Leishmania Infection-Induced Proteolytic Processing of SIRPα in Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice, Cells, and Parasites
2.2. Measurement of Soluble SIRPα
2.3. Western Blotting
2.4. ADAM10 Inhibition Assay
2.5. Quantitative RT-PCR
2.6. Statistical Analysis
3. Results
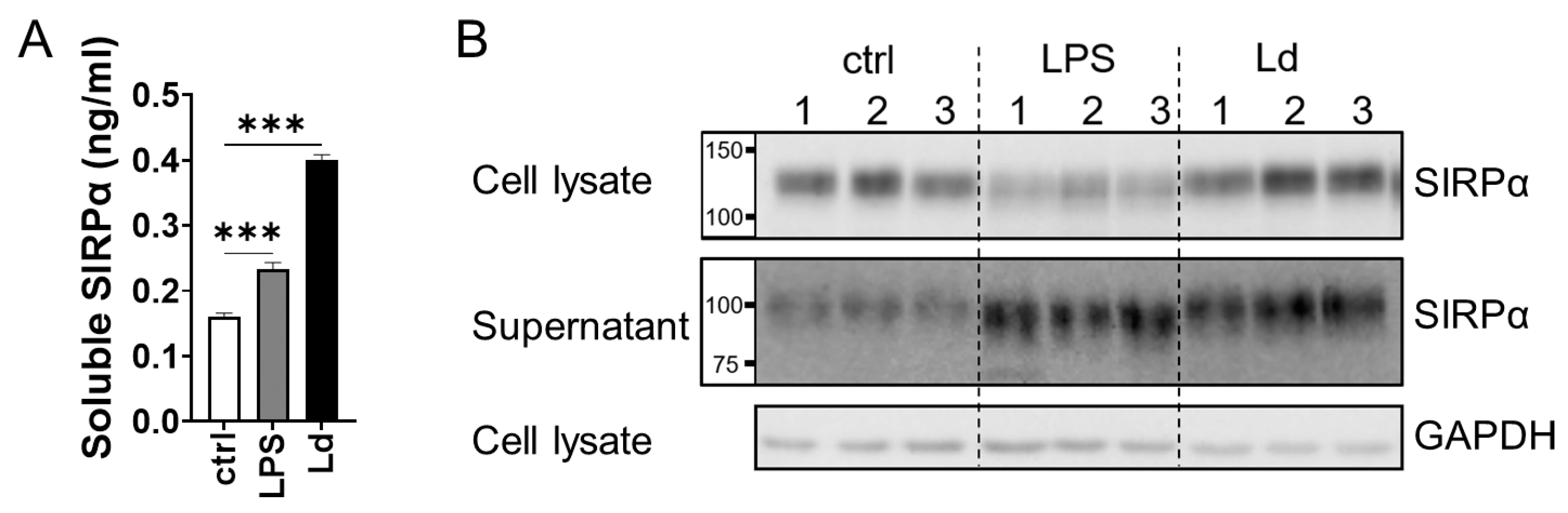
3.1. Increased Serum Soluble SIRPα in L. donovani-Infected BALB/cA Mice
3.2. Ectodomain Shedding of SIRPα in Macrophages Infected with L. donovani
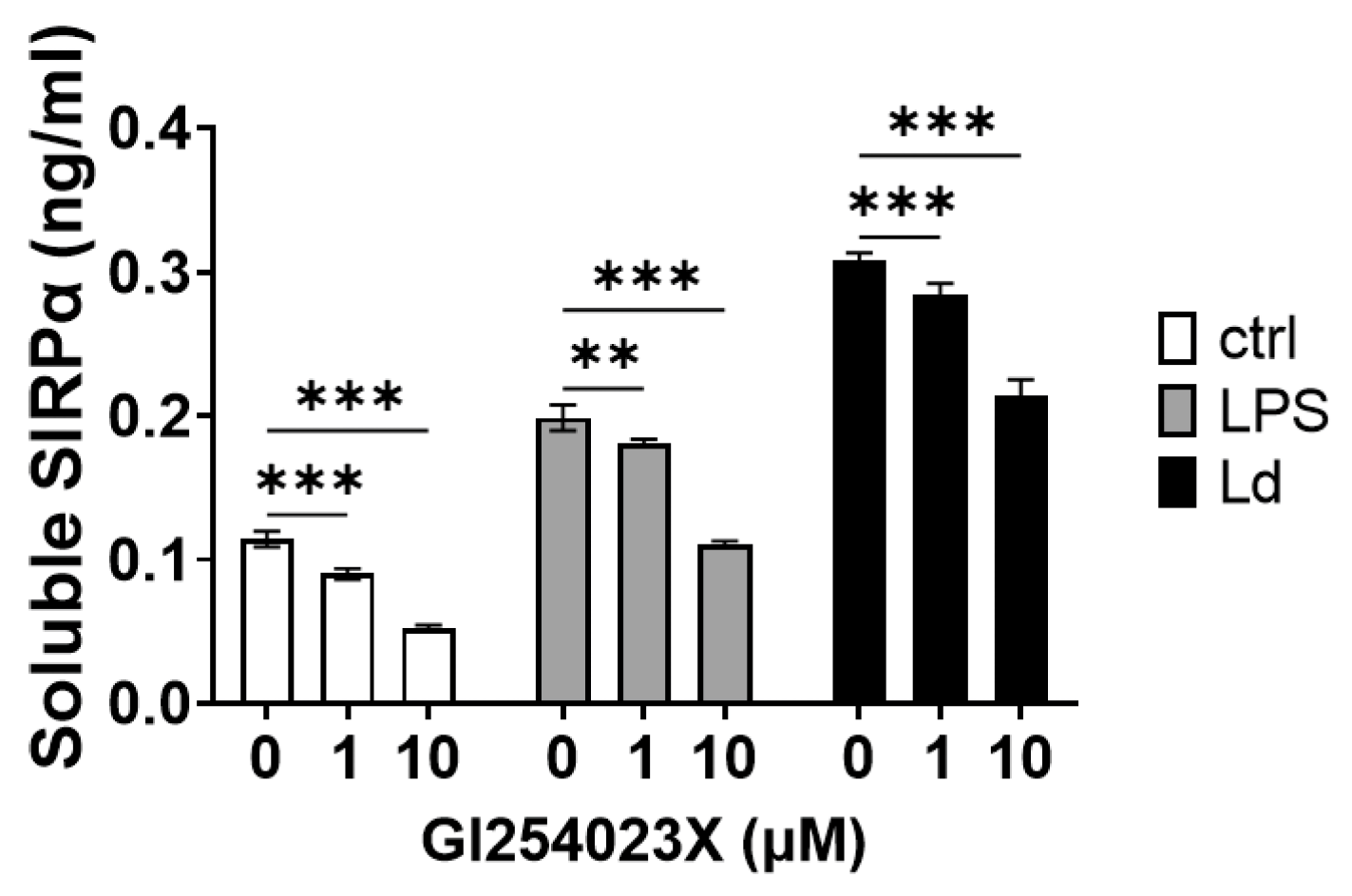
3.3. Involvement of ADAMs in SIRPα Ectodomain Shedding by L. donovani Infection
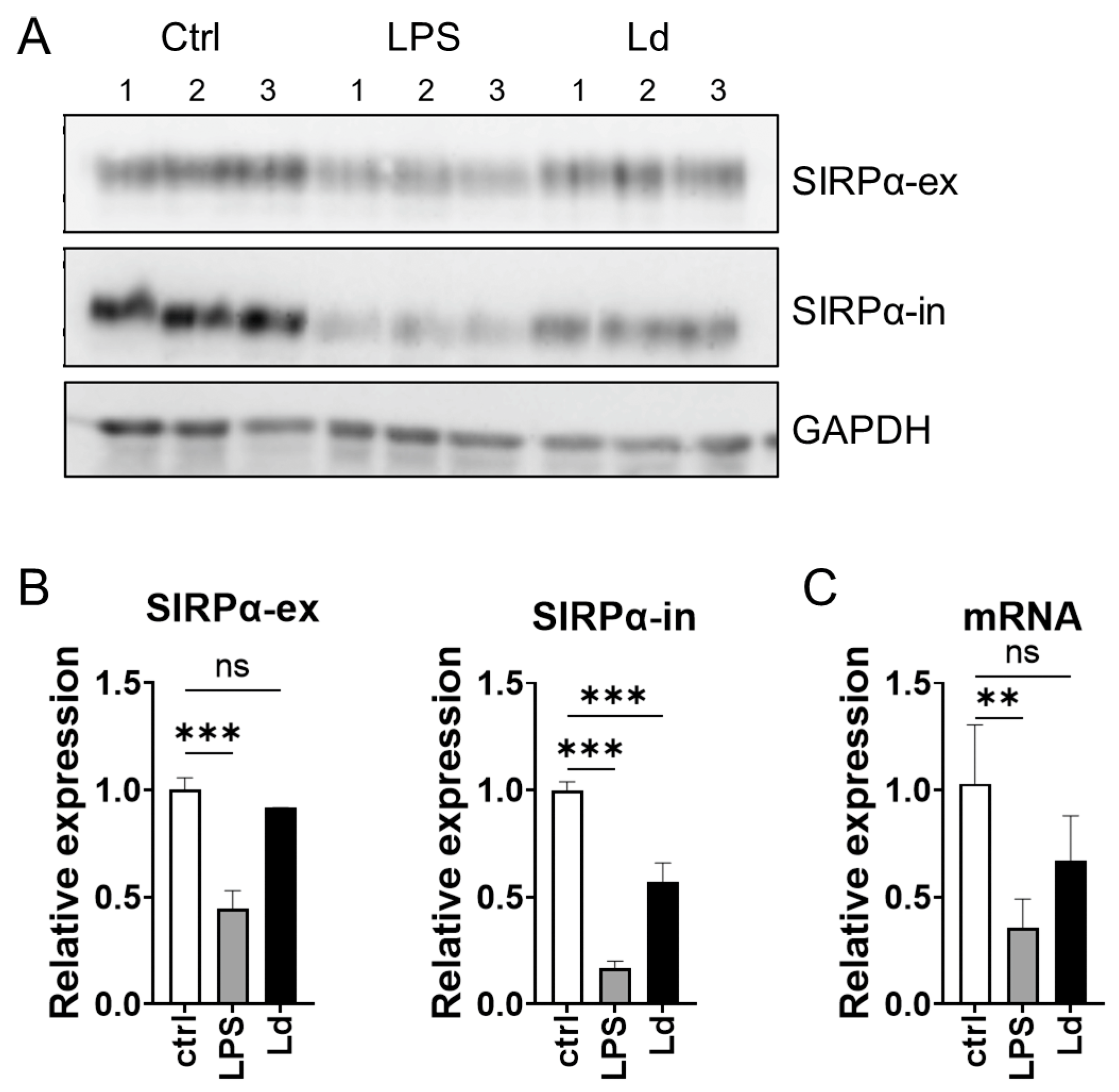
3.4. Loss of Cytoplasmic Portion of SIRPα during L. donovani Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goto, Y.; Cheng, J.; Omachi, S.; Morimoto, A. Prevalence, Severity, and Pathogeneses of Anemia in Visceral Leishmaniasis. Parasitol. Res. 2017, 116, 457–464. [Google Scholar] [CrossRef] [PubMed]
- La Gruta, N.L.; Kedzierska, K.; Stambas, J.; Doherty, P.C. A Question of Self-Preservation: Immunopathology in Influenza Virus Infection. Immunol. Cell Biol. 2007, 85, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Silva-Herzog, E.; Detweiler, C.S. Intracellular Microbes and Haemophagocytosis. Cell. Microbiol. 2008, 10, 2151–2158. [Google Scholar] [CrossRef]
- Colomba, C.; di Carlo, P.; Scarlata, F.; Iaria, C.; Barberi, G.; Famà, F.; Cama, V.; Cascio, A. Visceral Leishmaniasis, Hypertriglyceridemia and Secondary Hemophagocytic Lymphohistiocytosis. Infection 2016, 44, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Henter, J.I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and Therapeutic Guidelines for Hemophagocytic Lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef]
- Barclay, A.N.; van den Berg, T.K. The Interaction between Signal Regulatory Protein Alpha (SIRPα) and CD47: Structure, Function, and Therapeutic Target. Annu. Rev. Immunol. 2014, 32, 25–50. [Google Scholar] [CrossRef]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a Marker of Self on Red Blood Cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Oldenborg, P.A.; Gresham, H.D.; Lindberg, F.P. CD47-Signal Regulatory Protein Alpha (SIRPalpha) Regulates Fcgamma and Complement Receptor-Mediated Phagocytosis. J. Exp. Med. 2001, 193, 855–862. [Google Scholar] [CrossRef]
- Khandelwal, S.; van Rooijen, N.; Saxena, R.K. Reduced Expression of CD47 during Murine Red Blood Cell (RBC) Senescence and Its Role in RBC Clearance from the Circulation. Transfusion 2007, 47, 1725–1732. [Google Scholar] [CrossRef]
- de Back, D.Z.; Kostova, E.B.; van Kraaij, M.; van den Berg, T.K.; van Bruggen, R. Of Macrophages and Red Blood Cells; A Complex Love Story. Front. Physiol. 2014, 5, 9. [Google Scholar] [CrossRef]
- Ishikawa-Sekigami, T.; Kaneko, Y.; Okazawa, H.; Tomizawa, T.; Okajo, J.; Saito, Y.; Okuzawa, C.; Sugawara-Yokoo, M.; Nishiyama, U.; Ohnishi, H.; et al. SHPS-1 Promotes the Survival of Circulating Erythrocytes through Inhibition of Phagocytosis by Splenic Macrophages. Blood 2006, 107, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, A.; Omachi, S.; Osada, Y.; Chambers, J.K.; Uchida, K.; Sanjoba, C.; Matsumoto, Y.; Goto, Y. Hemophagocytosis in Experimental Visceral Leishmaniasis by Leishmania Donovani. PLoS Negl. Trop. Dis. 2016, 10, e0004505. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, A.; Uchida, K.; Chambers, J.K.; Sato, K.; Hong, J.; Sanjoba, C.; Matsumoto, Y.; Yamagishi, J.; Goto, Y. Hemophagocytosis Induced by Leishmania donovani Infection Is Beneficial to Parasite Survival within Macrophages. PLoS Negl. Trop. Dis. 2019, 13, e0007816. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.W.; Kong, X.N.; Yan, H.X.; Yu, L.X.; Chen, L.; Yang, W.; Liu, Q.; Huang, D.D.; Wu, M.C.; Wang, H.Y. Signal Regulatory Protein α Negatively Regulates Both TLR3 and Cytoplasmic Pathways in Type I Interferon Induction. Mol. Immunol. 2008, 45, 3025–3035. [Google Scholar] [CrossRef]
- Kong, X.N.; Yan, H.X.; Chen, L.; Dong, L.W.; Yang, W.; Liu, Q.; Yu, L.X.; Huang, D.D.; Liu, S.Q.; Liu, H.; et al. LPS-Induced down-Regulation of Signal Regulatory Protein α Contributes to Innate Immune Activation in Macrophages. J. Exp. Med. 2007, 204, 2719–2731. [Google Scholar] [CrossRef]
- Londino, J.D.; Gulick, D.; Isenberg, J.S.; Mallampalli, R.K. Cleavage of Signal Regulatory Protein α (Sirpα) Enhances Inflammatory Signaling. J. Biol. Chem. 2015, 290, 31113–31125. [Google Scholar] [CrossRef]
- Miller, M.A.; Sullivan, R.J.; Lauffenburger, D.A. Molecular Pathways: Receptor Ectodomain Shedding in Treatment, Resistance, and Monitoring of Cancer. Clin. Cancer Res. 2017, 23, 623–629. [Google Scholar] [CrossRef]
- Tajbakhsh, A.; Gheibi Hayat, S.M.; Butler, A.E.; Sahebkar, A. Effect of Soluble Cleavage Products of Important Receptors/Ligands on Efferocytosis: Their Role in Inflammatory, Autoimmune and Cardiovascular Disease. Ageing Res. Rev. 2019, 50, 43–57. [Google Scholar] [CrossRef]
- Garton, K.J. Emerging Roles for Ectodomain Shedding in the Regulation of Inflammatory Responses. J. Leukoc. Biol. 2006, 79, 1105–1116. [Google Scholar] [CrossRef]
- Levine, S.J. Mechanisms of Soluble Cytokine Receptor Generation. J. Immunol. 2004, 173, 5343–5348. [Google Scholar] [CrossRef]
- Toth, A.B.; Terauchi, A.; Zhang, L.Y.; Johnson-Venkatesh, E.M.; Larsen, D.J.; Sutton, M.A.; Umemori, H. Synapse Maturation by Activity-Dependent Ectodomain Shedding of SIRPα. Nat. Neurosci. 2013, 16, 1417–1425. [Google Scholar] [CrossRef]
- Umemori, H.; Sanes, J.R. Signal Regulatory Proteins (SIRPS) Are Secreted Presynaptic Organizing Molecules. J. Biol. Chem. 2008, 283, 34053–34061. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zhao, L.; Pan, L.; Li, D.; Chen, G.; Chen, Z.; Jiang, Z. Soluble SIRP-Alpha Promotes Murine Acute Lung Injury through Suppressing Macrophage Phagocytosis. Front. Immunol. 2022, 13, 865579. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yan, X.Q.; Yang, F.; Yang, X.W.; Jiang, X.; Zhao, X.C.; Zhu, B.K.; Liu, L.; Qin, H.Y.; Liang, Y.M.; et al. Soluble Extracellular Domains of Human SIRPα and CD47 Expressed in Escherichia coli Enhances the Phagocytosis of Leukemia Cells by Macrophages in Vitro. Protein Expr. Purif. 2012, 85, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, H.; Kobayashi, H.; Okazawa, H.; Ohe, Y.; Tomizawa, K.; Sato, R.; Matozaki, T. Ectodomain Shedding of SHPS-1 and Its Role in Regulation of Cell Migration. J. Biol. Chem. 2004, 279, 27878–27887. [Google Scholar] [CrossRef]
- Vladimirova, Y.V.; Mølmer, M.K.; Antonsen, K.W.; Møller, N.; Rittig, N.; Nielsen, M.C.; Møller, H.J. A New Serum Macrophage Checkpoint Biomarker for Innate Immunotherapy: Soluble Signal-Regulatory Protein Alpha (SSIRPα). Biomolecules 2022, 12, 937. [Google Scholar] [CrossRef]
- Cara-Fuentes, G.; Andres-Hernando, A.; Bauer, C.; Banks, M.; Garcia, G.E.; Cicerchi, C.; Kuwabara, M.; Shimada, M.; Johnson, R.J.; Lanaspa, M.A. Pulmonary Surfactants and the Respiratory-Renal Connection in Steroid-Sensitive Nephrotic Syndrome of Childhood. iScience 2022, 25, 104694. [Google Scholar] [CrossRef]
- Thomas, S.S.; Wu, J.; Davogustto, G.; Holliday, M.W.; Eckel-Mahan, K.; Verzola, D.; Garibotto, G.; Hu, Z.; Mitch, W.E.; Taegtmeyer, H. SIRPα Mediates IGF1 Receptor in Cardiomyopathy Induced by Chronic Kidney Disease. Circ. Res. 2022, 131, 207–221. [Google Scholar] [CrossRef]
- Wu, J.; Dong, J.; Verzola, D.; Hruska, K.; Garibotto, G.; Hu, Z.; Mitch, W.E.; Thomas, S.S. Signal Regulatory Protein Alpha Initiates Cachexia through Muscle to Adipose Tissue Crosstalk. J. Cachexia Sarcopenia Muscle 2019, 10, 1210–1227. [Google Scholar] [CrossRef]
- Pandey, K.; Yanagi, T.; Pandey, B.D.; Mallik, A.K.; Sherchand, J.B.; Kanbara, H. Characterization of Leishmania Isolates from Nepalese Patients with Visceral Leishmaniasis. Parasitol. Res. 2007, 100, 1361–1369. [Google Scholar] [CrossRef]
- Shirakabe, K.; Omura, T.; Shibagaki, Y.; Mihara, E.; Homma, K.; Kato, Y.; Yoshimura, A.; Murakami, Y.; Takagi, J.; Hattori, S.; et al. Mechanistic Insights into Ectodomain Shedding: Susceptibility of CADM1 Adhesion Molecule Is Determined by Alternative Splicing and O-Glycosylation. Sci. Rep. 2017, 7, 46174. [Google Scholar] [CrossRef] [PubMed]
- Zen, K.; Guo, Y.; Bian, Z.; Lv, Z.; Zhu, D.; Ohnishi, H.; Matozaki, T.; Liu, Y. Inflammation-Induced Proteolytic Processing of the SIRPα Cytoplasmic ITIM in Neutrophils Propagates a Proinflammatory State. Nat. Commun. 2013, 4, 2436. [Google Scholar] [CrossRef] [PubMed]
- Hundhausen, C.; Misztela, D.; Berkhout, T.A.; Broadway, N.; Saftig, P.; Reiss, K.; Hartmann, D.; Fahrenholz, F.; Postina, R.; Matthews, V.; et al. The Disintegrin-like Metalloproteinase ADAM10 Is Involved in Constitutive Cleavage of CX3CL1 (Fractalkine) and Regulates CX3CL1-Mediated Cell-Cell Adhesion. Blood 2003, 102, 1186–1195. [Google Scholar] [CrossRef]
- Kidder, K.; Bian, Z.; Shi, L.; Liu, Y. Inflammation Unrestrained by SIRPα Induces Secondary Hemophagocytic Lymphohistiocytosis Independent of IFN-γ. J. Immunol. 2020, 205, 2821–2833. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Shi, L.; Guo, Y.-L.; Lv, Z.; Tang, C.; Niu, S.; Tremblay, A.; Venkataramani, M.; Culpepper, C.; Li, L.; et al. Cd47-Sirpα Interaction and IL-10 Constrain Inflammation-Induced Macrophage Phagocytosis of Healthy Self-Cells. Proc. Natl. Acad. Sci. USA 2016, 113, E5434–E5443. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward | Reverse |
|---|---|---|
| Sirpa | TCGAGTGATCAAGGGAGCAT | CCTGGACACTAGCATACTCTGAG |
| Actb | GTTACCAACTGGGACGACA | TGGCCATCTCCTGCTCGAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirai, H.; Hong, J.; Fujii, W.; Sanjoba, C.; Goto, Y. Leishmania Infection-Induced Proteolytic Processing of SIRPα in Macrophages. Pathogens 2023, 12, 593. https://doi.org/10.3390/pathogens12040593
Hirai H, Hong J, Fujii W, Sanjoba C, Goto Y. Leishmania Infection-Induced Proteolytic Processing of SIRPα in Macrophages. Pathogens. 2023; 12(4):593. https://doi.org/10.3390/pathogens12040593
Chicago/Turabian StyleHirai, Hana, Jing Hong, Wataru Fujii, Chizu Sanjoba, and Yasuyuki Goto. 2023. "Leishmania Infection-Induced Proteolytic Processing of SIRPα in Macrophages" Pathogens 12, no. 4: 593. https://doi.org/10.3390/pathogens12040593
APA StyleHirai, H., Hong, J., Fujii, W., Sanjoba, C., & Goto, Y. (2023). Leishmania Infection-Induced Proteolytic Processing of SIRPα in Macrophages. Pathogens, 12(4), 593. https://doi.org/10.3390/pathogens12040593









