Abstract
No human vaccine is available for visceral leishmaniasis (VL). Live attenuated centrin gene-deleted L. donovani (LdCen−/−) parasite vaccine has been shown to induce robust innate immunity and provide protection in animal models. Toll-like receptors (TLRs) are expressed in innate immune cells and are essential for the early stages of Leishmania infection. Among TLRs, TLR-9 signaling has been reported to induce host protection during Leishmania infection. Importantly, TLR-9 ligands have been used as immune enhancers for non-live vaccination strategies against leishmaniasis. However, the function of TLR-9 in the generation of a protective immune response in live attenuated Leishmania vaccines remains unknown. In this study, we investigated the function of TLR-9 during LdCen−/− infection and found that it increased the expression of TLR-9 on DCs and macrophages from ear-draining lymph nodes and spleen. The increase in TLR-9 expression resulted in changes in downstream signaling in DCs mediated through signaling protein myeloid differentiation primary response 88 (MyD88), resulting in activation and nuclear translocation of nuclear factor-κB (NF-κB). This process resulted in an increase in the DC’s proinflammatory response, activation, and DC-mediated CD4+T cell proliferation. Further, LdCen−/− immunization in TLR-9−/− mice resulted in a significant loss of protective immunity. Thus, LdCen−/− vaccine naturally activates the TLR-9 signaling pathway to elicit protective immunity against virulent L. donovani challenge.
1. Introduction
Visceral leishmaniasis (VL) is caused by the protozoan parasite Leishmania donovani, which affects nations worldwide. An estimated 50,000–90,000 new VL cases are recorded every year from endemic areas of the world including Bangladesh, India, Nepal, Brazil, Ethiopia, Kenya, and Sudan [1].
Host defenses against Leishmania species are mainly initiated by innate immune cell-induced activation of an inflammatory response, followed by cell-mediated immune responses [2]. The innate immune system actively contributes to the rapid identification of pathogens, such as viruses and bacteria, by using a range of pattern recognition receptors (PRRs), such as Toll-like receptors [3,4,5,6,7]. TLRs are cellular receptors that detect common pathogen-associated molecules such as lipopeptides, peptidoglycan, flagellin, lipopolysaccharides, and nucleic acids and induce innate responses by several pathways, facilitating the generation of inflammatory cytokines by macrophages and dendritic cells (DCs) [8,9]. During Leishmania infection, the participation of TLR-9, TLR-4, TLR-2, and TLR-3 is pivotal for mediating a proinflammatory cytokine response and subsequent infection control [10,11,12,13].
The findings that resistance to Leishmania is dependent on parasite lipophosphoglycan (LPG) by TLR-2 [14], the generation of IL-12, and the establishment of a Th1 immune response [15] in addition to NO production have validated the significance of TLR-2 in infection control [16]. Previous studies also have demonstrated a significant function for TLR-2/TLR-3 in L. donovani recognition and parasite clearance by macrophages via secretion of nitric oxide (NO) and proinflammatory cytokines [13]. Similarly, TLR-4 signaling controls L. major parasite growth, and lack of TLR-4 signaling exacerbates parasite growth during both the innate and adaptive phase of the immune response as well as a delay in the healing of the cutaneous wounds [17,18]. Recent studies also provided novel insights into the mechanism of TLR-9-induced host protection during Leishmania infection. For example, TLR-9 recognizes CpG DNA sequences of L. major and activates the dendritic cell (DC) along with the generation of a Th1-dominant response that resolves the lesions [19]. Likewise, during L. infantum infection, TLR-9 activates CD11chigh DCs thereby triggering IFN-γ production and cytotoxic function in NK cell [20,21]. Notably, enhancing the effectiveness of immunization with parasite antigens against leishmaniasis by activating TLRs via exogenous agonists has emerged as a viable option [10,22,23].
Of the numerous vaccination methods against Leishmania, live attenuated vaccines showed great potential because of mimicking the natural infection [24,25,26]. Our laboratory created several Leishmania mutant strains including L. donovani (LdCen−/−), which lack the Centrin1 gene [27,28,29,30]. We showed that immunization with LdCen−/− resulted in protection against virulent L. donovani infection in a variety of animal models, including mice, hamsters, and dogs. Control of parasite burden and induction of an adaptive T cell response that is host-protective served as indicators of LdCen−/− induced protection [31,32,33,34]. We recently reported that immunization with LdCen−/− parasites and innate cells such as neutrophils, macrophages, and DCs are critical for eliciting a protective Th1 immune response [28,35,36,37]. Innate cell-mediated recognition of pathogens and subsequent initiation of signal transduction pathways that further instruct the development of antigen-specific adaptive immunity mostly depend on TLRs [38]. Several TLR ligands have been utilized as promising immune enhancers for vaccination against VL [39]. Specifically, TLR-9 ligand, CpG-ODN, has been extensively explored as a preventative vaccine adjuvant for Leishmania antigens and it has been demonstrated to provide protection following challenges with L. major and L. donovani [39,40,41,42,43,44].
Considering the beneficial effects of TLR-9 activation in Leishmania vaccine studies, we have investigated the role of TLR-9 in LdCen−/− induced immunity. We have demonstrated that LdCen−/− infection specifically upregulates TLR-9 expression and subsequent TLR-9-mediated downstream signaling in DCs compared to LdWT infection. These events enable DCs to produce an increased proinflammatory response and initiate CD4+Th1 cell proliferation. Importantly, significant loss of protection by LdCen−/− immunization in TLR-9−/− mice against virulent L. donovani challenge highlights the crucial role of TLR-9 activation for the generation of a host-protecting immune response by LdCen−/− vaccine.
2. Material and Methods
2.1. Animals and Parasites
Female C57BL/6 mice aged five to six weeks were purchased from The National Cancer Institute, National Institutes of Health, Bethesda, MD; TLR-9−/− mice were purchased from Jackson Laboratory. We performed all our tests on female mice that were 6 to 8 weeks old. Mice were kept in an environment that was appropriate for this species at the Food and Drug Administration/CBERAAALAC-accredited facility. The LdWT (MHOM/SD/62/1S) and LdCen−/− line of L. donovani (Ld1S2D) parasites were employed, and the parasite culture process and standard molecular biology practices were carried out as previously reported [31].
2.2. Ethics Statement
The study’s animal protocol (ASP 1995#26) received clearance from the Institutional Animal Care and Use Committee of the Center for Biologics Evaluation and Research of the Food and Drug Administration. Further, the animal protocol is in full accordance with the “Guide for the Care and Use 198 of Laboratory Animals” as described in the U.S. Public Health Service 199 Policy on Humane Care and Use of Laboratory Animals 2015. (http://grants.nih.gov/grants/olaw/references/phspolicylabanimals.pdf; accessed on 15 October 2021).
2.3. Infection of Mice and Isolation of Macrophages and DCs from Ear dLN and Spleen
Using a 29-gauge needle (BD Ultra-Fine) and a volume of 10 μL, 3 × 106 LdWT/LdCen−/− parasites were intradermally (ID) injected into C57BL/6 mice in the ear pinna. Each study employed a minimum of six mice per group. As a control group, naïve mice that were age-matched received PBS. Macrophages (MØ) (Cd11b+Ly6G−Ly6c−Cd11c−MHCII+) and dendritic cells (DC) (Cd11b+Ly6G−Ly6c−Cd11c+MHCIIhi) were sort selected from the spleen and ear dLN of LdWT and LdCen−/− infected mice at 72 h and 7 days after infection, respectively, by high-speed FACS cell sorter system (BD FACS ARIA IITM). Briefly, tweezers and a syringe plunger were used to physically separate and remove the retro maxillary (ear-draining) lymph nodes. Filtration of tissue homogenates using a 70-μm cell strainer was performed (Falcon Products, Corning, NY, USA). To create a single-cell suspension, mouse spleens were collected and processed with collagenase (1 mg/mL) and DNase I (20 mg/mL) (Thermo Fisher Scientific, Waltham, MA, USA). The single cell suspension from the spleen and dLN of the ear was labeled with anti-TCR-, anti-NK1.1, and anti-Cd1b that had been APC-tagged. To exclude certain cell types, anti-APC magnetic beads were utilized and run across LS columns. Flow through enriched population of macrophages and DCs were collected and stained with macrophages and DC-specific markers and then further sorted.
2.4. Cultivation of Bone Marrow-Derived Dendritic Cells (BMDCs)
In vitro dendritic cell culture was performed using bone marrow progenitors. Briefly, mice’s tibias and femurs were removed, cleared of tissue, and then cleansed with RPMI media. The erythrocytes were removed using ACK lysis buffer (Lonza, Rockville, MD, USA), and the isolated bone marrow was then cultured for 7 days with complete RPMI medium supplemented with 10% (v/v) fetal bovine serum (FBS) (R&D systems, Minneapolis, MN, USA), 1% penicillin (20 U/mL)/streptomycin (20 g/mL) (Thermo Fisher Scientific, Waltham, MA, USA ), and 20 ng/mL GM-CSF (Peprotech, Cranbury, NJ, USA) and IL-4 (Peprotech, Cranbury, NJ, USA) for obtaining >75% purity of DCs and analyzed by flow cytometry. BMDCs were transfected with TLR-9 siRNA/MyD88 siRNA or treated with/without JSH-23 (NF-κB transcriptional activity inhibitor) (Abcam, Waltham, MA, USA) followed by infection. In some experiments BMDCs were treated with LPS (Sigma, St. Louis, MO, USA) (1 μg/mL) for 45 min before siRNA transfection/JSH-23 treatment and infection. By using sandwich ELISA, mouse cytokines were detected in the conditioned medium of BMDC cultures. Using a sandwich ELISA kit (Thermo Fisher Scientific, Waltham, MA, USA), culture supernatants were collected 24 h after infection to assess cytokine production. Using flow cytometry, acquisitions of a million events were carried out to quantify the expression of costimulatory molecules on the surface of DCs. The flow cytometry section of the Material and Methods has a full description of the technique and antibodies.
2.5. TLR-9 siRNA and MyD88 siRNA Mediated Silencing in DC In Vitro
TLR-9 siRNA and control siRNA were purchased from Thermo Fisher Scientific (Waltham, MA, USA). MyD88 siRNA and control siRNA were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Cell transfections with siRNAs were performed according to the manufacturer’s protocol.
2.6. NF-κB Nuclear Translocation
DCs were isolated from mouse BM and stimulated with LPS (1 μg/mL) for 45 min followed by infection with LdWT/LdCen−/− parasites and recovered for analysis. After 6 h of incubation, Fc receptors were blocked with normal mouse serum for 15 min at 4 °C, and cells were fixed by 4% formaldehyde for 10 min at room temperature. Then, DCs were treated with monoclonal rabbit anti-mouse pNF-κBp65 (Cell signaling, Danvers, MA, USA) at a dilution of 1:50 in permeabilization buffer for 30 min at room temperature. Cells were then stained using a 1:200 dilution of an Alexa 647-conjugated goat anti-rabbit secondary antibody (Thermo Fisher Scientific, Waltham, MA, USA) for 30 min at room temperature. The cells were stained with DAPI (Thermo Fisher Scientific, Waltham, MA, USA) for the nucleus before being captured using Image stream AMNIS. By using the TransAM NF-κB activation assay kit (Active Motif, Carlsbad, CA, USA) NF-κBp65 activation in LPS-treated control and infected DCs was also quantitatively evaluated as per manufacturer’s instructions.
2.7. Antigen Presentation Assay: In Vitro DC and T Cell Co-Culture Studies
After being transfected with control siRNA/TLR-9 siRNA or treated or untreated with JSH-23 and an OVA peptide pulse (2 μg/mL; residues 323 to 339; Anaspec, Fremont, CA, USA), DCs were infected for 24 h with either LdWT or LdCen−/− parasites. From the spleens of DO11.10 transgenic mice, CD4+ T lymphocytes were isolated and stained with 5 μM carboxyfluorescein succinimidyl ester (CFSE) (Thermo Fisher Scientific, Waltham, MA, USA) for 10 min in RPMI 1640 without fetal calf serum (FCS) at 37 °C in a 5% CO2 humidified chamber. After that, cells were incubated with ice-cold RPMI 1640 and 10% FCS for 5 min to quench the CFSE, and cells were properly washed before being plated in 96-well tissue culture plates with OVA-pulsed BMDC. CD4 T cell proliferation was then calculated using flow cytometry by gating on CD4+T cells after 5 days at 37 °C with 5% CO2. An amount of 10,000 CD4-positive cells were counted in each sample. The program utilized was FlowJo version 9.7.5. Day 5 culture supernatants were collected for an ELISA test using a sandwich ELISA kit to assess cytokines (Thermo Fisher Scientific, Waltham, MA, USA). The assay was carried out in accordance with the manufacturer’s thorough instructions.
2.8. RT-PCR
Total RNA was extracted from the (1) macrophages and DCs recruited in ear dLN or spleen following ID injection of either PBS/LdWT or LdCen−/− parasites (2) DCs (3) spleen utilizing RNAqueous-Micro kit (AM1931; Ambion, Austin, Texas, USA), which additionally removes any contaminating DNA by using on-column PureLink DNase treatment during RNA purification. A high-capacity cDNA reverse transcription kit from Applied Biosystems was used to reverse transcribe aliquots (400 ng) of total RNA into cDNA via random hexamers. The TaqMan gene expression master mix and prepared TaqMan gene expression assays (Applied Biosystems) were used with a CFX96 Touch Real-Time System (Bio- Rad, Hercules, CA, USA) to measure the levels of cytokine gene expression. CFX Manager Software was used to evaluate the data. The CFX96 Touch Real-Time System was used to measure the expression of the following genes: TLR-2 (Mm00442346_m1); TLR-4 (Mm00445273_m1); TLR-9 (Mm00446193_m1); MyD88 (Mm00440338_m1); and GAPDH (Mm99999915_g1). The 2-DD Cycle threshold approach was used to determine the expression values. Samples were compared to the expression levels from untreated samples or animals that had received PBS injections, as necessary, after being normalized to GAPDH expression.
2.9. Flow Cytometry
During the surface labeling of BMDCs, rat anti-mouse CD16/32 (BD Biosciences) from BD Pharmingen was used to block cells for 20 min at 4 °C (5 μg/mL). Cells were then stained with the following antibodies: anti-mouse CD11b (Thermo Fisher Scientific, Waltham, MA, USA), anti-mouse CD3 (Thermo Fisher Scientific, Waltham, MA, USA), anti-mouse CD4(Biolegend, San Diego, CA, USA), anti-mouse Cd44 (Thermo Fisher Scientific, Waltham, MA, USA), anti-mouse Cd11c (Thermo Fisher Scientific, Waltham, MA, USA), anti-mouse Ly6G (Thermo Fisher Scientific, Waltham, MA, USA), anti-mouse Ly6C (Biolegend, San Diego, CA, USA), anti-mouse CD80 (BD Bioscience, San Jose, CA, USA), anti-mouse MHCII Class II (I-A/I-E) (Thermo Fisher Scientific, Waltham, MA, USA), anti-mouse CD40 (Thermo Fisher Scientific, Waltham, MA, USA), and anti-mouse CD200 (Thermo Fisher Scientific, Waltham, MA, USA); each with 1:100 dilution at 4 °C. All flow studies’ samples were stained with Live/Dead Fixable Aqua (Thermo Fisher Scientific, Waltham, MA, USA) to mark the dead cells. Cells were then fixed using a Fixation/Permeabilization Solution Kit (BD Bioscience, San Jose, CA, USA) for 20 min at room temperature after being washed twice with wash buffer. Cells were then acquired utilizing FACS Diva 6.1.2 software on an LSR II (BD Biosciences, San Jose, CA, USA) outfitted with laser lines of 407, 488, 532, and 633 nm. There were one million events acquired. Version 9.7.5 of the FlowJo program was used to analyze the data (Tree Star). First doublets were eliminated using the width parameter, and dead cells were disregarded based on Live/Dead Aqua dye (Thermo Fisher Scientific, Waltham, MA, USA) staining. Lymphocytes were classified using the characteristics of their light scattering. CD4 T cells were identified as CD3+ lymphocytes that express CD4 exclusively.
2.10. Immunization and Challenge Studies
Wild type (WT) (n = 13) or TLR-9−/− (n = 13) mice were immunized intradermally with 3 × 106 stationary-phase LdCen−/− promastigotes. On day 22, three animals from each experimental group were euthanized and the spleens were collected, and TLR-9 mRNA expression in splenocytes was evaluated by RT-PCR. On day 22, 105 virulent L. donovani (LdWT) metacyclic parasites were administered by tail vein to the remaining animals (n = 6). Density gradient centrifugation was used to separate L. donovani’s infectious stage metacyclic promastigotes from stationary cultures as described before [45]. Age-matched naïve mice used as controls received 105 virulent L. donovani metacyclic parasites in a similar manner. In order to quantify the parasite burden in the challenged mice’s spleens and livers at 6 weeks after the challenge, the separated host cell preparations were cultured using limiting dilutions as previously reported [31]. By using sandwich ELISA, IFN-γ and IL-10 were also detected in the Leishmania Ag-stimulated splenocyte culture supernatants 3 weeks after vaccination and 6 weeks after the challenge (Thermo Fisher Scientific, Waltham, MA, USA).
2.11. Statistical Analysis
GraphPad Prism 5.0 software was used to conduct an unpaired, two-tailed Student t-test to statistically analyze the differences in group mean values. A p value of 0.05 was regarded as statistically significant, and a p value of 0.005 was regarded as highly significant.
3. Results
3.1. Infection with LdCen−/− Induces TLR-9 mRNA Expression
TLR-2, 4, and 9 are primarily responsible for influencing the various immune reactions during Leishmania infections [10,46]. Hence, we investigated the expression of TLR- 2, 4, and 9 in ear dLN and spleen-derived macrophages and dendritic cells from mice infected with LdCen−/− and compared it to LdWT-infected mice by real-time-PCR. Macrophages (MØ) (Cd11b+Ly6G−Ly6c−Cd11c−MHCII+) and dendritic cells (DC) (Cd11b+Ly6G−Ly6c−Cd11c+MHCIIhi) were sort selected from ear dLN and the spleen of mice infected with LdWT and LdCen−/− at 72 h and 7 d post infection, respectively, and the expression profiles of TLR- 2, 4, and 9 were assessed. The representative sorting strategy has been displayed in Figure 1A,B showing the percentage of the positive population of macrophages and DCs from ear dLN of LdWT and LdCen−/− infected mice. TLR-9 expression was significantly higher in macrophages (Figure 1C,E) and DCs (Figure 1D,F) isolated from LdCen−/− infected mice’s ear dLN/spleen than in LdWT-infected animals. No significant difference in the expression of TLR-4 in these infections was observed. TLR-2 expression was significantly lower in macrophages and DCs from LdCen−/− infected mice compared to LdWT-infected mice (Figure 1C–F). These results demonstrate that there is an early induction of TLR-9 in the phagocytic cells following LdCen−/− infection.
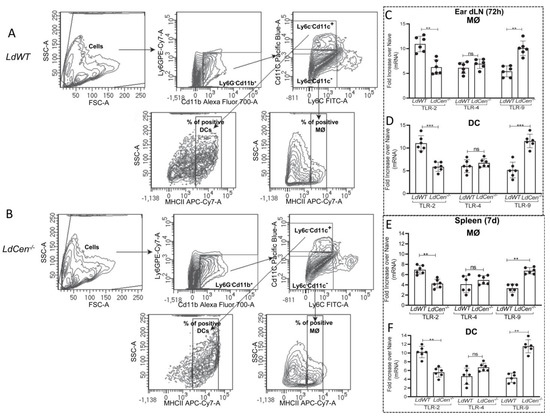
Figure 1.
LdCen−/− infection induces TLR-9 expression in ear dLN and spleen-derived macrophages and dendritic cells. Macrophages (MØ) (Cd11b+Ly6C−Ly6G−CD11c−MHCII+) and DCs (Cd11b+Ly6C−Ly6G−CD11c+MHCIIhi) were flow sorted from the ear dLN or spleen 72 h and 7 d post infection, respectively. (A,B) Sorting strategy showing the percentage of the positive population of macrophages and DCs from ear dLN of LdWT and LdCen−/− infected mice. (C–F) mRNA expression levels of TLR-2, 4, and 9 from sorted macrophages and DCs in (C,D) ear dLN and (E,F) spleen were estimated by qPCR as described in Materials and Methods and expressed as fold change from uninfected naive mice. The experiment was repeated three times with pooled digests from five to six ear dLNs per experiment. The data represent the mean values ± SD of results from three independent experiments that all yielded similar results (n = 6). ** p < 0.005; *** p < 0.0005 between the groups.
3.2. LdCen−/− Infected DCs Showed Heighted NF-κB Activation and Proinflammatory Cytokine Response via TLR-9- Myd88 Pathway
Since infection with LdCen−/− enhanced TLR-9 expression compared to LdWT infection, we investigated whether the TLR-9 mediated downstream signaling is also altered in LdCen−/− infected DCs. We have specifically chosen DCs for our experiment since it has been demonstrated to be crucial in coordinating the immune response in Leishmania infection [47]. Downstream TLR-9 signaling involves Myd88-dependent pathway-mediated regulation of NF-κB transcription factor activation, which controls the expression of proinflammatory mediators [10]. Hence, we first assessed the translocation of NF-κB in LdCen−/− infected DCs either untreated or transfected with TLR-9 siRNA/Myd88-siRNAs or treated with JSH-23, an inhibitor of NF-κB transcriptional activity. Upon transfection with TLR-9 siRNA/Myd88-siRNAs, expression levels of TLR-9 and Myd88 mRNA were significantly reduced in LdWT/LdCen−/− infected DCs compared to untreated DCs, and the representative data for the LdCen−/− infected group has been shown (Figure 2A). Quantitative ELISA indicated marked NF-κBp65 activation in LdCen−/− infected DCs with a 1.2-fold increase at 6 h post infection compared to LdWT (Figure 2B). Further, Image Flow analysis of DCs showed an active translocation of immunofluorescence of p65 from the cytoplasm to the nucleus (Figure 2C). Silencing of either TLR-9 or MyD88 using corresponding siRNAs, respectively, or inhibition of NF-κB signaling using JSH-23, significantly abrogated NF-κB p65 activation and translocation in LdCen−/− infected DCs (Figure 2B,C). Active translocation of NF-κB to the nucleus facilitates up-regulation of the proinflammatory response [48]. Corroborating the heightened NF-κB activation and nuclear translocation in LdCen−/− infected DCs, we observed considerably increased production of proinflammatory cytokines IL-12 (Figure 2D) and TNF-α (Figure 2E) than in LdWT infected DCs. Importantly, silencing of TLR-9 or Myd88 or treatment with JSH-23 significantly abrogated the expression of IL-12 and TNF-α in LdCen−/− infected DCs (Figure 2D,E). These data show that LdCen−/− infection elicits the TLR-9-Myd88 signaling pathway to regulate inflammatory cytokine responses through the activation of the NF-κB.
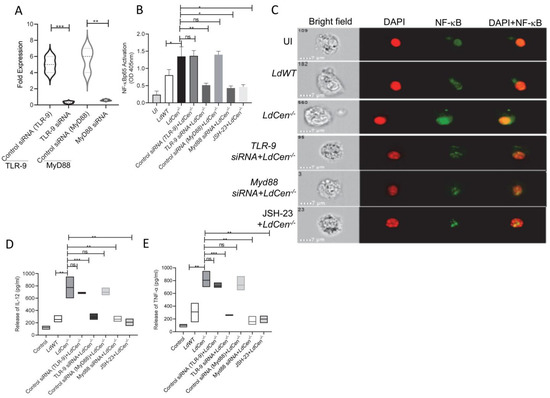
Figure 2.
LdCen−/− infected BMDCs showed heightened NF-kB activation and proinflammatory cytokine generation via the TLR-9-Myd88 pathway. (A) BMDCs were transfected with control siRNA (TLR-9)/TLR-9 siRNA or control siRNA (MyD88)/MyD88 siRNA or treated with JSH-23 followed by treatment with LPS (1 μg/mL) for 45 min and infected with LdCen−/− parasites for 6 h. The transfection efficiency of TLR-9 siRNA/Myd88 siRNA was checked for every sample by qPCR as described in Materials and Methods and the representative data for LdCen−/− infected group has been shown. The data represent the mean values ± SD of results from three independent experiments that all yielded similar results. ** p < 0.005; *** p < 0.0005 between the groups. (B) Cell extract was prepared, and NF-κB p65 activation was measured quantitatively using Trans AM NF-κB kit. Data for NF-κB p65 activation (Absorbance at 450 nM) are expressed as means ± standard deviations (SD) from triplicate experiments that yielded similar results. * p < 0.05; ** p < 0.005. (C) LPS (1 μg/mL) treated BMDCs were either infected with LdWT parasites or transfected with TLR-9 siRNA or MyD88 siRNA or treated with JSH-23 and infected with LdCen−/− parasites for 6 h, and nuclear translocation was analyzed in Flowsight. Representative images of treated cells. For improved visualization, red (DAPI) and green (NF-κB) colors were assigned in IDEAS software. Brightfield (BF), co-localization (yellow). (D,E) LPS (1 μg/mL) treated BMDCs were either infected with LdWT parasites or transfected with control siRNA (TLR-9)/ /TLR-9 siRNA or control siRNA (MyD88)/ /MyD88 siRNA or JSH-23 and infected LdCen−/− parasites for 24 h. Culture supernatants were collected to determine the (D) IL-12 and (E) TNF- α release by ELISA. The data represent the mean values ± SD of results from three independent experiments that all yielded similar results. * p < 0.05; ** p < 0.005; *** p < 0.0005 between the groups.
3.3. TLR-9 Silencing or Inhibition of NF-κB Transcriptional Activity Significantly Reduced the Expression of Costimulatory Molecules While Augmenting Expression of Coinhibitory Molecule in LdCen−/− Infected DCs
Next, we investigated the role of TLR-9 and its downstream NF-κB signaling pathway in eliciting DC function during LdCen−/− infection and compared it to LdWT infection. We assessed the expression of costimulatory and coinhibitory molecules in LdCen−/− infected DCs under either TLR-9 silenced or JSH-23 treated/untreated conditions and compared them to LdWT infection by flow cytometry. The strategy for gating and representative individual flow plots for one costimulatory (MHCII) as well as one coinhibitory (CD200) molecule have been shown in Figure 3A,B. LdCen−/− infected DCs showed a significant increase of co-stimulatory molecules such as MHCII (Figure 3A,C), CD40 (Figure 3D), and CD80 (Figure 3E) compared to LdWT infected mice under control siRNA or JSH-23 untreated conditions. A substantial decrease in the expression of MHCII, CD40, and CD80 was seen after TLR-9 silencing or JSH-23 treatment in DCs infected with LdCen−/− but not in LdWT infected DCs (Figure 3A,C–E). Additionally, we measured the expression of co-inhibitory molecules such as CD200 in both LdWT or LdCen−/− infected DCs under either TLR-9-silenced or JSH-23 treated conditions. LdCen−/−infected DCs exhibited significantly reduced expression of CD200 (Figure 3B,F) compared to LdWT under control siRNA or JSH-23 untreated conditions. On the contrary, TLR-9 silencing or NF-κB inhibition significantly increased CD200 in LdCen−/− infected DCs albeit it had no effect in LdWT-infected DCs (Figure 3B,F).
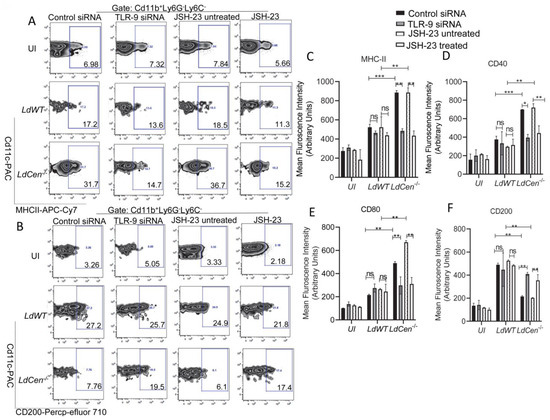
Figure 3.
TLR-9 silencing or inhibition of NF-kB transcriptional activity significantly reduced the expression of activation marker and costimulatory molecule expression while augmenting coinhibitory molecule expression in LdCen−/− infected DCs. BMDCs were transfected with control siRNA/TLR-9 siRNA or treated/untreated with JSH-23 followed by infection with LdWT/LdCen−/− parasites for 24 h. The expression of MHCII, CD40, CD80, and CD200 in the BMDCs was analyzed by flow cytometry. (A,B) The gating strategy and the individual flow plots for (A) MHCII and (B) CD200 have been shown. (C–F) Mean fluorescence intensity of (C) MHCII, (D) CD40, (E) CD80, and (F) CD200 expression in BMDCs have been represented by the bar diagram. The data represent the mean values ± SD of results from three independent experiments that all yielded similar results (n = 6). * p < 0.05; ** p < 0.005; *** p < 0.0005 between the groups.
3.4. TLR-9 Silencing or Inhibition of NF-κB Transcriptional Activity Significantly Reduced LdCen−/− Infected DC Mediated T Cell Proliferation and Altered Cytokine Expression
We analyzed the effect of TLR-9 silencing or inhibition of NF-κB transcriptional activity on the functional activity of DCs. We specifically examined the capacity of parasite antigens to be presented by infected DCs to CFSE-labeled naïve CD4+T cells to determine their antigen presentation capabilities under TLR-9 siRNA or JSH-23 treated/untreated conditions (Figure 4A). LdCen−/− infected DCs significantly increased antigen-specific CD4+T cell proliferation after 5 days of co-culture compared to those co-cultured with DCs from uninfected or LdWT infected under control siRNA or JSH-23 untreated conditions (Figure 4A). Following TLR-9 silencing/NF-κB inhibition, antigen-specific CD4+ T cell proliferation co-cultured with DCs from uninfected and LdWT-infected cultures did not vary (Figure 4A). However, the proliferation of Ag-specific CD4+T cells was significantly reduced in LdCen−/− infected DCs compared to the same population from control siRNA or JSH-23 untreated LdCen−/− infected DCs (Figure 4A). The generation of cytokines in the DC-CD4+ T cell co-cultures’ supernatants was then evaluated and showed that LdCen−/− infected DC with CD4T cell had a considerable increase in IL-12 p40 (Figure 4B) and TNF-α (Figure 4C) along with a significant decrease in IL-10 (Figure 4D) production compared to LdWT infection under control siRNA or JSH-23 untreated condition. Conversely, following TLR-9 silencing or NF-κB inactivation, there was a significant decrease in IL-12p40 (Figure 4B) and TNF-α levels (Figure 4C) while IL-10 levels were significantly elevated (Figure 4D) compared to control siRNA or the JSH-23 untreated condition. In contrast, TLR-9 siRNA or JSH-23 itself had no effect on the cytokine levels from LdWT-infected DC-CD4T cell co-cultures (Figure 4B–D).
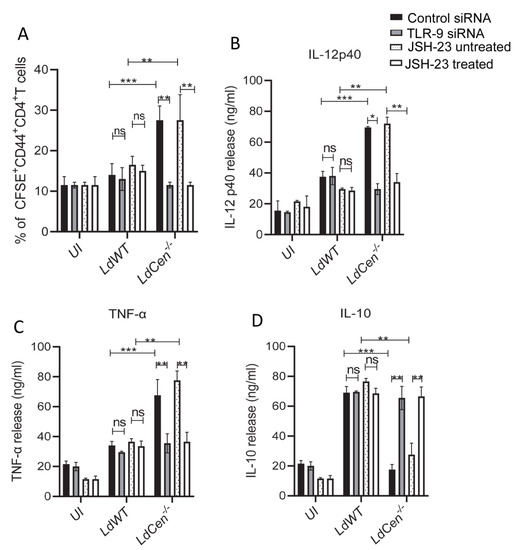
Figure 4.
TLR-9 silencing or inhibition of NF-kB transcriptional activity significantly reduced LdCen−/− infected DC mediated T cell proliferation and generates Th2 response. (A) BMDCs were transfected with control siRNA/TLR-9 siRNA or treated//untreated with JSH-23, pulsed with OVA peptide followed by infection with LdWT/LdCen−/− parasites for 24 h, and then co-cultured with purified CFSE-labeled CD4+T cells from DO11.10 transgenic mice for 5 d. OT-II T cells proliferation after 5 d culture with OVA peptide-pulsed DCs was estimated by flow cytometry by studying CFSE dilution of gated CD4+ T cells and is represented by the bar diagram. Cell proliferation was analyzed in triplicate experiments. The data represent the mean values ± SD of results from three independent experiments that all yielded similar results. ** p < 0.005; *** p < 0.0005 between the groups. (B–D) The cytokines released from the co-culture experiment were measured by sandwich ELISA as described in Materials and Methods. The data represent the mean values ± SD of results from three independent experiments that all yielded similar results. * p < 0.05; ** p < 0.005; *** p < 0.0005 between the groups.
3.5. Absence of TLR-9 Abrogates LdCen−/− Induced Protection against LdWT Infection
To ascertain the role of TLR-9 signaling in LdCen−/− mediated protection against virulent Leishmania challenge, wild type (WT) or TLR-9−/− mice were immunized with LdCen−/− for 21 days (3 weeks). The schematic for the treatment regimen has been shown in Figure 5A. TLR-9 mRNA expression in immunized WT and TLR-9−/− mice was determined in total spleen cells at day 22. TLR-9 mRNA expression was significantly higher in splenic cells at day 22 in immunized WT mice compared to TLR-9−/− mice (Figure 5B) confirming the abrogation of the TLR-9 gene in TLR-9−/− mice. Further, a significantly reduced IFN-γ:IL10 ratio in splenocytes from LdCen−/− immunized TLR-9−/− mice compared to WT immunized mice at 3 weeks post-immunization was observed (Figure 5C). At day 22 post-immunization, mice were challenged with virulent Leishmania donovani parasites and were monitored for 6 weeks. Age-matched naïve mice were challenged with virulent L. donovani parasites (i. v.). Significantly reduced IFN-γ: IL10 ratio in splenocytes from LdCen−/− immunized TLR-9−/− mice compared to WT immunized mice after 6 weeks post-challenge indicated a poor Th1 response (Figure 5C). Parasite burden in the spleen, as well as liver after 6 weeks of challenge with LdWT parasites, showed the absence of TLR-9 reversed LdCen−/− mediated parasite control as evidenced by substantially increased parasite burden at the 6-week post-challenge period in comparison to immunized challenged WT mice (Figure 5D,E). Thus, activation of the TLR-9 pathway is important for the LdCen−/− induced protective immunity.
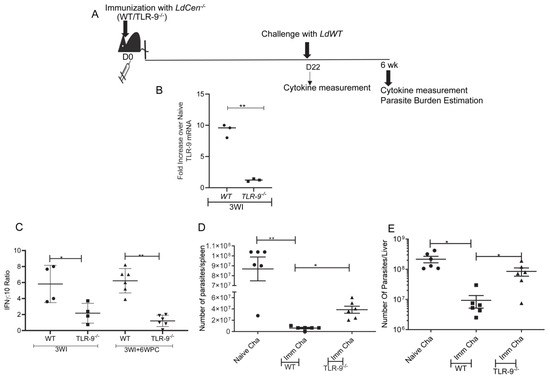
Figure 5.
Absence of TLR-9 abrogates the LdCen−/− induced host protective immunity. (A) Schematic diagram showing the treatment regimen. (B) TLR-9 mRNA expression levels in the spleens of immunized WT and TLR-9−/− mice at day 21 (3WI) was determined by qPCR as described in Materials and Methods and expressed as fold change from uninfected naive mice. The data represent the mean values ± SD of results from three independent experiments that all yielded similar results (n = 3). ** p < 0.005 between the groups. (C) Leishmania Ag–specific cytokines were measured from splenocytes of WT or TLR-9−/− LdCen−/− immunized mice at the time of challenge (3 WI, 3 wk post-immunization) and after challenge (3 WI+ 6 WPC: 6 wk post-challenge) by sandwich ELISA. The ratio of IFNγ/IL-10 is shown. The data represent the mean values ± SEM of results from two independent experiments. The mean and SEM of 4–6 mice in each group are shown. ** p < 0.005. (D) Splenic and (E) liver parasite burden were measured at 6 wk post-challenge in different groups of immunized-challenged and naive-challenged mice. The data represent the mean values ± SEM of results. The mean and SEM of 6 mice in each group are shown. * p < 0.05; ** p < 0.005 between the groups.
4. Discussion
TLRs, mostly found on phagocytes, play a crucial role in protecting the host against various pathogens [3,4,5,6,7]. During Leishmania infection, TLRs such as 2, 3, 4, 7, and 9 are essential for initiating an immunological defense response [10,11,12,49]. The addition of TLR ligands has been shown to enhance immunogenicity in experimental vaccination using soluble, heat-killed, or recombinant antigens from various Leishmania species, such as L. donovani, L. major, and L. amazonensis [39,40,41,42,43,50]. The live attenuated L. donovani parasite vaccine (LdCen−/−) without exogenous addition of TLR ligands has demonstrated efficacy in preclinical investigations, resulting in the induction of protective immunity in experimental animal models [31,32,33,34]. Therefore, we wanted to explore if LdCen−/− immunization inherently engages various TLRs towards inducing protective immunity.
Macrophages and DCs, two important components of the innate immune system, detect pathogens via TLRs. This activation is critical in initiating both the innate and acquired immune response [38]. Our previous studies have shown that after infection with LdCen−/− parasites, macrophages and DCs acquire a pro-inflammatory phenotype [28,36], which could be due to the recognition of parasite ligands by various TLRs. In this study, we analyzed the expression of TLRs 2, 4, and 9 in macrophages and DCs in response to LdCen−/− and LdWT infections and found an elevated expression of TLR-9 transcript in LdCen−/− infection compared to LdWT infection. Notably, DCs have been shown to be important in activating TLR-9 through its ligand, CpG oligodeoxynucleotides, and serve as an adjuvant for a vaccine against L. major [44]. Therefore, in the present study, we determined the function of TLR-9 and the signaling molecules that it triggers exclusively in DCs during LdCen−/− infection. We observed, upon LdCen−/− infection, enhanced expression of TLR-9 receptor on DCs stimulated NF-κB signaling protein through MyD88-dependent mechanism to produce proinflammatory cytokines such as IL-12 and TNF, which have a protective function during Leishmania infection. Further, by examining the signaling events, we determined that silencing either MyD88 or TLR-9 reduces the production of proinflammatory cytokines in DCs during LdCen−/− infection. This suggests that the generation of pro-inflammatory responses in LdCen−/−infected DCs is enabled by the activation of the MyD88 aided by TLR-9 downstream signaling. Notably, MyD88 assisted activation of TLR-9 signaling in DCs has been reported during L. infantum infection [3]. Our results also emphasize the importance of TLR-9 and NF-κB in LdCen−/− infection induced DC activation. The inactivation of either TLR-9 or NF-κB prevented the activation and maturation of DCs and the subsequent presentation of antigens to CD4+ T cells. Additionally, the considerably higher parasite load in LdCen−/− immunized TLR-9−/− animals after challenge further shows the impairment of vaccination immunity in the absence of TLR-9 signaling and highlights the importance of TLR-9 in LdCen−/− vaccine-induced immunity. Similarly, a recent study also showed that the TLR-9-binding L. amazonensis antigen vaccine LaAg comprising CpG motifs was ineffective in protecting against virulent challenges in TLR-9 deficient mice because of the lack of a protective Th1 response [51].
Studies have investigated TLR-9 expression during L. donovani infection, but the findings have been inconsistent. One study showed no variation in TLR-9 mRNA expression in splenic biopsies and PBMCs of VL patients’ before and after treatment, indicating that TLR-9 activation might be inhibited and not significant during L. donovani infection [52]. Thus, TLR-9 activation might not be essential in virulent infection but could play a crucial role in LdCen−/− vaccine-induced immunity. A separate study found a substantial increase in TLR-9 expression in whole blood samples from Sudanese VL patients. However, these results were considered inconclusive since they did not align with TLR-9′s established role in providing protection [53]. Nevertheless, it is important to note that in all these studies mentioned above, TLR-9 expression was studied during the active disease whereas our studies investigated TLR-9 expression in APCs at an early stage of infection, suggesting that there can be functional differences of TLR-9 during active disease verses in early stages of developing immune response.
Studies have shown that the detection of L. donovani CpG DNA by TLR-9 [54] can trigger a Th1-mediated immune response [55], and the anti-leishmanial effect of miltefosine in infected THP1 cells or peripheral blood mononuclear cells from VL patients has also been linked to proinflammatory responses driven by TLR-9, demonstrating that TLR-9 is necessary for parasite control [56]. Therefore, it is reasonable to suggest that the upregulation of TLR-9 mRNA expression and the consequent activation of downstream signaling in LdCen−/−infected DCs could be essential in the proinflammatory response triggered by the vaccination. This response might help to confer protection against a virulent challenge as observed in our study.
The impact of TLR-9 on infections caused by various species of Leishmania is variable and depends on the specific parasite species. For example, the activation and maturation of dendritic cells (DCs) during L. major infection is influenced by TLR-9 signaling. This leads to an increase in IFN-γ production by CD4+T cells, which in turn enhances the wound healing process during the infection. Thus, activation of DCs by TLR-9 and subsequent development of Th1 cells is a crucial factor in the overall immune response to L. major infection. [19]. Likewise, infection with L. infantum activates a protective type-I interferon response and production of IL-12 through TLR-9 activation [21]. However, L. guanenesis, which has an endogenous RNA virus, fails to activate TLR-9-Myd88, leading to increased IL-4 and IL-13 and reduced IL-12 levels, making the host more susceptible to disease [57]. Thus, the role of TLR-9 in the control of parasite infections is not unequivocal and may result in either protective or susceptibility responses depending on the Leishmania species.
Besides increased expression of TLR-9 upon LdCen−/− immunization, we observed decreased expression of TLR-2 in APCs compared to LdWT infection. This suggests that LdWT parasites may use LPG-TLR-2-dependent Th2 bias to weaken the host effector response, allowing the parasite to grow within the host, as has been shown during L. major or L. donovani infection [58,59]. Further, TLR-2 activation during L. major infection decreases the expression of TLR-9 and reduces anti-leishmanial responses [58]. Thus, reduced expression of TLR-2 in macrophages and DCs from mice infected with LdCen−/− likely led to increased expression of TLR-9. Thus, LdWT and LdCen−/− parasites manipulate DC function through different TLR signaling pathways. Even though TLR-4 is essential for causing antileishmanial activity in macrophages during experimental L. donovani infection [59], in our work, we did not detect any discernible differences in TLR-4 expression between LdWT and LdCen−/− infection. It appears likely that TLR-4-mediated activation is not necessary for vaccine-induced immunity.
Collectively, these in vitro and in vivo studies point towards a novel mechanism of TLR-9 mediated immunoprotection during LdCen−/− infection. Recent studies have hypothesized that activating TLR-9 with parasitic CpG islands might serve as possible adjuvants, especially against VL [54,60]. Further, the conventional Leishmania vaccines such as Leishmania soluble antigen, recombinant vaccines, heat-killed vaccines, and DNA vaccines require the presence of immunostimulatory TLR-9 ligand CpG-ODN to render protection against leishmaniasis [39,40,41,42,43,44,50]. In contrast, live attenuated LdCen−/− vaccine inherently elevates TLR-9 expression, eliminating the need for adjuvants and making it a more affordable option for vaccine formulation against different types of leishmaniasis. These findings have broad implications for the future development of vaccines against leishmaniasis and highlight the importance of the TLR-9 pathway in the innate immune response against this disease.
Author Contributions
Conceptualization, P.B. and H.L.N.; methodology, P.B.; software, A.S., A.A., M.K., P.K.D.; validation, P.B., N.I., S.G.; formal analysis, A.S., A.A., P.B., S.G.; investigation, P.B.; resources, H.L.N., M.K., A.S., P.K.D.; data curation, P.B., N.I.; writing—original draft preparation, P.B.; writing—review and editing, H.L.N., S.G., P.B.; visualization, P.B., N.I., P.K.D.; supervision, H.L.N.; project administration, P.B., H.L.N.; funding acquisition, H.L.N.; All authors have read and agreed to the published version of the manuscript.
Funding
Funding for these studies was provided by intramural funds of the Center for Biologics Evaluation and Research, U.S. Food and Drug Administration (FDA) for HLN. The funders had no role in the study design, the data collection and analysis, the decision to publish, or the preparation of the manuscript.
Institutional Review Board Statement
The study’s animal protocol (ASP 1995#26) received clearance from the Institutional Animal Care and Use Committee of the Center for Biologics Evaluation and Research of the Food and Drug Administration. Further, the animal protocol is in full accordance with the “Guide for the Care and Use 198 of Laboratory Animals” as described in the U.S. Public Health Service 199 Policy on Humane Care and Use of Laboratory Animals 2015. (http://grants.nih.gov/grants/olaw/references/phspolicylabanimals.pdf; accessed on 15 October 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are within the manuscript.
Acknowledgments
We thank Flow Cytometry Core, the National Heart, Lung, and Blood Institute, and the National Institutes of Health.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grifferty, G.; Shirley, H.; McGloin, J.; Kahn, J.; Orriols, A.; Wamai, R. Vulnerabilities to and the Socioeconomic and Psychosocial Impacts of the Leishmaniases: A Review. Res. Rep. Trop. Med. 2021, 12, 135–151. [Google Scholar] [CrossRef]
- Elmahallawy, E.K.; Alkhaldi, A.A.M.; Saleh, A.A. Host immune response against leishmaniasis and parasite persistence strategies: A review and assessment of recent research. Biomed. Pharmacother. 2021, 139, 111671. [Google Scholar] [CrossRef]
- Sacramento, L.; Trevelin, S.C.; Nascimento, M.S.; Lima-Junior, D.S.; Costa, D.L.; Almeida, R.P.; Cunha, F.Q.; Silva, J.S.; Carregaro, V. Toll-like receptor 9 signaling in dendritic cells regulates neutrophil recruitment to inflammatory foci following Leishmania infantum infection. Infect. Immun. 2015, 83, 4604–4616. [Google Scholar] [CrossRef] [PubMed]
- Bafica, A.; Santiago, H.C.; Goldszmid, R.; Ropert, C.; Gazzinelli, R.T.; Sher, A. Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J. Immunol. 2006, 177, 3515–3519. [Google Scholar] [CrossRef]
- Debierre-Grockiego, F.; Azzouz, N.; Schmidt, J.; Dubremetz, J.F.; Geyer, H.; Geyer, R.; Weingart, R.; Schmidt, R.R.; Schwarz, R.T. Roles of glycosylphosphatidylinositols of Toxoplasma gondii. Induction of tumor necrosis factor-alpha production in macrophages. J. Biol. Chem. 2003, 278, 32987–32993. [Google Scholar] [CrossRef]
- Shoda, L.K.; Kegerreis, K.A.; Suarez, C.E.; Roditi, I.; Corral, R.S.; Bertot, G.M.; Norimine, J.; Brown, W.C. DNA from protozoan parasites Babesia bovis, Trypanosoma cruzi, and T. brucei is mitogenic for B lymphocytes and stimulates macrophage expression of interleukin-12, tumor necrosis factor alpha, and nitric oxide. Infect. Immun. 2001, 69, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.A.; Almeida, I.C.; Takeuchi, O.; Akira, S.; Valente, E.P.; Procopio, D.O.; Travassos, L.R.; Smith, J.A.; Golenbock, D.T.; Gazzinelli, R.T. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 2001, 167, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Vijay, K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 2018, 59, 391–412. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Faria, M.S.; Reis, F.C.; Lima, A.P. Toll-like receptors in leishmania infections: Guardians or promoters? J. Parasitol. Res. 2012, 2012, 930257. [Google Scholar] [CrossRef]
- Muraille, E.; De Trez, C.; Brait, M.; De Baetselier, P.; Leo, O.; Carlier, Y. Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J. Immunol. 2003, 170, 4237–4241. [Google Scholar] [CrossRef] [PubMed]
- De Trez, C.; Brait, M.; Leo, O.; Aebischer, T.; Torrentera, F.A.; Carlier, Y.; Muraille, E. Myd88-dependent in vivo maturation of splenic dendritic cells induced by Leishmania donovani and other Leishmania species. Infect. Immun. 2004, 72, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Flandin, J.F.; Chano, F.; Descoteaux, A. RNA interference reveals a role for TLR2 and TLR3 in the recognition of Leishmania donovani promastigotes by interferon-gamma-primed macrophages. Eur. J. Immunol. 2006, 36, 411–420. [Google Scholar] [CrossRef]
- de Veer, M.J.; Curtis, J.M.; Baldwin, T.M.; DiDonato, J.A.; Sexton, A.; McConville, M.J.; Handman, E.; Schofield, L. MyD88 is essential for clearance of Leishmania major: Possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur. J. Immunol. 2003, 33, 2822–2831. [Google Scholar] [CrossRef]
- Osanya, A.; Song, E.H.; Metz, K.; Shimak, R.M.; Boggiatto, P.M.; Huffman, E.; Johnson, C.; Hostetter, J.M.; Pohl, N.L.; Petersen, C.A. Pathogen-derived oligosaccharides improve innate immune response to intracellular parasite infection. Am. J. Pathol. 2011, 179, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Bhattacharjee, S.; Gupta, G.; Majumder, S.; Adhikari, A.; Mukherjee, A.; Majumdar, S.B.; Saha, B.; Majumdar, S. Arabinosylated lipoarabinomannan-mediated protection in visceral leishmaniasis through up-regulation of toll-like receptor 2 signaling: An immunoprophylactic approach. J. Infect. Dis. 2010, 202, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Kropf, P.; Freudenberg, M.A.; Modolell, M.; Price, H.P.; Herath, S.; Antoniazi, S.; Galanos, C.; Smith, D.F.; Muller, I. Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect. Immun. 2004, 72, 1920–1928. [Google Scholar] [CrossRef]
- Kropf, P.; Freudenberg, N.; Kalis, C.; Modolell, M.; Herath, S.; Galanos, C.; Freudenberg, M.; Muller, I. Infection of C57BL/10ScCr and C57BL/10ScNCr mice with Leishmania major reveals a role for Toll-like receptor 4 in the control of parasite replication. J. Leukoc. Biol. 2004, 76, 48–57. [Google Scholar] [CrossRef]
- Abou Fakher, F.H.; Rachinel, N.; Klimczak, M.; Louis, J.; Doyen, N. TLR9-dependent activation of dendritic cells by DNA from Leishmania major favors Th1 cell development and the resolution of lesions. J. Immunol. 2009, 182, 1386–1396. [Google Scholar] [CrossRef]
- Liese, J.; Schleicher, U.; Bogdan, C. The innate immune response against Leishmania parasites. Immunobiology 2008, 213, 377–387. [Google Scholar] [CrossRef]
- Schleicher, U.; Liese, J.; Knippertz, I.; Kurzmann, C.; Hesse, A.; Heit, A.; Fischer, J.A.; Weiss, S.; Kalinke, U.; Kunz, S.; et al. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J. Exp. Med. 2007, 204, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Raman, V.S.; Duthie, M.S.; Fox, C.B.; Matlashewski, G.; Reed, S.G. Adjuvants for Leishmania vaccines: From models to clinical application. Front. Immunol. 2012, 3, 144. [Google Scholar] [CrossRef] [PubMed]
- Noorpisheh Ghadimi, S.; Farjadian, S.; Hatam, G.R.; Kalani, M.; Sarkari, B. Vaccination with Live Attenuated L. Major and TLR4 Agonist Promotes a Th1 Immune Response and Induces Protection against L. Major Infection in BALB/c Mice. Iran. J. Immunol. 2018, 15, 74–83. [Google Scholar] [PubMed]
- Saljoughian, N.; Taheri, T.; Rafati, S. Live vaccination tactics: Possible approaches for controlling visceral leishmaniasis. Front. Immunol. 2014, 5, 134. [Google Scholar] [CrossRef] [PubMed]
- Zabala-Penafiel, A.; Todd, D.; Daneshvar, H.; Burchmore, R. The potential of live attenuated vaccines against Cutaneous Leishmaniasis. Exp. Parasitol. 2020, 210, 107849. [Google Scholar] [CrossRef]
- Gannavaram, S.; Dey, R.; Avishek, K.; Selvapandiyan, A.; Salotra, P.; Nakhasi, H.L. Biomarkers of safety and immune protection for genetically modified live attenuated leishmania vaccines against visceral leishmaniasis—Discovery and implications. Front. Immunol. 2014, 5, 241. [Google Scholar] [CrossRef]
- Selvapandiyan, A.; Debrabant, A.; Duncan, R.; Muller, J.; Salotra, P.; Sreenivas, G.; Salisbury, J.L.; Nakhasi, H.L. Centrin gene disruption impairs stage-specific basal body duplication and cell cycle progression in Leishmania. J. Biol. Chem. 2004, 279, 25703–25710. [Google Scholar] [CrossRef]
- Dey, R.; Natarajan, G.; Bhattacharya, P.; Cummings, H.; Dagur, P.K.; Terrazas, C.; Selvapandiyan, A.; McCoy, J.P., Jr.; Duncan, R.; Satoskar, A.R.; et al. Characterization of cross-protection by genetically modified live-attenuated Leishmania donovani parasites against Leishmania mexicana. J. Immunol. 2014, 193, 3513–3527. [Google Scholar] [CrossRef]
- Zhang, W.W.; Karmakar, S.; Gannavaram, S.; Dey, R.; Lypaczewski, P.; Ismail, N.; Siddiqui, A.; Simonyan, V.; Oliveira, F.; Coutinho-Abreu, I.V.; et al. A second generation leishmanization vaccine with a markerless attenuated Leishmania major strain using CRISPR gene editing. Nat. Commun. 2020, 11, 3461. [Google Scholar] [CrossRef]
- Gannavaram, S.; Davey, S.; Lakhal-Naouar, I.; Duncan, R.; Nakhasi, H.L. Deletion of ubiquitin fold modifier protein Ufm1 processing peptidase Ufsp in L. donovani abolishes Ufm1 processing and alters pathogenesis. PLoS Negl. Trop. Dis. 2014, 8, e2707. [Google Scholar] [CrossRef]
- Selvapandiyan, A.; Dey, R.; Nylen, S.; Duncan, R.; Sacks, D.; Nakhasi, H.L. Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis. J. Immunol. 2009, 183, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, J.A.; Santiago Hda, C.; Selvapandiyan, A.; Gannavaram, S.; Ricci, N.D.; Bueno, L.L.; Bartholomeu, D.C.; Correa-Oliveira, R.; Nakhasi, H.L.; Fujiwara, R.T. Induction of immunogenicity by live attenuated Leishmania donovani centrin deleted parasites in dogs. Vaccine 2013, 31, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, J.A.; Gannavaram, S.; Santiago Hda, C.; Selvapandiyan, A.; Souza, D.M.; Passos, L.S.; de Mendonca, L.Z.; Lemos-Giunchetti Dda, S.; Ricci, N.D.; Bartholomeu, D.C.; et al. Vaccination using live attenuated Leishmania donovani centrin deleted parasites induces protection in dogs against Leishmania infantum. Vaccine 2015, 33, 280–288. [Google Scholar] [CrossRef]
- Fiuza, J.A.; Dey, R.; Davenport, D.; Abdeladhim, M.; Meneses, C.; Oliveira, F.; Kamhawi, S.; Valenzuela, J.G.; Gannavaram, S.; Nakhasi, H.L. Intradermal Immunization of Leishmania donovani Centrin Knock-Out Parasites in Combination with Salivary Protein LJM19 from Sand Fly Vector Induces a Durable Protective Immune Response in Hamsters. PLoS Negl. Trop. Dis. 2016, 10, e0004322. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Dey, R.; Saxena, A.; Karmakar, S.; Ismail, N.; Gannavaram, S.; Dagur, P.K.; Satoskar, M.; Satoskar, S.; De Paoli, S.; et al. Essential Role of Neutrophils in the Protective Immune Response Induced by a Live Attenuated Leishmania Vaccine. J. Immunol. 2020, 205, 3333–3347. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Dey, R.; Dagur, P.K.; Kruhlak, M.; Ismail, N.; Debrabant, A.; Joshi, A.B.; Akue, A.; Kukuruga, M.; Takeda, K.; et al. Genetically Modified Live Attenuated Leishmania donovani Parasites Induce Innate Immunity through Classical Activation of Macrophages That Direct the Th1 Response in Mice. Infect. Immun. 2015, 83, 3800–3815. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Ismail, N.; Saxena, A.; Gannavaram, S.; Dey, R.; Oljuskin, T.; Akue, A.; Takeda, K.; Yu, J.; Karmakar, S.; et al. Neutrophil-dendritic cell interaction plays an important role in live attenuated Leishmania vaccine induced immunity. PLoS Negl. Trop. Dis. 2022, 16, e0010224. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- Daifalla, N.S.; Bayih, A.G.; Gedamu, L. Leishmania donovani recombinant iron superoxide dismutase B1 protein in the presence of TLR-based adjuvants induces partial protection of BALB/c mice against Leishmania major infection. Exp. Parasitol. 2012, 131, 317–324. [Google Scholar] [CrossRef]
- Rhee, E.G.; Mendez, S.; Shah, J.A.; Wu, C.Y.; Kirman, J.R.; Turon, T.N.; Davey, D.F.; Davis, H.; Klinman, D.M.; Coler, R.N.; et al. Vaccination with heat-killed leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against leishmania major infection. J. Exp. Med. 2002, 195, 1565–1573. [Google Scholar] [CrossRef]
- Tewary, P.; Pandya, J.; Mehta, J.; Sukumaran, B.; Madhubala, R. Vaccination with Leishmania soluble antigen and immunostimulatory oligodeoxynucleotides induces specific immunity and protection against Leishmania donovani infection. FEMS Immunol. Med. Microbiol. 2004, 42, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Tewary, P.; Sukumaran, B.; Saxena, S.; Madhubala, R. Immunostimulatory oligodeoxynucleotides are potent enhancers of protective immunity in mice immunized with recombinant ORFF leishmanial antigen. Vaccine 2004, 22, 3053–3060. [Google Scholar] [CrossRef]
- Lanza, J.S.; Vucen, S.; Flynn, O.; Donadei, A.; Cojean, S.; Loiseau, P.M.; Fernandes, A.; Frezard, F.; Moore, A.C. A TLR9-adjuvanted vaccine formulated into dissolvable microneedle patches or cationic liposomes protects against leishmaniasis after skin or subcutaneous immunization. Int. J. Pharm. 2020, 586, 119390. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.A.; Darrah, P.A.; Ambrozak, D.R.; Turon, T.N.; Mendez, S.; Kirman, J.; Wu, C.Y.; Glaichenhaus, N.; Seder, R.A. Dendritic cells are responsible for the capacity of CpG oligodeoxynucleotides to act as an adjuvant for protective vaccine immunity against Leishmania major in mice. J. Exp. Med. 2003, 198, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Spath, G.F.; Beverley, S.M. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp. Parasitol. 2001, 99, 97–103. [Google Scholar] [CrossRef]
- Campos, M.B.; Lima, L.; de Lima, A.C.S.; Vasconcelos Dos Santos, T.; Ramos, P.K.S.; Gomes, C.M.C.; Silveira, F.T. Toll-like receptors 2, 4, and 9 expressions over the entire clinical and immunopathological spectrum of American cutaneous leishmaniasis due to Leishmania (V.) braziliensis and Leishmania (L.) amazonensis. PLoS ONE 2018, 13, e0194383. [Google Scholar] [CrossRef]
- Tiburcio, R.; Nunes, S.; Nunes, I.; Rosa Ampuero, M.; Silva, I.B.; Lima, R.; Machado Tavares, N.; Brodskyn, C. Molecular Aspects of Dendritic Cell Activation in Leishmaniasis: An Immunobiological View. Front. Immunol. 2019, 10, 227. [Google Scholar] [CrossRef]
- Reinhard, K.; Huber, M.; Lohoff, M.; Visekruna, A. The role of NF-kappaB activation during protection against Leishmania infection. Int. J. Med. Microbiol. 2012, 302, 230–235. [Google Scholar] [CrossRef]
- Martinez-Orellana, P.; Montserrat-Sangra, S.; Quirola-Amores, P.; Gonzalez, N.; Solano-Gallego, L. Cytokine Effect of TLR3, TLR4, and TLR7 Agonists Alone or Associated with Leishmania infantum Antigen on Blood from Dogs. BioMed Res. Int. 2018, 2018, 5693736. [Google Scholar] [CrossRef]
- Daifalla, N.S.; Bayih, A.G.; Gedamu, L. Immunogenicity of Leishmania donovani iron superoxide dismutase B1 and peroxidoxin 4 in BALB/c mice: The contribution of Toll-like receptor agonists as adjuvant. Exp. Parasitol. 2011, 129, 292–298. [Google Scholar] [CrossRef]
- Pratti, J.E.S.; da Fonseca Martins, A.M.; da Silva, J.P.; Ramos, T.D.; Pereira, J.C.; Firmino-Cruz, L.; Oliveira-Maciel, D.; Vieira, T.S.S.; Lacerda, L.L.; Vale, A.M.; et al. The role of TLR9 on Leishmania amazonensis infection and its influence on intranasal LaAg vaccine efficacy. PLoS Negl. Trop. Dis. 2019, 13, e0007146. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, O.P.; Gautam, S.; Nylen, S.; Sundar, S. Enhanced expression of Toll-like receptors 2 and 4, but not 9, in spleen tissue from patients with visceral leishmaniasis. Parasite Immunol. 2014, 36, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Babiker, D.T.; Bakhiet, S.M.; Mukhtar, M.M. Leishmania donovani influenced cytokines and Toll-like receptors expression among Sudanese visceral leishmaniasis patients. Parasite Immunol. 2015, 37, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.L.; Akhtar, S.; Waye, A.; Pandey, N.R.; Pathak, N.; Bajpai, P. Cross talk between Leishmania donovani CpG DNA and Toll-like receptor 9: An immunoinformatics approach. Biochem. Biophys. Res. Commun. 2015, 459, 424–429. [Google Scholar] [CrossRef]
- Bamigbola, I.E.; Ali, S. Paradoxical immune response in leishmaniasis: The role of toll-like receptors in disease progression. Parasite Immunol. 2022, 44, e12910. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Gupta, G.; Adhikari, A.; Majumder, S.; Kar Mahapatra, S.; Bhattacharyya Majumdar, S.; Majumdar, S. Miltefosine triggers a strong proinflammatory cytokine response during visceral leishmaniasis: Role of TLR4 and TLR9. Int. Immunopharmacol. 2012, 12, 565–572. [Google Scholar] [CrossRef]
- Ives, A.; Masina, S.; Castiglioni, P.; Prevel, F.; Revaz-Breton, M.; Hartley, M.A.; Launois, P.; Fasel, N.; Ronet, C. MyD88 and TLR9 dependent immune responses mediate resistance to Leishmania guyanensis infections, irrespective of Leishmania RNA virus burden. PLoS ONE 2014, 9, e96766. [Google Scholar] [CrossRef]
- Srivastava, S.; Pandey, S.P.; Jha, M.K.; Chandel, H.S.; Saha, B. Leishmania expressed lipophosphoglycan interacts with Toll-like receptor (TLR)-2 to decrease TLR-9 expression and reduce anti-leishmanial responses. Clin. Exp. Immunol. 2013, 172, 403–409. [Google Scholar] [CrossRef]
- Murray, H.W.; Zhang, Y.; Zhang, Y.; Raman, V.S.; Reed, S.G.; Ma, X. Regulatory actions of Toll-like receptor 2 (TLR2) and TLR4 in Leishmania donovani infection in the liver. Infect. Immun. 2013, 81, 2318–2326. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Gupta, C.L.; Bajpai, P. Impelling TLR9: Road to perspective vaccine for visceral leishmaniasis. Drug Dev. Res. 2022, 83, 222–224. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).