Timing of Symptoms of Early-Onset Sepsis after Intrapartum Antibiotic Prophylaxis: Can It Inform the Neonatal Management?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical and Microbiological Practices
2.3. Definitions
- Intrapartum antibiotic prophylaxis: intrapartum antibiotics administered i.v. for preventing GBS EOS;
- Adequate IAP: penicillin, ampicillin, or cefazolin administered at least 4 h prior to delivery;
- Active IAP: the pathogen yielded in cultures was susceptible to the antibiotic administered for IAP;
- Pre-term neonates: neonates born at <37 weeks’ gestation;
- Late preterm neonates: neonates born at 34–36 weeks’ gestation;
- Full term neonates: neonates born at ≥37 weeks’ gestation;
- Asymptomatic neonate: infant without any symptoms of EOS, with positive blood culture collected for maternal RFs.
2.4. Statistical Analysis
3. Results
3.1. IAP-Exposure in GBS EOS and Timing of Symptoms
3.2. IAP-Exposure in E. coli EOS and Timing of Symptoms
3.3. Comparison between GBS and E. coli EOS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puopolo, K.M.; Benitz, W.E.; Zaoutis, T.E.; Committee on Fetus and Newborn; Committee on Infectious Diseases. Management of Neonates Born at ≥35 0/7 Weeks’ Gestation with Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics 2018, 142, e20182894. [Google Scholar] [CrossRef] [PubMed]
- Benitz, W.E.; Gould, J.B.; Druzin, M.L. Risk Factors for Early-Onset Group B Streptococcal Sepsis: Estimation of Odds Ratios by Critical Literature Review. Pediatrics 1999, 103, e77. [Google Scholar] [CrossRef]
- Verani, J.R.; McGee, L.; Schrag, S.J.; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases; Centers for Disease Control and Prevention (CDC). Prevention of Perinatal Group B Streptococcal Disease--Revised Guidelines from CDC, 2010. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2010, 59, 1–36. [Google Scholar]
- Shane, A.L.; Sánchez, P.J.; Stoll, B.J. Neonatal Sepsis. Lancet 2017, 390, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Shah, V.S. Intrapartum Antibiotics for Known Maternal Group B Streptococcal Colonization. Cochrane Database Syst. Rev. 2014, CD007467. [Google Scholar] [CrossRef]
- Boyer, K.M.; Gotoff, S.P. Prevention of Early-Onset Neonatal Group B Streptococcal Disease with Selective Intrapartum Chemoprophylaxis. N. Engl. J. Med. 1986, 314, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.V.; Morales, W.J.; Walsh, A.F.; Kazanis, D. Reduction of Morbidity and Mortality Rates for Neonatal Group B Streptococcal Disease through Early Diagnosis and Chemoprophylaxis. J. Clin. Microbiol. 1986, 23, 489–492. [Google Scholar] [CrossRef]
- Matorras, R.; García-Perea, A.; Omeñaca, F.; Diez-Enciso, M.; Madero, R.; Usandizaga, J.A. Intrapartum Chemoprophylaxis of Early-Onset Group B Streptococcal Disease. Eur. J. Obstet. Gynecol. Reprod. Biol. 1991, 40, 57–62. [Google Scholar] [CrossRef]
- Tuppurainen, N.; Hallman, M. Prevention of Neonatal Group B Streptococcal Disease: Intrapartum Detection and Chemoprophylaxis of Heavily Colonized Parturients. Obstet. Gynecol. 1989, 73, 583–587. [Google Scholar]
- Garland, S.M.; Fliegner, J.R. Group B Streptococcus (GBS) and Neonatal Infections: The Case for Intrapartum Chemoprophylaxis. Aust. N. Z. J. Obstet. Gynaecol. 1991, 31, 119–122. [Google Scholar] [CrossRef]
- Schrag, S.J.; Zywicki, S.; Farley, M.M.; Reingold, A.L.; Harrison, L.H.; Lefkowitz, L.B.; Hadler, J.L.; Danila, R.; Cieslak, P.R.; Schuchat, A. Group B Streptococcal Disease in the Era of Intrapartum Antibiotic Prophylaxis. N. Engl. J. Med. 2000, 342, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, S.A.; Petit, S.; Smelser, C.; Apostol, M.; Alden, N.B.; Harrison, L.H.; Lynfield, R.; Vagnone, P.S.; Burzlaff, K.; Spina, N.L.; et al. Epidemiology of Invasive Early-Onset and Late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015: Multistate Laboratory and Population-Based Surveillance. JAMA Pediatr. 2019, 173, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Money, D.; Allen, V.M. No. 298-The Prevention of Early-Onset Neonatal Group B Streptococcal Disease. J. Obstet. Gynaecol. Can. 2018, 40, e665–e674. [Google Scholar] [CrossRef]
- Prevention of Early-Onset Neonatal Group B Streptococcal Disease: Green-Top Guideline No. 36. BJOG Int. J. Obstet. Gynaecol. 2017, 124, e280–e305. [CrossRef] [PubMed]
- Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion Summary, Number 782. Obstet. Gynecol. 2019, 134, 1. [CrossRef]
- Schrag, S.J.; Farley, M.M.; Petit, S.; Reingold, A.; Weston, E.J.; Pondo, T.; Hudson Jain, J.; Lynfield, R. Epidemiology of Invasive Early-Onset Neonatal Sepsis, 2005 to 2014. Pediatrics 2016, 138, e20162013. [Google Scholar] [CrossRef]
- Schrag, S.J.; Zell, E.R.; Lynfield, R.; Roome, A.; Arnold, K.E.; Craig, A.S.; Harrison, L.H.; Reingold, A.; Stefonek, K.; Smith, G.; et al. A Population-Based Comparison of Strategies to Prevent Early-Onset Group B Streptococcal Disease in Neonates. N. Engl. J. Med. 2002, 347, 233–239. [Google Scholar] [CrossRef]
- Illuzzi, J.L.; Bracken, M.B. Duration of Intrapartum Prophylaxis for Neonatal Group B Streptococcal Disease: A Systematic Review. Obstet. Gynecol. 2006, 108, 1254–1265. [Google Scholar] [CrossRef]
- Berardi, A.; Spada, C.; Vaccina, E.; Boncompagni, A.; Bedetti, L.; Lucaccioni, L. Intrapartum Beta-Lactam Antibiotics for Preventing Group B Streptococcal Early-Onset Disease: Can We Abandon the Concept of “inadequate” Intrapartum Antibiotic Prophylaxis? Expert Rev. Anti Infect. Ther. 2020, 18, 37–46. [Google Scholar] [CrossRef]
- Idsoe, O.; Guthe, T.; Willcox, R.R.; de Weck, A.L. Nature and Extent of Penicillin Side-Reactions, with Particular Reference to Fatalities from Anaphylactic Shock. Bull. World Health Organ. 1968, 38, 159–188. [Google Scholar]
- Kelkar, P.S.; Li, J.T. Cephalosporin Allergy. N. Engl. J. Med. 2001, 345, 804–809. [Google Scholar] [CrossRef]
- Tzialla, C.; Berardi, A.; Farina, C.; Clerici, P.; Borghesi, A.; Viora, E.; Scollo, P.; Stronati, M.; Task Force for group B streptococcal infections for the Italian Society of Neonatology; Italian Society of Obstetricians and Gynecologists; et al. Strategies for Preventing Group B Streptococcal Infections in Newborns: A Nation-Wide Survey of Italian Policies. Ital. J. Pediatr. 2017, 43, 98. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, E.; Dimopoulou, V.; Klingenberg, C.; Navér, L.; Nordberg, V.; Berardi, A.; El Helou, S.; Fusch, G.; Bliss, J.M.; Lehnick, D.; et al. Analysis of Antibiotic Exposure and Early-Onset Neonatal Sepsis in Europe, North America, and Australia. JAMA Netw. Open 2022, 5, e2243691. [Google Scholar] [CrossRef] [PubMed]
- Puopolo, K.M.; Lynfield, R.; Cummings, J.J.; Committee on Fetus And Newborn; Committee on Infectious Diseases. Management of Infants at Risk for Group B Streptococcal Disease. Pediatrics 2019, 144, e20191881. [Google Scholar] [CrossRef] [PubMed]
- Berardi, A.; Di Fazzio, G.; Gavioli, S.; Di Grande, E.; Groppi, A.; Papa, I.; Piccinini, G.; Simoni, A.; Tridapalli, E.; Volta, A.; et al. Universal Antenatal Screening for Group B Streptococcus in Emilia-Romagna. J. Med. Screen. 2011, 18, 60–64. [Google Scholar] [CrossRef]
- Berardi, A.; Lugli, L.; Baronciani, D.; Creti, R.; Rossi, K.; Ciccia, M.; Gambini, L.; Mariani, S.; Papa, I.; Serra, L.; et al. Group B Streptococcal Infections in a Northern Region of Italy. Pediatrics 2007, 120, e487–e493. [Google Scholar] [CrossRef]
- Berardi, A.; Baroni, L.; Bacchi Reggiani, M.L.; Ambretti, S.; Biasucci, G.; Bolognesi, S.; Capretti, M.G.; Carretto, E.; Ciccia, M.; Fiorini, V.; et al. The Burden of Early-Onset Sepsis in Emilia-Romagna (Italy): A 4-Year, Population-Based Study. J. Matern.-Fetal Neonatal Med. 2016, 29, 3126–3131. [Google Scholar] [CrossRef]
- Miselli, F.; Cuoghi Costantini, R.; Creti, R.; Sforza, F.; Fanaro, S.; Ciccia, M.; Piccinini, G.; Rizzo, V.; Pasini, L.; Biasucci, G.; et al. Escherichia Coli Is Overtaking Group B Streptococcus in Early-Onset Neonatal Sepsis. Microorganisms 2022, 10, 1878. [Google Scholar] [CrossRef]
- Weston, E.J.; Pondo, T.; Lewis, M.M.; Martell-Cleary, P.; Morin, C.; Jewell, B.; Daily, P.; Apostol, M.; Petit, S.; Farley, M.; et al. The Burden of Invasive Early-Onset Neonatal Sepsis in the United States, 2005–2008. Pediatr. Infect. Dis. J. 2011, 30, 937–941. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Sánchez, P.J.; Faix, R.G.; Poindexter, B.B.; Van Meurs, K.P.; Bizzarro, M.J.; Goldberg, R.N.; Frantz, I.D.; Hale, E.C.; et al. Early Onset Neonatal Sepsis: The Burden of Group B Streptococcal and E. Coli Disease Continues. Pediatrics 2011, 127, 817–826. [Google Scholar] [CrossRef]
- Ganatra, H.A.; Stoll, B.J.; Zaidi, A.K.M. International Perspective on Early-Onset Neonatal Sepsis. Clin. Perinatol. 2010, 37, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Schrag, S.; Gorwitz, R.; Fultz-Butts, K.; Schuchat, A. Prevention of Perinatal Group B Streptococcal Disease. Revised Guidelines from CDC. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2002, 51, 1–22. [Google Scholar]
- Kuzniewicz, M.W.; Walsh, E.M.; Li, S.; Fischer, A.; Escobar, G.J. Development and Implementation of an Early-Onset Sepsis Calculator to Guide Antibiotic Management in Late Preterm and Term Neonates. Jt. Comm. J. Qual. Patient Saf. 2016, 42, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Puopolo, K.M.; Benitz, W.E.; Zaoutis, T.E.; Committee on Fetus and Newborn; Committee on Infectious Diseases. Management of Neonates Born at ≤34 6/7 Weeks’ Gestation With Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics 2018, 142, e20182896. [Google Scholar] [CrossRef]
- Berardi, A.; Pietrangiolillo, Z.; Bacchi Reggiani, M.L.; Bianco, V.; Gallesi, D.; Rossi, K.; Facchinetti, F.; Ferrari, F. Are Postnatal Ampicillin Levels Actually Related to the Duration of Intrapartum Antibiotic Prophylaxis Prior to Delivery? A Pharmacokinetic Study in 120 Neonates. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F152–F156. [Google Scholar] [CrossRef]
- Scasso, S.; Laufer, J.; Rodriguez, G.; Alonso, J.G.; Sosa, C.G. Vaginal Group B Streptococcus Status during Intrapartum Antibiotic Prophylaxis. Int. J. Gynecol. Obstet. 2015, 129, 9–12. [Google Scholar] [CrossRef] [PubMed]
- McNanley, A.R.; Glantz, J.C.; Hardy, D.J.; Vicino, D. The Effect of Intrapartum Penicillin on Vaginal Group B Streptococcus Colony Counts. Am. J. Obstet. Gynecol. 2007, 197, 583.e1–583.e4. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Berardi, A.; Rossi, C.; Biasini, A.; Minniti, S.; Venturelli, C.; Ferrari, F.; Facchinetti, F. Efficacy of Intrapartum Chemoprophylaxis Less than 4 Hours Duration. J. Matern.-Fetal Neonatal Med. 2011, 24, 619–625. [Google Scholar] [CrossRef]
- Berardi, A.; Spada, C.; Reggiani, M.L.B.; Creti, R.; Baroni, L.; Capretti, M.G.; Ciccia, M.; Fiorini, V.; Gambini, L.; Gargano, G.; et al. Group B Streptococcus Early-Onset Disease and Observation of Well-Appearing Newborns. PLoS ONE 2019, 14, e0212784. [Google Scholar] [CrossRef]
- Berardi, A.; Bedetti, L.; Spada, C.; Lucaccioni, L.; Frymoyer, A. Serial Clinical Observation for Management of Newborns at Risk of Early-Onset Sepsis. Curr. Opin. Pediatr. 2020, 32, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Vergnano, S.; Menson, E.; Kennea, N.; Embleton, N.; Russell, A.B.; Watts, T.; Robinson, M.J.; Collinson, A.; Heath, P.T. Neonatal Infections in England: The NeonIN Surveillance Network. Arch. Dis. Child. Fetal Neonatal Ed. 2011, 96, F9–F14. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Barnes, E.H.; Isaacs, D.; Australian Study Group for Neonatal Infections. Early-Onset Neonatal Infections in Australia and New Zealand, 2002–2012. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F248–F252. [Google Scholar] [CrossRef] [PubMed]
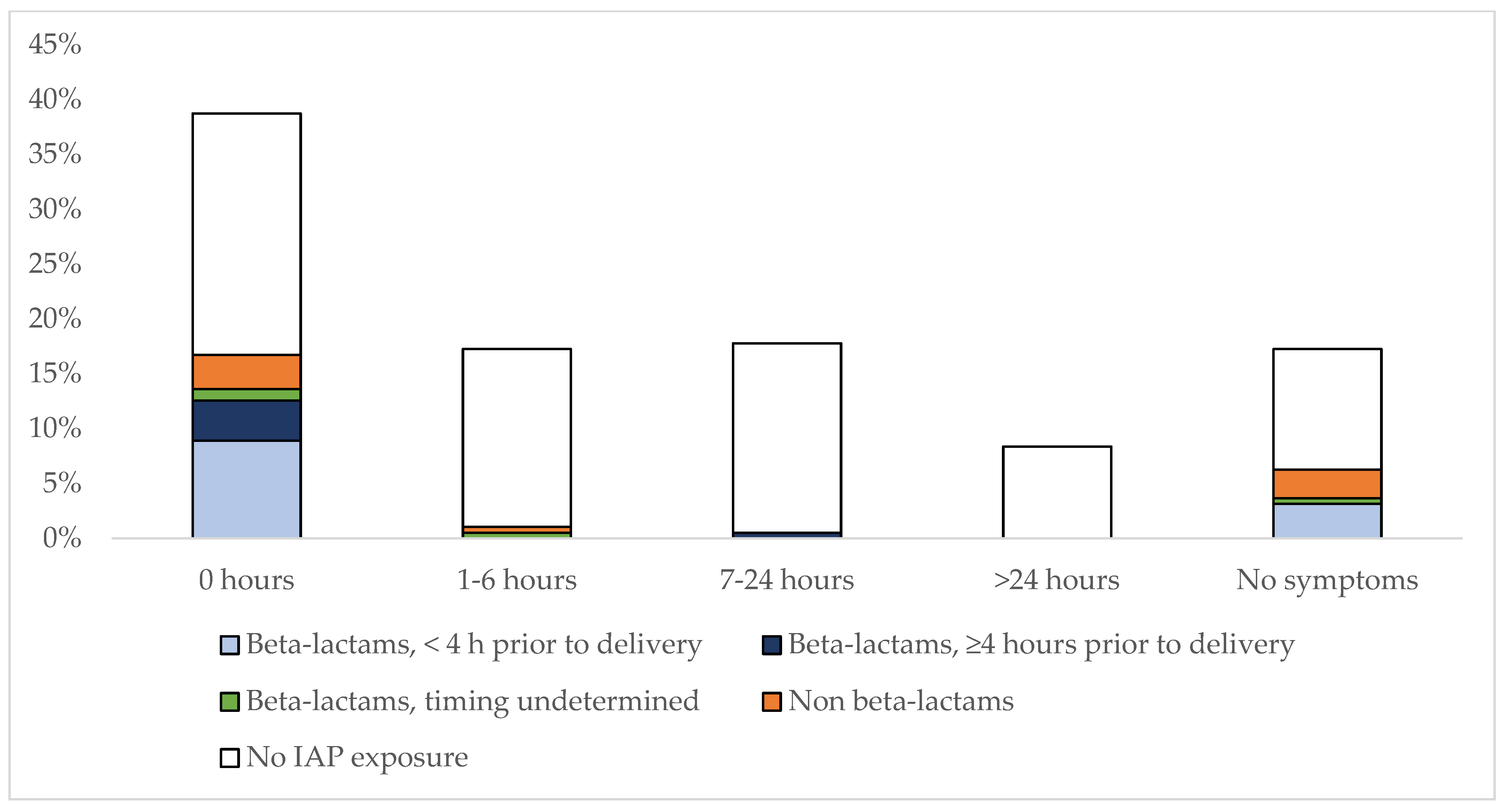
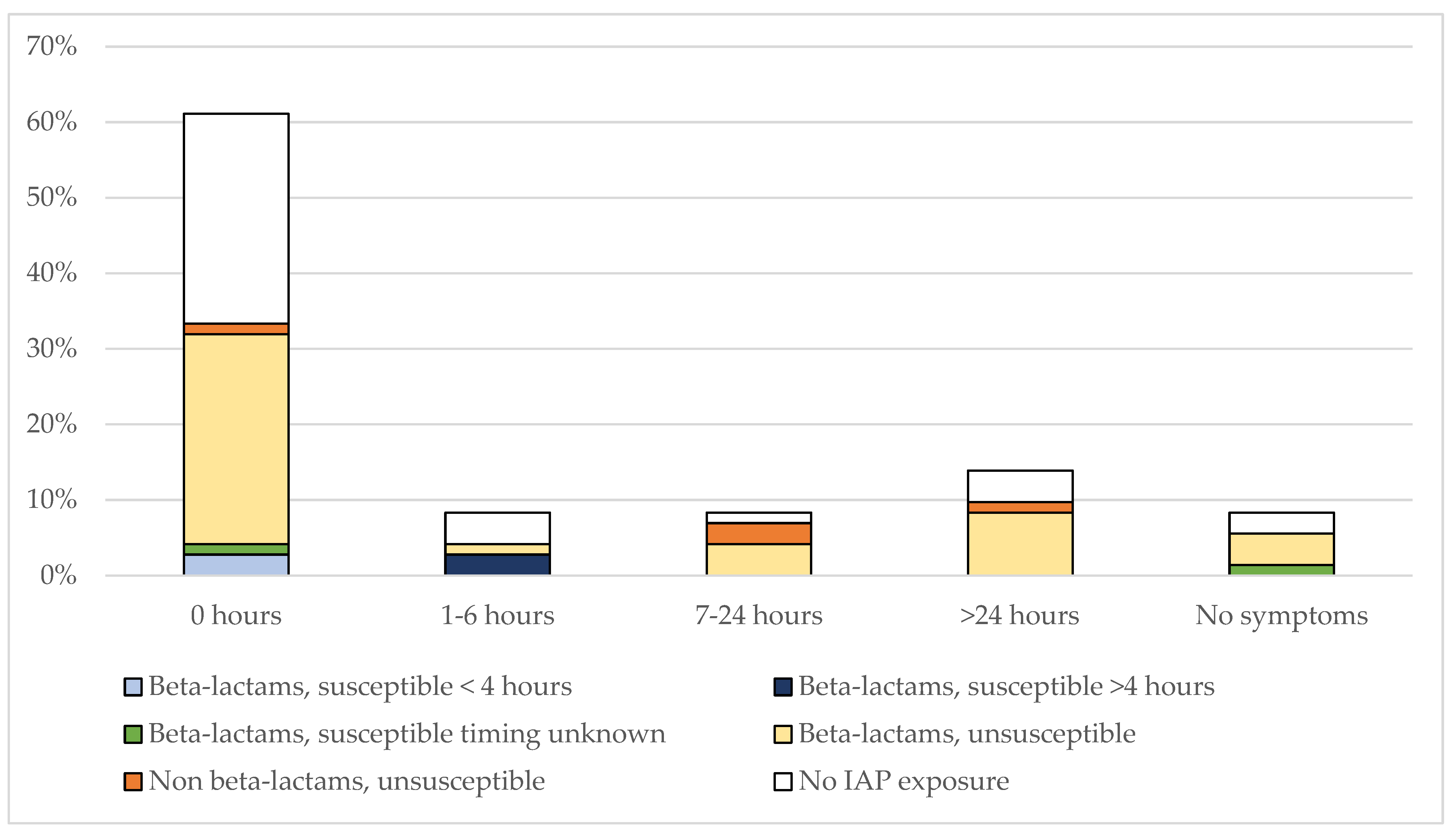
| All Cases (n = 263) | GBS (n = 191) | E. coli (n = 72) | p | |
|---|---|---|---|---|
| Median gestational age, wks (IQR) | 38 (34–40) | 39 (37–40) | 34 (28.5–39) | <0.0001 |
| Median birth weight, g (IQR) | 3100 (2208.75–3500.00) | 3220 (2776.25–3607.50) | 2160 (1110.00–3127.50) | <0.0001 |
| Preterm neonates, n (%) | 85 (32.3) | 42 (22.0) | 43 (59.7) | <0.0001 |
| Male sex, n (%) | 134 (51) | 97 (50.8) | 37 (51.4) | 0.9593 |
| Symptomatic neonates, hours at presentation (IQR) | 0.50 (0.00–8.00) | 1.50 (0.00–4.00) | 0.00 (0.00–0.00) | 0.1004 |
| All asymptomatic neonates (0–72 h of life), n (%) | 39 (14.8) | 33 (17.3) | 6 (8.3) | 0.1041 |
| Asymptomatic full-term neonates, n (%) | 38 (14.5) | 32 (16.8) | 6 (8.3) | 0.1247 |
| Asymptomatic preterm neonates, n (%) | 1 (0.4) | 1 (0.5) | 0 (0) | 0.6112 |
| All Cases (n = 263) | GBS (n = 191) | E. coli (n = 72) | p | |
|---|---|---|---|---|
| All IAP, n (%) | 91 (34.6) | 48 (25.1) | 43 (59.7) | <0.0001 |
| IAP with beta-lactams, n (%) | 69 (26.2) | 35 (18.3) | 34 (47.2) | <0.0001 |
| IAP with non beta-lactams, n (%) | 22 (8.4) | 13 (6.8) | 9 (12.5) | <0.0001 |
| Cases exposed to an active IAP, n (%) | 47 (17.9) | 41 (21.5) | 6 (8.3) | 0.0215 |
| 41 (15.6) | 35 (18.3) | 6 (8.3) | 0.0717 |
| 6 (2.3) | 6 (3.1) | 0 (0) | 0.2899 |
| No IAP Administration (n = 122) | All IAP Administered (n = 36) | Non Beta-Lactams (n = 8) | p1 | p2 | |
|---|---|---|---|---|---|
| Full-term neonates, median hours at the onset of symptoms (IQR) | 6.00 (0.75–12.50) | 0.00 (0.00–0.00) | 0.00 (0.00–4.00) | <0.0001 | 0.0197 |
| Preterm neonates, median hours at the onset of symptoms (IQR) | 0.00 (0.00–2.75) | 0.00 (0.00–0.00) | ND | 0.0856 | 0.4213 |
| No IAP Exposure (n = 10) | IAP Exposure (n = 8) | p | |
|---|---|---|---|
| Full-term neonates, median hours at the onset of symptoms (IQR) | 1.00 (0.00–6.00) | 8.00 (0.00–45.00) | 0.6965 |
| GBS (n = 158) | E. coli (n = 66) | p | |
|---|---|---|---|
| All IAP-unexposed neonates, median hours (IQR) | 4 (0.00–12.00) | 0 (0.00–1.50) | 0.0041 |
| Full term IAP-unexposed neonates, median hours (IQR) | 6 (4.00–8.00) | 1 (0.00–56.22) | 0.2143 |
| Preterm IAP-unexposed neonates, median hours (IQR) | 0 (0.00–2.75) | 0 (0.00–0.00) | 0.0957 |
| All IAP-exposed neonates, median hours (IQR) | 0 (0.00–0.00) | 0 (0.00–5.58) | 0.0267 |
| Full term IAP-exposed neonates, median hours (IQR) | 0 (0.00–0.00) | 8 (0.00–45.00) | 0.0296 |
| Preterm IAP-exposed neonates, median hours (IQR) | 0 (0.00–2.00) | 0 (0.00–0.00) | 0.0957 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berardi, A.; Trevisani, V.; Di Caprio, A.; Caccamo, P.; Latorre, G.; Loprieno, S.; Foglianese, A.; Laforgia, N.; Perrone, B.; Nicolini, G.; et al. Timing of Symptoms of Early-Onset Sepsis after Intrapartum Antibiotic Prophylaxis: Can It Inform the Neonatal Management? Pathogens 2023, 12, 588. https://doi.org/10.3390/pathogens12040588
Berardi A, Trevisani V, Di Caprio A, Caccamo P, Latorre G, Loprieno S, Foglianese A, Laforgia N, Perrone B, Nicolini G, et al. Timing of Symptoms of Early-Onset Sepsis after Intrapartum Antibiotic Prophylaxis: Can It Inform the Neonatal Management? Pathogens. 2023; 12(4):588. https://doi.org/10.3390/pathogens12040588
Chicago/Turabian StyleBerardi, Alberto, Viola Trevisani, Antonella Di Caprio, Paola Caccamo, Giuseppe Latorre, Sabrina Loprieno, Alessandra Foglianese, Nicola Laforgia, Barbara Perrone, Giangiacomo Nicolini, and et al. 2023. "Timing of Symptoms of Early-Onset Sepsis after Intrapartum Antibiotic Prophylaxis: Can It Inform the Neonatal Management?" Pathogens 12, no. 4: 588. https://doi.org/10.3390/pathogens12040588
APA StyleBerardi, A., Trevisani, V., Di Caprio, A., Caccamo, P., Latorre, G., Loprieno, S., Foglianese, A., Laforgia, N., Perrone, B., Nicolini, G., Ciccia, M., Capretti, M. G., Giugno, C., Rizzo, V., Merazzi, D., Fanaro, S., Taurino, L., Pulvirenti, R. M., Orlandini, S., ... Lugli, L. (2023). Timing of Symptoms of Early-Onset Sepsis after Intrapartum Antibiotic Prophylaxis: Can It Inform the Neonatal Management? Pathogens, 12(4), 588. https://doi.org/10.3390/pathogens12040588








