Relevant Brachycera (Excluding Oestroidea) for Horses in Veterinary Medicine: A Systematic Review
Abstract
1. Introduction
2. Results
2.1. Results of the Literature Research
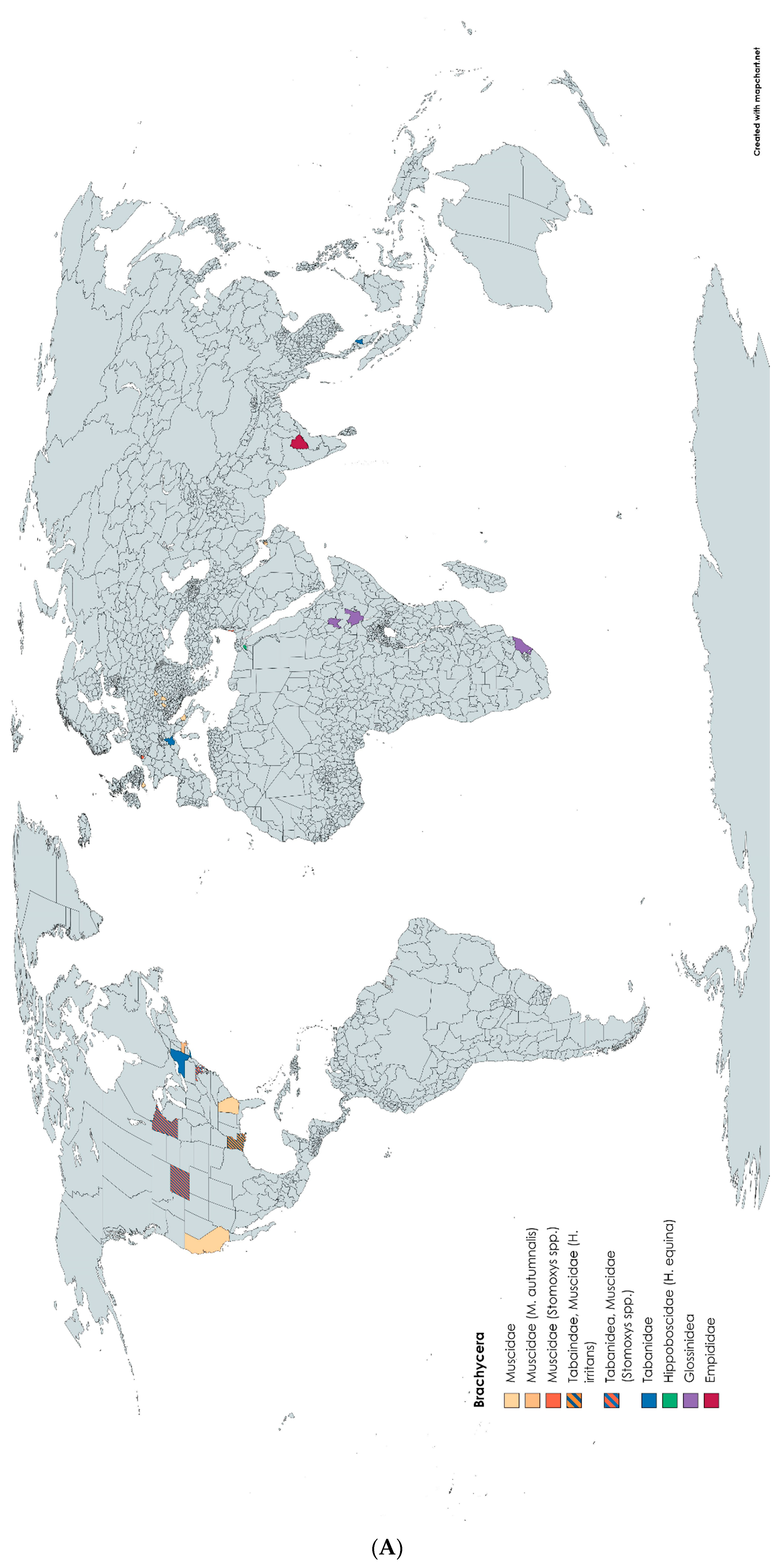
2.2. Results Concerning the Research Questions
2.2.1. Role of Tabanidae in Transmission of Pathogens
Role of Tabanidae in Transmission of Viruses
Role of Tabanidae in Transmission of Bacteria
Role of Tabanidae in Transmission of Parasites
2.2.2. Role of Muscidae in Transmission of Pathogens and as Pests
Role of Muscidae in Transmission of Viruses
Role of Muscidae in Transmission of Bacteria
Role of Muscidae in Transmission of Parasites
Muscidae as Dermatological Pests
2.2.3. Role of Glossinidae in Transmission of Pathogens
Role of Glossinidae in Transmission of Parasites
2.2.4. Role of Hippoboscidae in Transmission of Pathogens
Role of Hippoboscidae in Transmission of Bacteria
3. Discussion
4. Materials and Methods
4.1. Literature Search
4.2. Inclusion Criteria
4.3. Data Extraction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, R.D.; Gerhardt, R.R. 8—Flies (Diptera). In Medical and Veterinary Entomology; Mullen, G.R., Durden, L., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 127–145. ISBN 9780125104517. [Google Scholar]
- Integrated Taxonomic Information System—Report—Brachycera: Taxonomic Serial No.: 130052. Available online: https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&anchorLocation=SubordinateTaxa&credibilitySort=TWG%20standards%20met&rankName=Family&search_value=130052&print_version=SCR&source=from_print#SubordinateTaxa (accessed on 16 March 2023).
- Chvala, M. Fauna Europaea: Tabanidae. Fauna Europaea Version 2022.02. Available online: https://fauna-eu.org (accessed on 10 August 2022).
- Issel, C.J.; Foil, L.D. Equine infectious anaemia and mechanical transmission: Man and the wee beasties. Rev. Sci. Technol. 2015, 34, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Baldacchino, F.; Desquesnes, M.; Mihok, S.; Foil, L.D.; Duvallet, G.; Jittapalapong, S. Tabanids: Neglected subjects of research, but important vectors of disease agents! Infect. Genet. Evol. 2014, 28, 596–615. [Google Scholar] [CrossRef] [PubMed]
- Baldacchino, F.; Muenworn, V.; Desquesnes, M.; Desoli, F.; Charoenviriyaphap, T.; Duvallet, G. Transmission of pathogens by Stomoxys flies (Diptera, Muscidae): A review. Parasite 2013, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, W.L. Animal disease agents transmitted by horse flies and deer flies (Diptera: Tabanidae). J. Med. Entomol. 1976, 13, 225–275. [Google Scholar] [CrossRef]
- Cook, R.F.; Leroux, C.; Issel, C.J. Equine infectious anemia and equine infectious anemia virus in 2013: A review. Vet. Microbiol. 2013, 167, 181–204. [Google Scholar] [CrossRef]
- Issel, C.J.; Foil, L.D. Studies on equine infectious anemia virus transmission by insects. J. Am. Vet. Med. Assoc. 1984, 184, 293–297. [Google Scholar]
- Rochon, K.; Hogsette, J.A.; Kaufman, P.E.; Olafson, P.U.; Swiger, S.L.; Taylor, D.B. Stable Fly (Diptera: Muscidae)—Biology, Management, and Research Needs. J. Integr. Pest Manag. 2021, 12, 38. [Google Scholar] [CrossRef]
- Geden, C.J.; Nayduch, D.; Scott, J.G.; Burgess, E.R.; Gerry, A.C.; Kaufman, P.E.; Thomson, J.; Pickens, V.; Machtinger, E.T. House Fly (Diptera: Muscidae): Biology, Pest Status, Current Management Prospects, and Research Needs. J. Integr. Pest Manag. 2021, 12, 39. [Google Scholar] [CrossRef]
- Fryxell, R.T.; Moon, R.D.; Boxler, D.J.; Watson, D.W. Face Fly (Diptera: Muscidae) Biology, Pest Status, Current Management Prospects, and Research Needs. J. Integr. Pest Manag. 2021, 12, 5. [Google Scholar] [CrossRef]
- Otranto, D.; Stevens, J.R.; Cantacessi, C.; Gasser, R.B. Parasite transmission by insects: A female affair? Trends Parasitol. 2008, 24, 116–120. [Google Scholar] [CrossRef]
- Holmes, P. Tsetse-transmitted trypanosomes—Their biology, disease impact and control. J. Invertebr. Pathol. 2013, 112, S11–S14. [Google Scholar] [CrossRef]
- Onmaz, A.C.; Beutel, R.G.; Schneeberg, K.; Pavaloiu, A.N.; Komarek, A.; van den Hoven, R. Vectors and vector-borne diseases of horses. Vet. Res. Commun. 2013, 37, 65–81. [Google Scholar] [CrossRef]
- Bruce, D. Preliminary Report on the Tsetse Fly Disease or Nagana, in Zululand; Harrison and Sons: London, UK, 1895. [Google Scholar]
- Stringer, A.P. Infectious diseases of working equids. Vet. Clin. N. Am. Equine Pract. 2014, 30, 695–718. [Google Scholar] [CrossRef]
- Peña-Espinoza, M.; Em, D.; Shahi-Barogh, B.; Berer, D.; Duscher, G.G.; van der Vloedt, L.; Glawischnig, W.; Rehbein, S.; Harl, J.; Unterköfler, M.S.; et al. Molecular screening of keds (Diptera: Hippoboscidae) from domestic and wild ruminants in Austria reveals the presence of human and veterinary pathogens. Parasit. Vectors 2023. submitted. [Google Scholar]
- Dehio, C.; Sauder, U.; Hiestand, R. Isolation of Bartonella schoenbuchensis from Lipoptena cervi, a blood-sucking arthropod causing deer ked dermatitis. J. Clin. Microbiol. 2004, 42, 5320–5323. [Google Scholar] [CrossRef]
- Carn, V.M. The role of dipterous insects in the mechanical transmission of animal viruses. Br. Vet. J. 1996, 152, 377–393. [Google Scholar] [CrossRef]
- Issel, C.J.; Rushlow, K.; Foil, L.D.; Montelaro, R.C. A perspective on equine infectious anemia with an emphasis on vector transmission and genetic analysis. Vet. Microbiol. 1988, 17, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Foil, L.D.; Adams, W.V.; McManus, J.M.; Issel, C.J. Bloodmeal residues on mouthparts of Tabanus fuscicostatus (Diptera: Tabanidae) and the potential for mechanical transmission of pathogens. J. Med. Entomol. 1987, 24, 613–616. [Google Scholar] [CrossRef]
- Chambers, G.; Ellsmore, V.A.; O’Brien, P.M.; Reid, S.W.J.; Love, S.; Campo, M.S.; Nasir, L. Association of bovine papillomavirus with the equine sarcoid. J. Gen. Virol. 2003, 84, 1055–1062. [Google Scholar] [CrossRef]
- Haspeslagh, M.; Vlaminck, L.; Martens, A. The possible role of Stomoxys calcitrans in equine sarcoid transmission. Vet. J. 2018, 231, 8–12. [Google Scholar] [CrossRef]
- Nasir, L.; Campo, M.S. Bovine papillomaviruses: Their role in the aetiology of cutaneous tumours of bovids and equids. Vet. Dermatol. 2008, 19, 243–254. [Google Scholar] [CrossRef]
- Peck, D.E.; Reeves, W.K.; Pelzel-McCluskey, A.M.; Derner, J.D.; Drolet, B.; Cohnstaedt, L.W.; Swanson, D.; McVey, D.S.; Rodriguez, L.L.; Peters, D. Management Strategies for Reducing the Risk of Equines Contracting Vesicular Stomatitis Virus (VSV) in the Western United States. J. Equine Vet. Sci. 2020, 90, 103026. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; Mellor, P.S.; Fall, A.G.; Garros, C.; Venter, G.J. African horse sickness virus: History, transmission, and current status. Annu. Rev. Entomol. 2017, 62, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Foil, L.D. Tabanids as vectors of disease agents. Parasitol. Today 1989, 5, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.; Panella, N.; Hale, K.; Komar, N. Detection of West Nile virus in stable flies (Diptera: Muscidae) parasitizing juvenile american white pelicans. J. Med. Entomol. 2010, 47, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.S.; Swope, B.N.; Hogsette, J.A.; Burkhalter, K.L.; Savage, H.M.; Nasci, R.S. Vector competence of the stable fly (Diptera: Muscidae) for West Nile virus. J. Med. Entomol. 2011, 48, 656–668. [Google Scholar] [CrossRef]
- Gancz, A.Y.; Barker, I.K.; Lindsay, R.; Dibernardo, A.; McKeever, K.; Hunter, B. West Nile virus outbreak in North American owls, Ontario, 2002. Emerg. Infect. Dis. 2004, 10, 2135–2142. [Google Scholar] [CrossRef]
- Farajollahi, A.; Crans, W.J.; Nickerson, D.; Bryant, P.; Wolf, B.; Glaser, A.; Andreadis, T.G. Detection of West Nile virus RNA from the louse fly Icosta americana (Diptera: Hippoboscidae). J. Am. Mosq. Control Assoc. 2005, 21, 474–476. [Google Scholar] [CrossRef]
- Chung, C.Y.; Kasten, R.W.; Paff, S.M.; van Horn, B.A.; Vayssier-Taussat, M.; Boulouis, H.J.; Chomel, B.B. Bartonella spp. DNA associated with biting flies from California. Emerg. Infect. Dis. 2004, 10, 1311–1313. [Google Scholar] [CrossRef]
- Ford, R.B.; Cairns, R.A.; Short, C.D. Equine dermatophilosis: A two-year clinico-pathologic study. Vet. Med. Small Anim. Clin. 1974, 69, 1557–1561. [Google Scholar]
- Burg, J.G.; Neely, D.M.; Williams, N.M.; Knapp, F.W. Retention and attempted mechanical transmission of Ehrlichia risticii by Stomoxys calcitrans. Med. Vet. Entomol. 1994, 8, 43–46. [Google Scholar] [CrossRef]
- Spier, S.J.; Leutenegger, C.M.; Carroll, S.P.; Loye, J.E.; Pusterla, J.B.; Carpenter, T.E.; Mihalyi, J.E.; Madigan, J.E. Use of a real-time polymerase chain reaction-based fluorogenic 5′ nuclease assay to evaluate insect vectors of Corynebacterium pseudotuberculosis infections in horses. Am. J. Vet. Res. 2004, 65, 829–834. [Google Scholar] [CrossRef]
- DeLoache, P.; Whelchel, D.; Beetz, R.; Carter, J.; Eichelberger, A.; Pusterla, N. Guttural pouch empyema caused by Corynebacterium pseudotuberculosis in a pregnant mare. Equine Vet. Educ. 2018, 30, 76–79. [Google Scholar] [CrossRef]
- Barba, M.; Stewart, A.J.; Passler, T.; Hathcock, T.; Wooldridge, A.A.; van Santen, E.; Chamorro, M.F.; Cattley, R.C.; Hogsette, J.A.; Hu, X.P. Experimental inoculation of house flies Musca domestica with Corynebacterium pseudotuberculosis biovar equi. Bull. Insect. 2015, 68, 39–44. [Google Scholar]
- Hadi, A.M. Study of fly borne parasites (BRACHYCERA): A review. Plant Arch. 2020, 20, 2419–2428. [Google Scholar]
- Büscher, P.; Gonzatti, M.I.; Hébert, L.; Inoue, N.; Pascucci, I.; Schnaufer, A.; Suganuma, K.; Touratier, L.; Van Reet, N. Equine trypanosomosis: Enigmas and diagnostic challenges. Parasit. Vectors 2019, 12, 234. [Google Scholar] [CrossRef]
- Antoine-Moussiaux, N.; Desmecht, D. Epidemiology of Trypanosoma evansi infection. Ann. Med. Vet. 2008, 152, 191–201. [Google Scholar]
- Mihok, S.; Maramba, O.; Munyoki, E.; Kagoiya, J. Mechanical transmission of Trypanosoma spp. by African Stomoxyinae (Diptera: Muscidae). Trop. Parasitol. 1995, 46, 103–105. [Google Scholar]
- Sumba, A.L.; Mihok, S.; Oyieke, F.A. Mechanical transmission of Trypanosoma evansi and T. congolense by Stomoxys niger and S. taeniatus in a laboratory mouse model. Med. Vet. Entomol. 1998, 12, 417–422. [Google Scholar] [CrossRef]
- Desquesnes, M.; Dia, M.; Acapovi, G.; Yoni, W. Les Vecteurs Mécaniques des Trypanosomoses Animales: Généralités, Morphologie, Biologie, Impacts et Contrôle. Identification des Espèces les Plus Abondantes en Afrique de l’Ouest; CIRAD & CIRDES: Montpellier, France, 2005. [Google Scholar]
- Desquesnes, M.; Dargantes, A.; Lai, D.H.; Lun, Z.R.; Holzmuller, P.; Jittapalapong, S. Trypanosoma evansi and Surra: A Review and Perspectives on Transmission, Epidemiology and Control, Impact, and Zoonotic Aspects. BioMed Res. Int. 2013, 2013, 321237. [Google Scholar] [CrossRef]
- Fetene, E.; Leta, S.; Regassa, F.; Büscher, P. Global distribution, host range and prevalence of Trypanosoma vivax: A systematic review and meta-analysis. Parasit. Vectors 2021, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Barlaam, A.; Traversa, D.; Papini, R.; Giangaspero, A. Habronematidosis in Equids: Current Status, Advances, Future Challenges. Front. Vet. Sci. 2020, 7, 358. [Google Scholar] [CrossRef] [PubMed]
- Krecek, R.C. Habronema malani sp. n. and Habronema tomasi sp. n. (Nematoda: Habronematidae) from the Burchell’s Zebras and Hartmann’s Mountain Zebras in Southern Africa. Proc. Helminthol. Soc. Wash. 1989, 56, 183–191. [Google Scholar]
- Ransom, B.H. The Life History of Habronema muscae (Carter), a Prasite of the Horse Transmitted by the House Fly. Ph.D. Thesis, U.S. Department of Agriculture, Washington, DC, USA, 1913. [Google Scholar]
- Pugh, D.G.; Hu, X.P.; Blagburn, B. Habronemiasis: Biology, signs, and diagnosis, and treatment and prevention of the nematodes and vector flies. J. Equine Vet. Sci. 2014, 34, 241–248. [Google Scholar] [CrossRef]
- Schuster, R.K.; Sivakumar, S.; Kinne, J.; Babiker, H.; Traversa, D.; Buzzell, G.R. Cutaneous and pulmonal habronemosis transmitted by Musca domestica in a stable in the United Arab Emirates. Vet. Parasitol. 2010, 174, 170–174. [Google Scholar] [CrossRef]
- Pusterla, N.; Watson, J.L.; Wilson, W.D.; Affolter, V.K.; Spier, S.J. Cutaneous and ocular habronemiasis in horses: 63 cases (1988–2002). J. Am. Vet. Med. Assoc. 2003, 222, 978–982. [Google Scholar] [CrossRef]
- Otranto, D.; Traversa, D. Thelazia eyeworm: An original endo- and ecto-parasitic nematode. Trends Parasitol. 2005, 21, 1–4. [Google Scholar] [CrossRef]
- Barker, I.K. Case report. Thelazia lacrymalis from the eyes of an Ontario horse. Can. Vet. J. 1970, 11, 186–189. [Google Scholar]
- Foil, L.; Foil, C. Parasitic skin diseases. Vet. Clin. N. Am. Equine Pract. 1986, 2, 403–437. [Google Scholar] [CrossRef]
- Foil, L.D.; Foil, C.S. Arthropod pests of horses. Compend. Contin. Educ. Pract. Vet. 1990, 12, 723–731. [Google Scholar]
- Ross, H.H. The Rocky Mountain “Black Fly,” Symphoromyia Atripes (Diptera: Rhagionidae). Ann. Entomol. Soc. Am. 1940, 33, 254–257. [Google Scholar] [CrossRef]
- Stuckenberg, B.R. A new genus and species of Athericidae (Diptera: Tabanoidea) from Cape York Peninsula. Rec. Aust. Mus. 2000, 52, 151–159. [Google Scholar] [CrossRef]
- Machtinger, E.T.; Weeks, E.N.; Geden, C.J.; Lacher, E. (Eds.) Pests and Parasites of Horses; Wageningen Academic Publishers: Wageningen, The Netherlands, 2022; ISBN 978-90-8686-371-6. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 105906. [Google Scholar] [CrossRef]
- Finlay, M.; Yuan, Z.; Burden, F.; Trawford, A.; Morgan, I.M.; Saveria Campo, M.; Nasir, L. The detection of Bovine Papillomavirus type 1 DNA in flies. Virus Res. 2009, 144, 315–317. [Google Scholar] [CrossRef]
- Abel-Reichwald, H.; Hainisch, E.K.; Zahalka, S.; Corteggio, A.; Borzacchiello, G.; Massa, B.; Merlone, L.; Nasir, L.; Burden, F.; Brandt, S. Epidemiologic analysis of a sarcoid outbreak involving 12 of 111 donkeys in Northern Italy. Vet. Microbiol. 2016, 196, 85–92. [Google Scholar] [CrossRef]
- Kemen, M.J.; McClain, D.S.; Matthysse, J.G. Role of horse flies in transmission of wquine infectious anemia from carrier ponies. J. Am. Vet. Med. Assoc. 1978, 172, 360–362. [Google Scholar]
- Hawkins, J.A.; Adams, W.V.; Cook, L.; Wilson, B.H.; Roth, E.E. Transmission of equine infectious anemia with horse fly Tabanus fuscicostatus Hine. Proc. United States Anim. Health Assoc. 1972, 76, 227–230. [Google Scholar]
- Hawkins, J.A.; Adams, W.V., Jr.; Wilson, B.H.; Issel, C.J.; Roth, E.E. Transmission of equine infectious anemia virus by Tabanus fuscicostatus. J. Am. Vet. Med. Assoc. 1976, 168, 63–64. [Google Scholar]
- Foil, L.D.; Meek, C.L.; Adams, W.V.; Issel, C.J. Mechanical transmission of equine infectious anemia virus by deer flies (Chrysops flavidus) and stable flies (Stomoxys calcitrans). Am. J. Vet. Res. 1983, 44, 155–156. [Google Scholar]
- Foil, L.; Adams, W.V., Jr.; Issel, C.J.; Pierce, R. Tabanid (Diptera) populations associated with an equine infectious anemia outbreak in an inapparently infected herd of horses. J. Med. Entomol. 1984, 21, 28–30. [Google Scholar] [CrossRef]
- Issel, C.J.; Adams Jr, W.V.; Meek, L.; Ochoa, R. Transmission of equine infectious anemia virus from horses without clinical signs of disease. J. Am. Vet. Med. Assoc. 1982, 180, 272–275. [Google Scholar] [PubMed]
- Scott, J.W. Experimental Transmission of Swamp Fever or Infectious Anemia by Means of Insects. J. Am. Vet. Med. Assoc. 1920, 56, 448–454. [Google Scholar]
- Stein, C.D.; Lotze, J.C.; Mott, L.O. Transmission of Equine Infectious Anemia by the Stablefly, Stomoxys calcitrans, the Horsefly, Tabanus sulcifrons (Macquart), and by Injection of minute Amounts of Virus. Am. J. Vet. Res. 1942, 3, 183–193. [Google Scholar]
- Tan, L.P.; Mohd Rajdi, N.Z.I.; Mohamad, M.A.; Mohamed, M.; Hamdan, R.H.; Goriman Khan, M.A.K.; Ahmad Syazwan, S.; Seng Hua, L. First report of Trypanosoma theileri in equine host and Tabanus sp. in Malaysia. J. Equine Vet. Sci. 2022, 108, 103807. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.W. Insect Transmission of Infectious Anemia of Horses. J. Parasitol. 1917, 4, 70–79. [Google Scholar] [CrossRef]
- Pusterla, N.; Bowers, J.; Barnum, S.; Hall, J.A. Molecular detection of Streptococcus equi subspecies equi in face flies (Musca autumnalis) collected during a strangles outbreak on a Thoroughbred farm. Med. Vet. Entomol. 2020, 34, 120–122. [Google Scholar] [CrossRef]
- Traversa, D.; Otranto, D.; Iorio, R.; Carluccio, A.; Contri, A.; Paoletti, B.; Bartolini, R.; Giangaspero, A. Identification of the intermediate hosts of Habronema microstoma and Habronema muscae under field conditions. Med. Vet. Entomol. 2008, 22, 283–287. [Google Scholar] [CrossRef]
- Schuster, R.K.; Sivakumar, S. A xenodiagnostic method using Musca domestica for the diagnosis of gastric habronemosis and examining the anthelmintic efficacy of moxidectin. Vet. Parasitol. 2013, 197, 176–181. [Google Scholar] [CrossRef]
- Muralidhar, P.; Rao, P.N. A note on a new vector of horse nematode, Habronema sp. from a Dipteran host (Empididae) from Hyderabad, A.P. Curr. Nematol. 1991, 2, 85–86. [Google Scholar]
- Branch, G.J.; Stoffolano, J.G., Jr. Face fly: Invertebrate vector and host of a mammalian eye worm in Massachusetts. J. Econ. Entomol. 1974, 67, 304–305. [Google Scholar] [CrossRef]
- Conn, D.B.; Weaver, J.; Tamang, L.; Graczyk, T.K. Synanthropic flies as vectors of Cryptosporidium and Giardia among livestock and wildlife in a multispecies agricultural complex. Vector Borne Zoonotic Dis. 2007, 7, 643–651. [Google Scholar] [CrossRef]
- Hornok, S.; Takács, N.; Szekeres, S.; Szoke, K.; Kontschán, J.; Horváth, G.; Sugár, L. DNA of Theileria orientalis, T. equi and T. capreoli in stable flies (Stomoxys calcitrans). Parasit. Vectors 2020, 13, 186. [Google Scholar] [CrossRef]
- Foil, L.D.; Foil, C.S.; French, D.D.; Klei, T.R.; Miller, R.I. The role of horn fly feeding and the management of seasonal equine ventral midline dermatitis. Equine Prat. 1990, 12, 6–13. [Google Scholar]
- Yeruham, I.; Braverman, Y. Skin lesions in dogs, horses and calves caused by the stable fly Stomoxys calcitrans (L.) (Diptera: Muscidae). Rev. Elev. Med. Vet. Pays Trop. 1995, 48, 347–349. [Google Scholar] [CrossRef]
- Abebe, R.; Wolde, A. Preliminary survey on equine trypanosomosis and its vectors in Asosa and Homosha districts in Benishangul Gumuz Regional State, northwest Ethiopia. Livest. Res. Rural. Dev. 2010, 22, 140–145. [Google Scholar]
- Mekuria, S.; Eyob, A.; Regassa, A.; Tadesse, A.; Mekibib, B.; Abebe, R. A cross-sectional study of equine trypanosomosis and its vectors in Wolayta Zone, Southern Ethiopia. J. Anim. Vet. Adv. 2010, 9, 2061–2066. [Google Scholar] [CrossRef]
- Abdullah, H.; Aboelsoued, D.; Farag, T.K.; Abdel-Shafy, S.; Abdel Megeed, K.N.; Parola, P.; Raoult, D.; Mediannikov, O. Molecular characterization of some equine vector-borne diseases and associated arthropods in Egypt. Acta Trop. 2022, 227, 106274. [Google Scholar] [CrossRef]
- Scott, J.W. Swamp Fever in Wyoming: Economic Importance, General Characteristics and Control; Agriculture Experiment Station Bulletins, University of Wyoming: Laramie, WY, USA, 1919; pp. 117–118. [Google Scholar]
- Kraneveld, F.C.; Djaenoedin, R. Test on the dissemination of anthrax by Tabanus rubidus in horses and buffalo. Overgedrukt Ned. Indische Bl. Voor Diergeneeskd. 1940, 52, 339–380. [Google Scholar]
- Mitzmain, M.B. The mechanical transmission of surra by Tabanus striatus Fabricus. Philip. J. Sci. 1913, 3, 223–229. [Google Scholar]
- Schuhberg, A.; Kuhn, P. Über die Übertragung von Krankheiten durch einheimische stechende Insekten.: 3. Die Übertragung von südwestafrikanischer Pferdesterbe. Arb. Kais. Gesundh. 1912, 40, 209–234. [Google Scholar]
- Cupp, E.W.; Kemen, M.J. The role of stable flies and mosquitoes in the transmission of equine infectious anemia virus. Proc. United States Anim. Health Assoc. 1980, 84, 362–367. [Google Scholar] [PubMed]
- Barba, M.; Stewart, A.J.; Passler, T.; Wooldridge, A.A.; van Santen, E.; Chamorro, M.F.; Cattley, R.C.; Hathcock, T.; Hogsette, J.A.; Hu, X.P. Experimental transmission of Corynebacterium pseudotuberculosis biovar equi in horses by house flies. J. Vet. Intern. Med. 2015, 29, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Amado, S.; Silveira, A.K.; Vieira, F.D.; Traversa, D. Habronema muscae (Nematoda: Habronematidae) larvae: Developmental stages, migration route and morphological changes in Musca domestica (Diptera: Muscidae). Exp. Parasitol. 2014, 136, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Lyons, E.T.; Drudge, J.H.; Tolliver, S.C. Thelazia lacrymalis in horses in Kentucky and observations on the face fly (Musca autumnalis) as a probable intermediate host. J. Parasitol. 1976, 62, 877–880. [Google Scholar] [CrossRef]
- Lyons, E.T.; Drudge, J.H.; Tolliver, S.C. Experimental infections of Thelazia lacrymalis: Maturation of third-stage larvae from face flies (Musca autumnalis) in eyes of ponies. J. Parasitol. 1980, 66, 181–182. [Google Scholar] [CrossRef]
- Rogers, L. Note on the Rôle of the Horse Fly in the Transmission of Trypanosoma Infection, with a Reply to Colonel Bruce’s Criticisms. Br. Med. J. 1904, 2, 1454–1455. [Google Scholar] [CrossRef]
- Bouet, G.; Roubaud, E. Expériences de transmission des trypanosomiases animales d’Afrique occidentale française par les stomoxes. Bull. Socie’Te’ Pathol. Exot. 1912, 5, 544–550. [Google Scholar]
- Bogaert, L.; Martens, A.; Depoorter, P.; Gasthuys, F. Equine sarcoids, part 1: Clinical presentation and epidemiology. Vlaams Diergeneeskd. Tijdschr. 2008, 77, 2–9. [Google Scholar]
- Stoffolano, J.G.; Haselton, A.T. The adult Dipteran crop: A unique and overlooked organ. Annu. Rev. Entomol. 2013, 58, 205–225. [Google Scholar] [CrossRef]
- Stoffolano, J.G. Fly foregut and transmission of microbes. Adv. Insect Phys. 2019, 57, 27–95. [Google Scholar] [CrossRef]
- Mellor, P.S.; Boorman, J. The transmission and geographical spread of African horse sickness and bluetongue viruses. Ann. Trop. Med. Parasitol. 1995, 89, 1–15. [Google Scholar] [CrossRef]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.-F.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- Dominguez, M.; Münstermann, S.; de Guindos, I.; Timoney, P. Equine disease events resulting from international horse movements: Systematic review and lessons learned. Equine Vet. J. 2016, 48, 641–653. [Google Scholar] [CrossRef]
- Majewska, A.C.; Werner, A.; Sulima, P.; Luty, T. Survey on equine cryptosporidiosis in Poland and the possibility of zoonotic transmission. Ann. Agric. Environ. Med. 1999, 6, 161–165. [Google Scholar]
- Thein, P. Vector-borne diseases in equidae: Factors, vectors, germs. Pferdeheilkunde 2009, 25, 345–353. [Google Scholar] [CrossRef]
- De Jong, Y.; Verbeek, M.; Michelsen, V.; Bjørn, P.D.P.; Los, W.; Steeman, F.; Bailly, N.; Basire, C.; Chylarecki, P.; Stloukal, E.; et al. Fauna Europaea—All European animal species on the web. Biodivers. Data J. 2014, 2, e4034. [Google Scholar] [CrossRef]
- Beuk, P.; Pape, T. Fauna Europaea: Oestridae. Fauna Europaea Version 2022.02. Available online: https://fauna-eu.org (accessed on 10 August 2022).
- Beuk, P.; Pape, T. Fauna Europaea: Brachycera. Fauna Europaea Version 2022.02. Available online: https://fauna-eu.org (accessed on 10 August 2022).
- Beuk, P.; Pape, T. Fauna Europaea: Sarcophagidae. Fauna Europaea Version 2022.02. Available online: https://fauna-eu.org (accessed on 10 August 2022).
- Adrian, C. Pont. Fauna Europaea: Muscidae. Fauna Europaea Version 2022.02. Available online: https://fauna-eu.org (accessed on 10 August 2022).
- Rognes, K. Fauna Europaea: Calliphoridae. Fauna Europaea Version 2022.02. Available online: https://fauna-eu.org (accessed on 10 August 2022).
- Petersen, F.T. Fauna Europaea: Hippoboscidae. Dr Thomas Fauna Europaea Version 2022.02. Available online: https://fauna-eu.org (accessed on 10 August 2022).







| Infectious Agent | Vector | Incidence | Prevalence | Location | Study |
|---|---|---|---|---|---|
| Anaplasma sp. | Hippobosca equina | 3.8% | Cairo | Abdullah et al., 2022 [84] | |
| Borrelia sp. | Hippobosca equina | 2.9% | Cairo | Abdullah et al., 2022 [84] | |
| Corynebacterium pseudotuberculosis | Haematobia irritans | 2.4% | Northern California | Spier et al., 2004 [36] | |
| Corynebacterium pseudotuberculosis | Musca domestica Stomoxys calcitrans | 20% | Northern California | Spier et al., 2004 [36] | |
| Corynebacterium pseudotuberculosis | Musca domestica Stomoxys calcitrans | 19.3% | Northern California | Spier et al., 2004 [36] | |
| Corynebacterium pseudotuberculosis | Musca domestica | 0.3% | Northern California | Spier et al., 2004 [36] | |
| Cryptosporidium | Flies (97.11% Muscidae) | 50% | Northwest Georgia | Conn et al., 2007 [78] | |
| Habronema microstoma | Stomoxys calcitrans | 1.5–7.6% (estimated) | Teramo, central Italy | Traversa et al., 2008 [74] | |
| Habronema muscae | Musca domestica | 1.7–8.5% (estimated) | Teramo, central Italy | Traversa et al., 2008 [74] | |
| Habronema muscae | Musca domestica | 25.8% | Dubai | Schuster et al., 2013 [75] | |
| Habronema muscae | Musca domestica | 16.2% | Sharjah Emirate of the United Arab Emirates | Schuster et al., 2010 [51] | |
| Streptococcus equi spp. equi | Musca autumnalis | 0.54% | Central California | Pusterla et al., 2020 [73] | |
| Theileria equi | Stomoxys calcitrans | 3.2% | Hungary | Hornok et al., 2020 [79] |
| Equidae, Einhufer, Equiden, équidés |
| Horse, Pferd, cheval, chevaux |
| Donkey, Esel, âne |
| Order | Diptera Zweiflügler Diptères | ||||||
| Suborder | Brachycera Fliege Brachycère | ||||||
| Family | Glossinidae | Hippoboscidae louse flies Lausfliege hippoboscidés | Calliphoridae blow flies Schmeißfliege | Sarcophagida flesh fly Fleischfliege | Muscidae “echte Fliege” | Oestridae Botfly Dasselfliege Oestridés | Tabanidae Horsefly Bremse taon |
| Genus | Glossina Tsetse fly Zungenfliege Tsetsefliege glossines | Hippobosca hippobosques Lipoptena | Muscinae | Oestrinae Nasendassel Hypodermatinae warble flies Hautdasseln Hypodermes Gasterophilinae Magendassel | Atylotus Chrysops Dasyrhamphis Glaucops Haematopota Heptatoma Hybomitra Nemorius Pangonius Philipomomyi Silvius Tabanus Therioplectes | ||
| Species | Hydrotaea Musca Stomoxys Haematobia Haematobosca | Cephenemyia Oestrus Pharyngomyia Rhinoestrus Hypoderma Oestromyia Portschinskia Bumblebee bot flies Przhevalskiana Gasterophilus |
| What is the title of the study? |
| Who are the authors who contributed to the study? |
| In which year was the paper received, accepted and published? |
| In which journal was the paper published? |
| Which study design was used to collect and analyze the data? |
| What was the aim of the study? |
| Where was the study carried out (city, country, continent)? |
| What species are the host animals reported in the study? |
| Which methods are used to identify the vectors, the infectious agents and the transmission? |
| How did the authors identify the vectors morphologically? |
| Which species were detected as vectors or pests? |
| Which infectious agents transmitted by Brachycera were detected? |
| What is the prevalence of the vectors that tested positive? |
| Which is the prevalence of the equids that tested positive? |
| What were the pathological findings or clinical signs detected? |
| Are any prophylactic measures or therapies described to prevent or treat an infection or damage caused by Brachycera? |
| What were the results? |
| Can the transmission events be paired with a particular season? |
| Comments |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frisch, V.; Fuehrer, H.-P.; Cavalleri, J.-M.V. Relevant Brachycera (Excluding Oestroidea) for Horses in Veterinary Medicine: A Systematic Review. Pathogens 2023, 12, 568. https://doi.org/10.3390/pathogens12040568
Frisch V, Fuehrer H-P, Cavalleri J-MV. Relevant Brachycera (Excluding Oestroidea) for Horses in Veterinary Medicine: A Systematic Review. Pathogens. 2023; 12(4):568. https://doi.org/10.3390/pathogens12040568
Chicago/Turabian StyleFrisch, Vicky, Hans-Peter Fuehrer, and Jessika-M. V. Cavalleri. 2023. "Relevant Brachycera (Excluding Oestroidea) for Horses in Veterinary Medicine: A Systematic Review" Pathogens 12, no. 4: 568. https://doi.org/10.3390/pathogens12040568
APA StyleFrisch, V., Fuehrer, H.-P., & Cavalleri, J.-M. V. (2023). Relevant Brachycera (Excluding Oestroidea) for Horses in Veterinary Medicine: A Systematic Review. Pathogens, 12(4), 568. https://doi.org/10.3390/pathogens12040568







