The question of the chronological and geographical origin of this potential clonal outbreak will most probably remain unanswered. The assessment of patient residence data of the national study cohort revealed no geographical accumulation of invasive SDSE stG62647 cases in German federal states (Figure 3a).
The analysis of additional patient data available in the national study cohort showed that around 90% of stG62647 and non-stG62647 isolates had been identified in blood cultures, indicating that the majority of reported SDSE isolates had indeed caused not only invasive but also systemic infections, which argues against the hypothesis that non-stG62647 emm types per se exhibit lower pathogenicity (Figure 3b).
The majority of invasive SDSE was isolated from male patients, independently of the emm type. However, the percentage of affected males was slightly higher when infected by non-stG62647 strains (Figure 3c). A comparable trend was observed in the single-center cohort of Ingolstadt Hospital, where the proportion of male patients affected by stG62647 was lower than the percentage of males affected by other emm types (Figure 4b). However, in contrast to the national cohort, in Ingolstadt Hospital stG62647 was isolated more frequently from female patients, whereas the resulting low numbers of female non-stG62647 cases may explain the observed differences in emm type diversity (Figure 4c). Nevertheless, the reason for this discrepancy between the two study cohorts is not clear. In Ingolstadt Hospital, the ratio of male to female patients suffering from stG62647 bloodstream infections was almost similar to that of non-stG62647 affected patients (Figure 4e). Taking the patient sex into account, invasive SDSE were more frequently isolated in the blood of male patients, than of females, but only males affected by non-stG62647 emm types showed a higher number of systemic infections, whereas the stG64647 cases were more frequently associated with fascial infections (Figure 5a) accompanied by a slightly higher age (Figure 5c). Unfortunately, the only other single-center study from Germany assessing the emm type distribution of SDSE infections did not describe the ratio of affected male and female patients nor did it address infection severity [46]. Since skin and mucous membranes of males and females exhibit significant differences in their composition [63,64,65], the molecular interactions of SDSE virulence factors, such as M protein, might be affected, which makes a sexual preference of particular emm types possible. Therefore, further studies are needed to examine whether particular emm types prefer colonization of male or female humans.
In the single-center cohort from Ingolstadt Hospital, female patients affected by invasive SDSE showed significant emm-type-dependent age differences, with females affected by non-stG62647 isolates being about 20 years younger than females suffering from stG62647 infections, and males in general (Figure 4d). This effect could not be explained by differences in isolation material (Figure 4e) nor infection severity (Figure 5a) and had no obvious influence on the duration of hospitalization (Figure 5b). However, the combined analysis of emm type, patient sex, and infection severity clearly showed that this age effect was mainly based on non-stG62647 superficial and fascial infections affecting younger women (Figure 5c). In addition to that, a general trend of increasing infection severity with patient age could be observed. Unfortunately, the emm-type-dependent age effect in females as well as the general trend of increasing infection severity with growing age was not reflected by infection markers such as leukocyte count (Figure 5d) or CRP concentration (Figure 5e), probably because of immense variation between patients, induced by low case numbers in the subgroups of the single-center cohort. Additionally, it needs to be argued that the applied infection severity classification, even though based on diagnostic data, isolation material information, and clinical infection values, represents a subjective categorization, prone to bias. However, the observation that females are less affected by non-stG62647 SDSE but at a younger age is of high importance and needs to be further elucidated by other single-center studies.
The presented data clearly show a dominance of stG62647 in invasive SDSE infections in Germany. However, a sudden drop in 2022 might indicate a reverse trend in the coming years (Figure 1c). The assessment of antimicrobial resistance data shows slightly lower non-susceptibility to erythromycin, clindamycin, and tetracycline in stG62647 isolates, compared to other emm types. Before 2013, stG62647 was only rarely identified in invasive SDSE infections. The first time isolates of this emm type were received at the GNRCS was 2005 (n = 2) and in the literature, it was first mentioned in 2016 [48]. Therefore, stG62647 was most likely not frequently confronted with antibiotics, a potential explanation for the higher susceptibility observed for erythromycin, clindamycin, and tetracycline. However, whether this leads to a substantial advantage in the treatment of invasive SDSE infections remains to be shown.
A much more concerning observation is the potential replacement of infections, usually associated with β-hemolytic pathogens such as S. pyogenes and S. agalactiae by S. dysgalactiae ssp. equisimilis, especially considering the observed increase in case numbers (Figure 1c). It is well known that SDSE shows a similar pathogenic potential as S. pyogenes, inducing severe tissue infections, sepsis, and toxic shock [9,10,11,12,13,14]. However, the isolation of invasive SDSE strains from diseases commonly caused by S. agalactiae such as mastitis or infections in newborns [66,67], as described here (Section 3.5) and also reported to the GNRCS, gives rise to serious concern. The impact of this potential pathogenicity shift on public health is currently not assessable but needs close monitoring.
5. Conclusions
In recent years, the number of invasive Streptococcus dysgalactiae subspecies equisimilis isolates reported to the GNRCS has continuously increased. However, it is not clear whether this increase is based on a biological phenomenon, a change in clinical awareness, or population structure. Within the observation period, the proportion of emm type stG62647 increased from 23% to 46% with a drop to 37% in 2022, and this type seems to exhibit increased virulence. In older patients, infections were more severe, and men are more prone to invasive SDSE infections than women, although in the single-center cohort, this trend was reversed in patients with stG62647, who mainly developed fascial infections. Women suffering from superficial and fascial infections by non-stG62647 SDSE were significantly younger than other patients. The observed sex-associated differences need further studies to elucidate the epidemiology and underlying mechanisms of the suggested clonal outbreak scenario.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens12040589/s1, Figure S1. Numbers of total SDSE infection cases treated at Ingolstadt Hospital between 2010 and 2022. Table S1. Patients colonized and/or infected with SDSE at Ingolstadt Hospital admitted between December 2016 and November 2022. Table S2. Invasive SDSE infections in Germany from 2010 to 2022 reported to the GNRCS.
Author Contributions
Conceptualization, A.I., M.v.d.L. and S.B.; data curation, A.I., V.W., D.M., B.R., M.v.d.L. and S.B.; formal analysis, A.I., V.W., B.R., M.v.d.L. and S.B.; investigation, A.I., V.W., D.M., B.R., M.v.d.L. and S.B.; project administration, A.I., M.v.d.L. and S.B.; resources, A.I., M.v.d.L. and S.B.; software, A.I., D.M., M.v.d.L. and S.B.; supervision, M.v.d.L.; validation, A.I., D.M., M.v.d.L. and S.B.; visualization, A.I. and S.B.; writing—original draft, A.I. and S.B.; writing—review and editing, A.I., D.M., M.v.d.L. and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
The surveillance of invasive streptococcal infections in Germany at the German National Reference Center for Streptococci was funded by the Robert Koch Institute (funding number: 1369-235), Berlin, Germany.
Institutional Review Board Statement
The present analysis is a retrospective study. The analyses performed at the GNRCS comprise no patient data allowing the identification of patients or individual clinical courses. Furthermore, all bacterial isolates and corresponding information arrive at the GNRCS on an anonymized basis, and consequently, ethical approval or patient consent was not required. At Ingolstadt Hospital, only diagnostic samples and clinical data which had occurred during patient treatment were used. No additional samples have been taken to perform the analysis. On the 15th of September 2022, the ethics committee of the Bavarian Medical Association (Bayerische Landesärztekammer), which has jurisdiction over medical matters in the Federal State of Bavaria, was asked if an ethics vote would be necessary. Based on the approval of the data protection officer of Ingolstadt Hospital, on the 30th of September 2022, the ethics committee replied that a vote would not be necessary. On the 6th of October 2022, the data protection officer confirmed that an ethics vote is not required. Therefore, an ethical review was waived for this study.
Informed Consent Statement
Written informed consent has been obtained from the patients indexed in Table S1 to publish this paper. If patients had not been able to give informed consent, their legal representatives were asked. If no legal representative was available, first-degree relatives were asked to give consent. This study was conducted in accordance with the Declaration of Helsinki.
Data Availability Statement
All data are included within the article and the Supplementary Materials.
Acknowledgments
The authors deeply thank their colleagues from the primary diagnostic laboratories in Germany for their cooperation in submitting invasive Streptococcus dysgalactiae subspecies equisimilis isolates to the GNRCS. The authors also thank the infection control nurses and microbiological laboratory staff at Ingolstadt Hospital for their excellent work in support of the study. We also thank Kerstin Becker and Bruno Weidenhaupt for excellent technical assistance in routine diagnostics at the GNRCS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baracco, G.J. Infections Caused by Group C and G Streptococcus (Streptococcus dysgalactiae subsp. equisimilis and Others): Epidemiological and Clinical Aspects. Microbiol. Spectr. 2019, 7. Part HISTORY AND TAXONOMY; HUMAN INFECTIONS. [Google Scholar] [CrossRef]
- Frost, W.D.; Engelbrecht, M.A. The Streptococci. Their Descriptions, Classification, and Distribution, with Special Reference to Those in Milk; Willdof Book Company: Madison, WI, USA, 1940; Volume 1. [Google Scholar]
- Vandamme, P.; Pot, B.; Falsen, E.; Kersters, K.; Devriese, L.A. Taxonomic Study of Lancefield Streptococcal Groups C, G, and L (Streptococcus dysgalactiae) and Proposal of S. dysgalactiae subsp. equisimilis subsp. nov. Int. J. Syst. Evol. Microbiol. 1996, 46, 774–781. [Google Scholar] [CrossRef]
- Vieira, V.V.; Teixeira, L.M.; Zahner, V.; Momen, H.; Facklam, R.R.; Steigerwalt, A.G.; Brenner, D.J.; Castro, A.C.D. Genetic relationships among the different phenotypes of Streptococcus dysgalactiae strains. Int. J. Syst. Evol. Microbiol. 1998, 48, 1231–1243. [Google Scholar] [CrossRef]
- Garvie, E.I.; Farrow, J.A.E.; Bramley, A.J. Streptococcus dysgalactiae (Diernhofer) nom. rev. Int. J. Syst. Evol. Microbiol. 1983, 33, 404–405. [Google Scholar] [CrossRef]
- Ciszewski, M.; Zegarski, K.; Szewczyk, E.M. Streptococcus dysgalactiae subsp. equisimilis Isolated From Infections in Dogs and Humans: Are Current Subspecies Identification Criteria accurate? Curr. Microbiol. 2016, 73, 684–688. [Google Scholar] [CrossRef]
- Porcellato, D.; Smistad, M.; Skeie, S.B.; Jørgensen, H.J.; Austbø, L.; Oppegaard, O. Whole genome sequencing reveals possible host species adaptation of Streptococcus dysgalactiae. Sci. Rep. 2021, 11, 17350. [Google Scholar] [CrossRef]
- Oppegaared, O. Streptococcus Dysgalactiae—Just One Small Strep for Mankind? (#55). In Proceedings of the 21st Lancefield International Symposium for Streptococci and Streptococcal Diseases, Stockholm, Sweden, 7–10 June 2022. [Google Scholar]
- Bruun, T.; Kittang, B.R.; de Hoog, B.; Aardal, S.; Flaatten, H.K.; Langeland, N.; Mylvaganam, H.; Vindenes, H.A.; Skrede, S. Necrotizing soft tissue infections caused by Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis of groups C and G in western Norway. Clin. Microbiol. Infect. 2013, 19, E545–E550. [Google Scholar] [CrossRef]
- Izumi, M.; Hiraiwa, T.; Tomioka, H.; Komura, M.; Hayashi, Y. Fatal necrotizing myositis from fulminant Streptococcus dysgalactiae subspecies equisimilis (SDSE) infection: A case report of autopsy images. J. Gen. Fam. Med. 2018, 19, 50–52. [Google Scholar] [CrossRef]
- Saintot, C.; André, J.; Hentgen, C.; Jacques, L.; Barrans, A. Meningitis and septic shock caused by Streptococcus dysgalactiae subsp. equisimilis. Med. Mal. Infect. 2018, 48, 221–222. [Google Scholar] [CrossRef]
- Trell, K.; Sendi, P.; Rasmussen, M. Recurrent bacteremia with Streptococcus dysgalactiae: A case–control study. Diagn. Microbiol. Infect. Dis. 2016, 85, 121–124. [Google Scholar] [CrossRef]
- Hashikawa, S.; Iinuma, Y.; Furushita, M.; Ohkura, T.; Nada, T.; Torii, K.; Hasegawa, T.; Ohta, M. Characterization of Group C and G Streptococcal Strains That Cause Streptococcal Toxic Shock Syndrome. J. Clin. Microbiol. 2004, 42, 186–192. [Google Scholar] [CrossRef]
- Rodríguez-Muñoz, L.; García-Galván, M.Á.; González-Soto, M.A.; Echaniz-Aviles, G.; Solórzano-Santos, F. Toxic shock syndrome caused by Streptococcus dysgalactiae subsp. equisimilis in a Mexican preschool patient. Bol. Med. Hosp. Infant Mex. 2019, 76, 237–240. [Google Scholar] [CrossRef]
- Waltereit, R.; Herrlinger, U.; Stark, M.; Borgmann, S. Meningitis in a pregnant woman caused by Streptococcus dysgalactiae subspecies equisimilis. Pol. J. Microbiol. 2013, 62, 217–219. [Google Scholar] [CrossRef]
- Barnham, M.R.D.; Weightman, N.C. Changing incidence of detected streptococcal bacteraemia in North Yorkshire, England. Indian J. Med. Res. 2004, 119, 160–163. [Google Scholar]
- Ekelund, K.; Skinhøj, P.; Madsen, J.; Konradsen, H. Invasive group A, B, C and G streptococcal infections in Denmark 1999–2002: Epidemiological and clinical aspects. Clin. Microbiol. Infect. 2005, 11, 569–576. [Google Scholar] [CrossRef]
- Gajdács, M.; Ábrók, M.; Lázár, A.; Burián, K. Beta-Haemolytic Group A, C and G Streptococcal Infections in Southern Hungary: A 10-Year Population-Based Retrospective Survey (2008–2017) and a Review of the Literature. Infect. Drug Resist. 2020, 13, 4739–4749. [Google Scholar] [CrossRef]
- Lambertsen, L.M.; Ingels, H.; Schønheyder, H.C.; Hoffmann, S. Nationwide laboratory-based surveillance of invasive beta-haemolytic streptococci in Denmark from 2005 to 2011. Clin. Microbiol. Infect. 2014, 20, O216–O223. [Google Scholar] [CrossRef]
- Rantala, S.; Vuopio-Varkila, J.; Vuento, R.; Huhtala, H.; Syrjänen, J. Clinical presentations and epidemiology of β-haemolytic streptococcal bacteraemia: A population-based study. Clin. Microbiol. Infect. 2009, 15, 286–288. [Google Scholar] [CrossRef]
- Broyles, L.N.; Van Beneden, C.; Beall, B.; Facklam, R.; Shewmaker, P.L.; Malpiedi, P.; Daily, P.; Reingold, A.; Farley, M.M. Population-Based Study of Invasive Disease Due to β-Hemolytic Streptococci of Groups Other than A and B. Clin. Infect. Dis. 2009, 48, 706–712. [Google Scholar] [CrossRef]
- Oppegaard, O.; Mylvaganam, H.; Kittang, B. Beta-haemolytic group A, C and G streptococcal infections in Western Norway: A 15-year retrospective survey. Clin. Microbiol. Infect. 2015, 21, 171–178. [Google Scholar] [CrossRef]
- Wajima, T.; Morozumi, M.; Hanada, S.; Sunaoshi, K.; Chiba, N.; Iwata, S.; Ubukata, K. Molecular Characterization of Invasive Streptococcus dysgalactiae subsp. equisimilis, Japan. Emerg. Infect. Dis. 2016, 22, 247–254. [Google Scholar] [CrossRef]
- Rantala, S. Streptococcus dysgalactiae subsp. equisimilis bacteremia: An emerging infection. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1303–1310. [Google Scholar] [CrossRef]
- Ferretti, J.J.; Stevens, D.L.; Fischetti, V.A. (Eds.) Streptococcus Pyogenes: Basic Biology to Clinical Manifestations, 2nd ed.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2022. [Google Scholar]
- Collins, C.M.; Kimura, A.; Bisno, A.L. Group G streptococcal M protein exhibits structural features analogous to those of class I M protein of group A streptococci. Infect. Immun. 1992, 60, 3689–3696. [Google Scholar] [CrossRef]
- Vasi, J.; Frykberg, L.; Carlsson, L.E.; Lindberg, M.; Guss, B. M-Like Proteins of Streptococcus dysgalactiae. Infect. Immun. 2000, 68, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.N.; Flicker, P.F.; Cohen, C.; Manjula, B.N.; Fischetti, V.A. Streptococcal M protein: Alpha-helical coiled-coil structure and arrangement on the cell surface. Proc. Natl. Acad. Sci. USA 1981, 78, 4689–4693. [Google Scholar] [CrossRef]
- Fischetti, V.; Pancholi, V.; Schneewind, O. Conservation of a hexapeptide sequence in the anchor region of surface proteins from Gram-positive cocci. Mol. Microbiol. 1990, 4, 1603–1605. [Google Scholar] [CrossRef]
- Swanson, J.; Hsu, K.C.; Gotschlich, E.C. Electron microscopic studies on streptococci: I. M antigen. J. Exp. Med. 1969, 130, 1063–1091. [Google Scholar] [CrossRef]
- Anderson, E.L.; Cole, J.N.; Olson, J.; Ryba, B.; Ghosh, P.; Nizet, V. The Fibrinogen-binding M1 Protein Reduces Pharyngeal Cell Adherence and Colonization Phenotypes of M1T1 Group A Streptococcus. J. Biol. Chem. 2014, 289, 3539–3546. [Google Scholar] [CrossRef]
- Laabei, M.; Ermert, D. Catch Me if You Can: Streptococcus pyogenes Complement Evasion Strategies. J. Innate Immun. 2018, 11, 3–12. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Terao, Y.; Kawabata, S. Pleiotropic virulence factor -Streptococcus pyogenes fibronectin-binding proteins. Cell. Microbiol. 2012, 15, 503–511. [Google Scholar] [CrossRef]
- Horstmann, R.D.; Sievertsen, H.J.; Knobloch, J.; Fischetti, V.A. Antiphagocytic activity of streptococcal M protein: Selective binding of complement control protein factor H. Proc. Natl. Acad. Sci. USA 1988, 85, 1657–1661. [Google Scholar] [CrossRef]
- McArthur, J.D.; Walker, M.J. Domains of group A streptococcal M protein that confer resistance to phagocytosis, opsonization and protection: Implications for vaccine development. Mol. Microbiol. 2006, 59, 1–4. [Google Scholar] [CrossRef]
- Cue, D.; Dombek, P.E.; Lam, H.; Cleary, P.P. Streptococcus pyogenes Serotype M1 Encodes Multiple Pathways for Entry into Human Epithelial Cells. Infect. Immun. 1998, 66, 4593–4601. [Google Scholar] [CrossRef]
- Cue, D.; Lam, H.; Cleary, P. Genetic dissection of the Streptococcus pyogenes M1 protein: Regions involved in fibronectin binding and intracellular invasion. Microb. Pathog. 2001, 31, 231–242. [Google Scholar] [CrossRef]
- Nerlich, A.; Rohde, M.; Talay, S.R.; Genth, H.; Just, I.; Chhatwal, G.S. Invasion of Endothelial Cells by Tissue-invasive M3 Type Group A Streptococci Requires Src Kinase and Activation of Rac1 by a Phosphatidylinositol 3-Kinase-independent Mechanism. J. Biol. Chem. 2009, 284, 20319–20328. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.; Liszewski, M.K.; Atkinson, J.P.; Caparon, M. Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A streptococcus. Proc. Natl. Acad. Sci. USA 1995, 92, 2489–2493. [Google Scholar] [CrossRef]
- Beachey, E.H.; Seyer, J.M.; Dale, J.B.; Simpson, W.A.; Kang, A.H. Type-specific protective immunity evoked by synthetic peptide of Streptococcus pyogenes M protein. Nature 1981, 292, 457–459. [Google Scholar] [CrossRef]
- Campbell, P.T.; Tong, S.Y.C.; Geard, N.; Davies, M.; Worthing, K.A.; Lacey, J.A.; Smeesters, P.; Batzloff, M.R.; Kado, J.; Jenney, A.W.J.; et al. Longitudinal Analysis of Group A Streptococcus emm Types and emm Clusters in a High-Prevalence Setting: Relationship between Past and Future Infections. J. Infect. Dis. 2020, 221, 1429–1437. [Google Scholar] [CrossRef]
- Lancefield, R.C. Current knowledge of type-specific M antigens of group A streptococci. J. Immunol. 1962, 89, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Beall, B.; Facklam, R.; Thompson, T. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J. Clin. Microbiol. 1996, 34, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Facklam, R.; Beall, B.; Efstratiou, A.; Fischetti, V.; Johnson, D.; Kaplan, E.; Kriz, P.; Lovgren, M.; Martin, D.; Schwartz, B.; et al. Emm Typing and Validation of Provisional M Types for Group A Streptococci1. Emerg. Infect. Dis. 1999, 5, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Streptococci Group a Subtyping Database. Available online: https://ftp.cdc.gov/pub/infectious_diseases/biotech/tsemm/ (accessed on 1 February 2023).
- Rößler, S.; Berner, R.; Jacobs, E.; Toepfner, N. Prevalence and molecular diversity of invasive Streptococcus dysgalactiae and Streptococcus pyogenes in a German tertiary care medical centre. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1325–1332. [Google Scholar] [CrossRef]
- German National Center for Streptococci. Streptococcal Surveillance. Available online: https://www.ukaachen.de/kliniken-institute/institut-fuer-medizinische-mikrobiologie/forschung/nationales-referenzzentrum-fuer-streptokokken/publikationen/surveillance/ (accessed on 1 February 2023).
- Traverso, F.; Blanco, A.; Villalón, P.; Beratz, N.; Nieto, J.A.S.; Lopardo, H. Molecular characterization of invasive Streptococcus dysgalactiae subsp. equisimilis. Multicenter study: Argentina 2011–2012. Rev. Argent. Microbiol. 2016, 48, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Ruppen, C.; Rasmussen, M.; Casanova, C.; Sendi, P. A 10-year observational study of Streptococcus dysgalactiae bacteraemia in adults: Frequent occurrence among female intravenous drug users. Swiss Med. Wkly. 2017, 147, w14469. [Google Scholar] [CrossRef] [PubMed]
- Oppegaard, O.; Mylvaganam, H.; Skrede, S.; Lindemann, P.C.; Kittang, B.R. Emergence of a Streptococcus dysgalactiae subspecies equisimilis stG62647-lineage associated with severe clinical manifestations. Sci. Rep. 2017, 7, 7589. [Google Scholar] [CrossRef] [PubMed]
- Bläckberg, A.; Nilson, B.; Özenci, V.; Olaison, L.; Rasmussen, M. Infective endocarditis due to Streptococcus dysgalactiae: Clinical presentation and microbiological features. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2261–2272. [Google Scholar] [CrossRef]
- Bruun, T.; Rath, E.; Madsen, M.B.; Oppegaard, O.; Nekludov, M.; Arnell, P.; Karlsson, Y.; Babbar, A.; Bergey, F.; Itzek, A.; et al. Risk Factors and Predictors of Mortality in Streptococcal Necrotizing Soft-tissue Infections: A Multicenter Prospective Study. Clin. Infect. Dis. 2021, 72, 293–300. [Google Scholar] [CrossRef]
- Rojo-Bezares, B.; Toca, L.; Azcona-Gutiérrez, J.M.; Ortega-Unanue, N.; Toledano, P.; Sáenz, Y. Streptococcus dysgalactiae subsp. equisimilis from invasive and non-invasive infections in Spain: Combining epidemiology, molecular characterization, and genetic diversity. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1013–1021. [Google Scholar] [CrossRef]
- Yajko, D.M.; Lawrence, J.; Nassos, P.; Young, J.; Hadley, W.K. Clinical trial comparing bacitracin with Strep-A-Chek for accuracy and turnaround time in the presumptive identification of Streptococcus pyogenes. J. Clin. Microbiol. 1986, 24, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Wellstood, S.A. Rapid, cost-effective identification of group A streptococci and enterococci by pyrrolidonyl-beta-naphthylamide hydrolysis. J. Clin. Microbiol. 1987, 25, 1805–1806. [Google Scholar] [CrossRef] [PubMed]
- Podbielski, A.; Melzer, B.; Lütticken, R. Application of the polymerase chain reaction to study the M protein(-like) gene family in beta-hemolytic streptococci. Med. Microbiol. Immunol. 1991, 180, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Protocol for Emm Typing. Available online: https://www.cdc.gov/streplab/groupa-strep/emm-typing-protocol.html (accessed on 1 February 2023).
- Fan, W.-T.; Qin, T.-T.; Bi, R.-R.; Kang, H.-Q.; Ma, P.; Gu, B. Performance of the matrix-assisted laser desorption ionization time-of-flight mass spectrometry system for rapid identification of streptococci: A review. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1005–1012. [Google Scholar] [CrossRef]
- Borgmann, S.; Pfeifer, Y.; Becker, L.; Rieß, B.; Siegmund, R.; Sagel, U. Findings from an outbreak of carbapenem-resistant Klebsiella pneumoniae emphasize the role of antibiotic treatment for cross transmission. Infection 2017, 46, 103–112. [Google Scholar] [CrossRef]
- Destatis. Statistisches Bundesamt. Bevölkerung Im Wandel—Annahmen Und Ergebnisse Der 14. Koordinierten Bevölkerungsvorausberechnung. Available online: https://www.destatis.de/DE/Presse/Pressekonferenzen/2019/Bevoelkerung/pressebroschuere-bevoelkerung.pdf?__blob=publicationFile (accessed on 1 February 2023).
- Kline, K.A.; Bowdish, D.M. Infection in an aging population. Curr. Opin. Microbiol. 2016, 29, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Leitner, E.; Zollner-Schwetz, I.; Zarfel, G.; Masoud-Landgraf, L.; Gehrer, M.; Wagner-Eibel, U.; Grisold, A.J.; Feierl, G. Prevalence of emm types and antimicrobial susceptibility of Streptococcus dysgalactiae subsp. equisimilis in Austria. Int. J. Med. Microbiol. 2015, 305, 918–924. [Google Scholar] [CrossRef]
- Madalli, S.; Beyrau, M.; Whiteford, J.; Duchene, J.; Nandhra, I.S.; Patel, N.S.A.; Motwani, M.P.; Gilroy, D.W.; Thiemermann, C.; Nourshargh, S.; et al. Sex-specific regulation of chemokine Cxcl5/6 controls neutrophil recruitment and tissue injury in acute inflammatory states. Biol. Sex Differ. 2015, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Cascella, F.; Mellai, M.; Barizzone, N.; Mignone, F.; Massa, N.; Nobile, V.; Bona, E. Influence of Sex on the Microbiota of the Human Face. Microorganisms 2022, 10, 2470. [Google Scholar] [CrossRef]
- Steglińska, A.; Jachowicz, A.; Szulc, J.; Adamiak, J.; Otlewska, A.; Pielech-Przybylska, K.; Gutarowska, B. Factors Influencing Microbiological Biodiversity of Human Foot Skin. Int. J. Environ. Res. Public Health 2019, 16, 3503. [Google Scholar] [CrossRef]
- Miselli, F.; Frabboni, I.; Di Martino, M.; Zinani, I.; Buttera, M.; Insalaco, A.; Stefanelli, F.; Lugli, L.; Berardi, A. Transmission of Group B Streptococcus in late-onset neonatal disease: A narrative review of current evidence. Ther. Adv. Infect. Dis. 2022, 9, 20499361221142732. [Google Scholar] [CrossRef]
- Shabayek, S.; Spellerberg, B. Group B Streptococcal Colonization, Molecular Characteristics, and Epidemiology. Front. Microbiol. 2018, 9, 437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).