Epidemiology and Economic Cost Analysis of Microbial Keratitis from a Tertiary Referral Hospital in Australia
Abstract
1. Introduction
2. Materials and Methods
2.1. Cost Calculation
2.2. Statistical Analysis
3. Results
3.1. Demographics
3.2. Microbiological Profile
3.3. Clinical Outcomes
3.4. Economic Burden
3.5. Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeng, B.H.; McLeod, S.D. Microbial keratitis. Br. J. Ophthalmol. 2003, 87, 805–806. [Google Scholar] [CrossRef]
- Robaei, D.; Watson, S. Corneal blindness: A global problem. Clin. Exp. Ophthalmol. 2014, 42, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Lakhundi, S.; Siddiqui, R.; Khan, N.A. Pathogenesis of microbial keratitis. Microb. Pathog. 2017, 104, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Khoo, P.; Cabrera-Aguas, M.; Robaei, D.; Lahra, M.M.; Watson, S. Microbial Keratitis and Ocular Surface Disease: A 5-Year Study of the Microbiology, Risk Factors and Clinical Outcomes in Sydney, Australia. Curr. Eye Res. 2019, 44, 1195–1202. [Google Scholar] [CrossRef]
- Willcox, M.D.P.; Holden, B.A. Contact Lens Related Corneal Infections. Biosci. Rep. 2001, 21, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Eagle, R.C. The eye and ocular adnexa. In Silverberg’s Principles and Practice of Surgical Pathology and Cytopathology, 5th ed.; Stelow, E.B., Pfeifer, J.D., Wick, M.R., Wakely, J.P.E., LiVolsi, V.A., Eds.; Cambridge University Press: Cambridge, UK, 2015; pp. 3126–3184. [Google Scholar] [CrossRef]
- Al-Mujaini, A.; Al-Kharusi, N.; Thakral, A.; Wali, U. Bacterial Keratitis: Perspective on Epidemiology, Clinico-Pathogenesis, Diagnosis and Treatment. Sultan Qaboos Univ. Med. J. 2009, 9, 184–195. [Google Scholar] [PubMed]
- Cabrera-Aguas, M.; Khoo, P.; George, C.R.R.; Lahra, M.M.; Watson, S.L. Antimicrobial resistance trends in bacterial keratitis over 5 years in Sydney, Australia. Clin. Exp. Ophthalmol. 2020, 48, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Robaei, D.; Carnt, N.; Watson, S. Established and emerging ancillary techniques in management of microbial keratitis: A review. Br. J. Ophthalmol. 2016, 100, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.; Cairns, J.; Gopal, B.; Ho, C.; Krstic, L.; Elsahn, A.; Lister, M.; Said, D.; Dua, H. Risk Factors, Clinical Outcomes, and Prognostic Factors of Bacterial Keratitis: The Nottingham Infectious Keratitis Study. Front. Med. 2021, 8, 715118. [Google Scholar] [CrossRef]
- Watson, S.; Cabrera-Aguas, M.; Khoo, P.; Pratama, R.; Gatus, B.J.; Gulholm, T.; El-Nasser, J.; Lahra, M.M. Keratitis antimicrobial resistance surveillance program, Sydney, Australia: 2016 Annual Report. Clin. Exp. Ophthalmol. 2019, 47, 20–25. [Google Scholar] [CrossRef]
- Green, M.; Carnt, N.; Apel, A.; Stapleton, F. Queensland Microbial Keratitis Database: 2005–2015. Br. J. Ophthalmol. 2019, 103, 1481. [Google Scholar] [CrossRef]
- Koh, Y.-Y.; Sun, C.-C.; Hsiao, C.-H. Epidemiology and the Estimated Burden of Microbial Keratitis on the Health Care System in Taiwan: A 14-Year Population-Based Study. Am. J. Ophthalmol. 2020, 220, 152–159. [Google Scholar] [CrossRef]
- Ashfaq, H.; Maganti, N.; Ballouz, D.; Feng, Y.; Woodward, M.A. Procedures, Visits, and Procedure Costs in the Management of Microbial Keratitis. Cornea 2021, 40, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.A.; Gronostaj, M.P.; MacGurn, A.K.; Cope, J.R.; Awsumb, K.L.; Yoder, J.S.; Beach, M.J. Estimated burden of keratitis—United States, 2010. Morb. Mortal. Wkly. Rep. 2014, 63, 1027–1030. [Google Scholar]
- Moussa, G.; Hodson, J.; Gooch, N.; Virdee, J.; Penaloza, C.; Kigozi, J.; Rauz, S. Calculating the economic burden of presumed microbial keratitis admissions at a tertiary referral centre in the UK. Eye 2021, 35, 2146–2154. [Google Scholar] [CrossRef]
- Radhakrishnan, N.; Pathak, N.; Raja Subramanian, K.; Das, D.R.; Ningombam, R.; Khaitan, I.; Gandhi, N.; Rahul, R.; Prajna, N.V. Comparative study on costs incurred for treatment of patients with bacterial and fungal keratitis—A retrospective analysis. Indian J. Ophthalmol. 2022, 70, 1191–1195. [Google Scholar] [CrossRef]
- Lee, J.W.Y.; Lai, J.S.M.; Yick, D.W.F.; Tse, R.K.K. Retrospective case series on the long-term visual and intraocular pressure outcomes of phacomorphic glaucoma. Eye 2010, 24, 1675–1680. [Google Scholar] [CrossRef]
- Cabrera-Aguas, M.; Khoo, P.; George, C.R.R.; Lahra, M.M.; Watson, S.L. Predisposing factors, microbiological features and outcomes of patients with clinical presumed concomitant microbial and herpes simplex keratitis. Eye 2022, 36, 86–94. [Google Scholar] [CrossRef]
- Independent Hospital Pricing Authority. Australian Hospital Patient Costing Standards. Part 1: Standards; Independent Hospital Pricing Authority: Sydney, Australia, 2021. [Google Scholar]
- Independent Hospital Pricing Authority. National Hospital Cost Data Collection Report Public Sector, Round 24 (Financial Year 2019–2020); Independent Hospital Pricing Authority: Sydney, Australia, 2021. [Google Scholar]
- Lee, B.W.H.; Hunter, D.; Robaei, D.S.; Samarawickrama, C. Open globe injuries: Epidemiology, visual and surgical predictive variables, prognostic models, and economic cost analysis. Clin. Exp. Ophthalmol. 2021, 49, 336–346. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Average Weekly Earnings, Australia. Available online: https://www.abs.gov.au/media-centre/media-releases/average-full-time-earnings-21-cent-over-2021#:~:text=Average%20weekly%20ordinary%20time%20earnings,Bureau%20of%20Statistics%20(ABS) (accessed on 17 August 2022).
- Australian Bureau of Statistics. Income and Work. Available online: https://www.abs.gov.au/statistics/labour/earnings-and-working-conditions/income-and-work-census/2021 (accessed on 31 August 2022).
- Health Policy Analysis. Australian Emergency Care Classification Final Report; Independent Hospital Pricing Authority: Sydney, Australia, 2019. [Google Scholar]
- Health Policy Analysis. Australian Emergency Care Classification: Definitions Manual; Independent Hospital Pricing Authority: Sydney, Australia, 2019. [Google Scholar]
- Independent Hospital Pricing Authority. Australian Hospital Patient Costing Standards. Part 3: Costing Guidelines—Version 4.1; Independent Hospital Pricing Authority: Sydney, Australia, 2021. [Google Scholar]
- Independent Hospital Pricing Authority. Tier 2 Outpatient Clinic Definitions, 1.2 ed.; Independent Hospital Pricing Authority: Sydney, Australia, 2012. [Google Scholar]
- Department of Health and Aged Care. Medicare Benefits Schedule—Item 104. Available online: http://www9.health.gov.au/mbs/fullDisplay.cfm?type=item&q=104&qt=item (accessed on 17 August 2022).
- Fong, L.P.; Fong, L.P. Eye injuries in Victoria, Australia. Med. J. Aust. 1995, 162, 64–68. [Google Scholar] [CrossRef]
- Bharathi, M.J.; Ramakrishnan, R.; Meenakshi, R.; Padmavathy, S.; Shivakumar, C.; Srinivasan, M. Microbial Keratitis in South India: Influence of Risk Factors, Climate, and Geographical Variation. Ophthalmic Epidemiol. 2007, 14, 61–69. [Google Scholar] [CrossRef]
- Tan, S.Z.; Walkden, A.; Au, L.; Fullwood, C.; Hamilton, A.; Qamruddin, A.; Armstrong, M.; Brahma, A.K.; Carley, F. Twelve-year analysis of microbial keratitis trends at a UK tertiary hospital. Eye 2017, 31, 1229–1236. [Google Scholar] [CrossRef]
- Mun, Y.; Kim, M.K.; Oh, J.Y. Ten-year analysis of microbiological profile and antibiotic sensitivity for bacterial keratitis in Korea. PLoS ONE 2019, 14, e0213103. [Google Scholar] [CrossRef] [PubMed]
- Leibovitch, I.; Lai, T.F.; Senarath, L.; Hsuan, J.; Selva, D. Infectious Keratitis in South Australia: Emerging Resistance to Cephazolin. Eur. J. Ophthalmol. 2005, 15, 23–26. [Google Scholar] [CrossRef]
- Cabrera-Aguas, M.; Khoo, P.; Watson, S.L. Outcomes of Microbial Keratitis Cases Resistant to Antimicrobials in Sydney, Australia. Cornea 2022, 41, 572–578. [Google Scholar] [CrossRef]
- Samarawickrama, C.; Chan, E.; Daniell, M. Rising fluoroquinolone resistance rates in corneal isolates: Implications for the wider use of antibiotics within the community. Healthc. Infect. 2015, 20, 128–133. [Google Scholar] [CrossRef]
- Chandra, V.; Chan, E.; Cabrera-Aguas, M.; Bloch, A.; Waters, M.J.; Daniell, M.; Watson, S.L. Organisms causing microbial keratitis and antibiotic resistance patterns in Australia. Clin. Amp. Exp. Ophthalmol. 2021, 49, 1111–1113. [Google Scholar] [CrossRef]
- Cheng, A.C.; Turnidge, J.; Collignon, P.; Looke, D.; Barton, M.; Gottlieb, T. Control of Fluoroquinolone Resistance through Successful Regulation, Australia. Emerg. Infect. Dis. 2012, 18, 1453–1460. [Google Scholar] [CrossRef]
- Srinivasan, M.; Mascarenhas, J.; Rajaraman, R.; Ravindran, M.; Lalitha, P.; O’Brien, K.S.; Glidden, D.V.; Ray, K.J.; Oldenburg, C.E.; Zegans, M.E.; et al. The Steroids for Corneal Ulcers Trial (SCUT): Secondary 12-Month Clinical Outcomes of a Randomized Controlled Trial. Am. J. Ophthalmol. 2014, 157, 327–333.e323. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the Management of Infectious Keratitis. Ophthalmology 2017, 124, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.T.; Tran, T.; Lim, L.L.; Samarawickrama, C.; Arnold, J.; Gillies, M.; Catt, C.; Mitchell, L.; Symons, A.; Buttery, R.; et al. Local delivery of corticosteroids in clinical ophthalmology: A review. Clin. Amp. Exp. Ophthalmol. 2020, 48, 366–401. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Hughes, I.; Hogden, J.; Sara, S.; Apel, A.; Stapleton, F. High-Dose Steroid Treatment of Bacterial Keratitis. Cornea 2019, 38, 135–140. [Google Scholar] [CrossRef]
- Herretes, S.; Wang, X.; Reyes, J.M.G. Topical corticosteroids as adjunctive therapy for bacterial keratitis. Cochrane Database Syst. Rev. 2014, 10, CD005430. [Google Scholar] [CrossRef] [PubMed]
- Khoo, P.; Cabrera-Aguas, M.; Watson, S.L. Topical Steroids as Adjunctive Therapy for Bacterial Keratitis: Evidence From a Retrospective Case Series of 313 Cases. Asia-Pac. J. Ophthalmol. 2020, 9, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Keay, L.; Edwards, K.; Dart, J.; Stapleton, F. Grading Contact Lens-related Microbial Keratitis: Relevance to Disease Burden. Optom. Vis. Sci. 2008, 85, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Keay, L.; Edwards, K.; Naduvilath, T.; Taylor, H.R.; Snibson, G.R.; Forde, K.; Stapleton, F. Microbial Keratitis: Predisposing Factors and Morbidity. Ophthalmology 2006, 113, 109–116. [Google Scholar] [CrossRef]
| Demographics | |
| Age (mean ± SD, range years) | 57.6 ± 24.6, range 5–101 |
| Female, n (%) | 68 (42.5%) |
| Admission, n (%) | 95 (59.3%) |
| Median length of admission (days) (IQR) | 7 (4–12) |
| Clinical Features at Presentation | |
| Initial median VA logMAR; Snellen equivalent, logMAR (IQR) | 1.00; 20/200 (0.18–2.0) |
| ED 1 present (%) | 139 (86.9%) |
| ED 1 size, median, mm2 | 4.3 |
| Infiltrate present (%) | 119 (74.4%) |
| Hypopyon present (%) | 44 (27.5%) |
| Corneal thinning present (%) | 61 (38.1%) |
| Final median VA; Snellen equivalent, logMAR (IQR) | 0.48, ~20/40 (0.04–1.7) |
| Risk Factors | |
| Existing corneal disease | 25 (15.6%) |
| Existing external eye disease | 25 (15.6%) |
| Previous keratitis | 24 (15%) |
| Contact lens use | 20 (12.5%) |
| Foreign body | 13 (8.1%) |
| Ocular Trauma | 6 (3.8%) |
| Name of Organism | N (%) |
|---|---|
| Gram positive | 55/94 (58.5) |
| Staphylococcus aureus (incl. MRSA 1) | 18 (15.1) |
| Streptococcus pneumoniae | 10 (8.4) |
| Propionibacterium acnes | 9 (7.6) |
| Coagulase-negative staphylococci | 6 (5.0) |
| Corynebacterium macginleyi | 4 (3.4) |
| Other Gram-positive: Streptococcus sanguinis/milleri/dysgalactiae/viridans, Actinomyces spp., Bacillus spp., Staphylococcus lugdunensis, Enterococcus faecalis | 8 (6.7) |
| Gram Negative | 39/94 (41.5) |
| Pseudomonas aeruginosa | 17 (14.3) |
| Moraxella spp. | 11 (9.2) |
| Serratia marcescens | 4 (3.4) |
| Other Gram-negative: Enterobacter cloacae, Proteus mirabillis, Escherichia coli, Pseudomonas oryzihabitans, Haemophilus influenzae, Morganella morganii, Stenotrophomonas maltophilia | 7 (5.9) |
| Fungi | |
| Candida parapsilosis, Exserohilium, Aspergilus ruber, Fusarium spp. | 4 (3.4) |
| Virus | |
| Herpes Simplex Virus | 19 (16.0) |
| Varicella Zoster Virus | 2 (1.7) |
| Total | 119 (100) |
| Organism | No. of Isolates | Sensitivity | ||||
|---|---|---|---|---|---|---|
| Ciprofloxacin | Flucloxacillin | Penicillin * | Vancomycin | Gentamicin | ||
| Gram positive | 37 | 3/3 (100%) | 13/18 (72%) | 15/25 (60%) | 9/9 (100%) | 1/1 (100%) |
| MSSA 1 | 13 | 1/1 (100%) | 12/12 (100%) | 1/5 (20%) | - | - |
| MRSA 2 | 5 | - | 0/5 (0%) | 0/5 (0%) | 5/5 (100%) | - |
| Staphylococcus lugdunensis | 1 | - | 1/1 (100%) | - | - | - |
| Streptococcus pneumoniae | 10 | - | - | 9/9 (100%) | - | - |
| Streptococcus milleri | 1 | - | - | 1/1 (100%) | - | - |
| Streptococcus dysgalactiae | 1 | - | - | 1/1 (100%) | - | - |
| Corynebacterium macginleyi | 4 | 1/1 (100%) | - | 1/2 (50%) | 2/2 (100%) | - |
| Bacillus spp. | 1 | 1/1 (100%) | - | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) |
| Enterococcus faecalis | 1 | - | - | 1/1 (100%) | 1/1 (100%) | - |
| Gram negative | 38 | 26/27 (96%) | - | 1/14 (7%) | - | 25/26 (96%) |
| Pseudonomas spp. | 18 | 17/17 (100%) | - | - | - | 18/18 (100%) |
| Moraxella spp. | 11 | 2/2 (100%) | - | 0/7 (0%) | - | - |
| Proteus mirabilis | 1 | - | - | 1/1 (100%) | - | 1/1 (100%) |
| Serratia marcescens | 4 | 4/4 (100%) | - | 0/4 (0%) | - | 4/4 (100%) |
| Enterobacter cloacae | 1 | 1/1 (100%) | - | - | - | 1/1 (100%) |
| Escherichia coli | 1 | 1/1 (100%) | - | 0/1 (0%) | - | 1/1 (100%) |
| Morganella morganii | 1 | 0/1 (0%) | - | 0/1 (0%) | - | 0/1 (0%) |
| Haemophilus influenzae | 1 | 1/1 (100%) | - | - | - | - |
| Inpatient Treatment Costs AUD (USD) | Outpatient Treatment Costs AUD (USD) | |||
|---|---|---|---|---|
| Median | Total | Median | Total | |
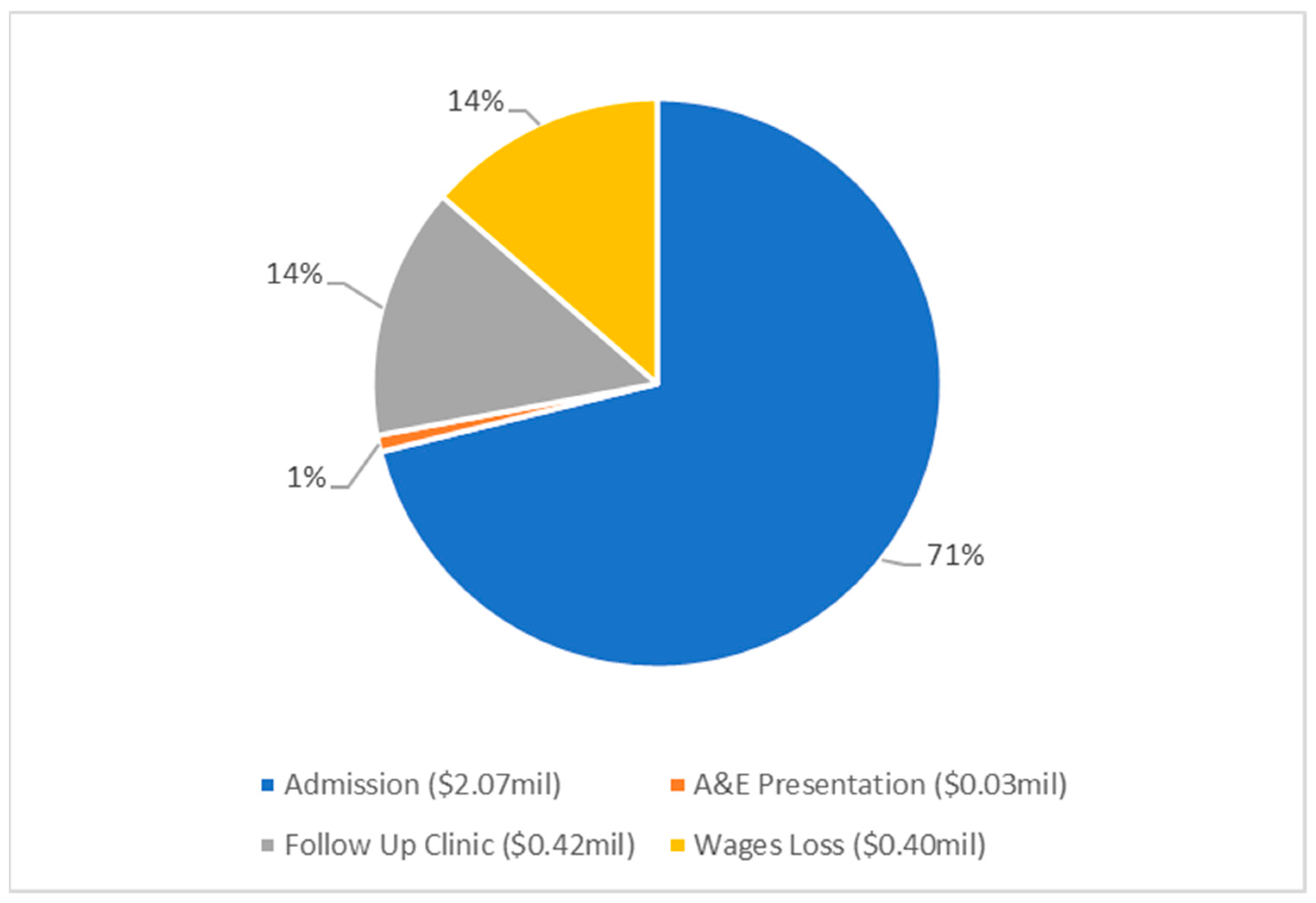
| Admission | 11,998 (8159) | 2,069,386 (1,407,182) | - | - |
| A&E presentation | - | - | 388 (264) | 27,252 (18,531) |
| Follow Up Clinics | 2079 (1414) | 297,123 (202,044) | 1485 (1010) | 119,721 (81,410) |
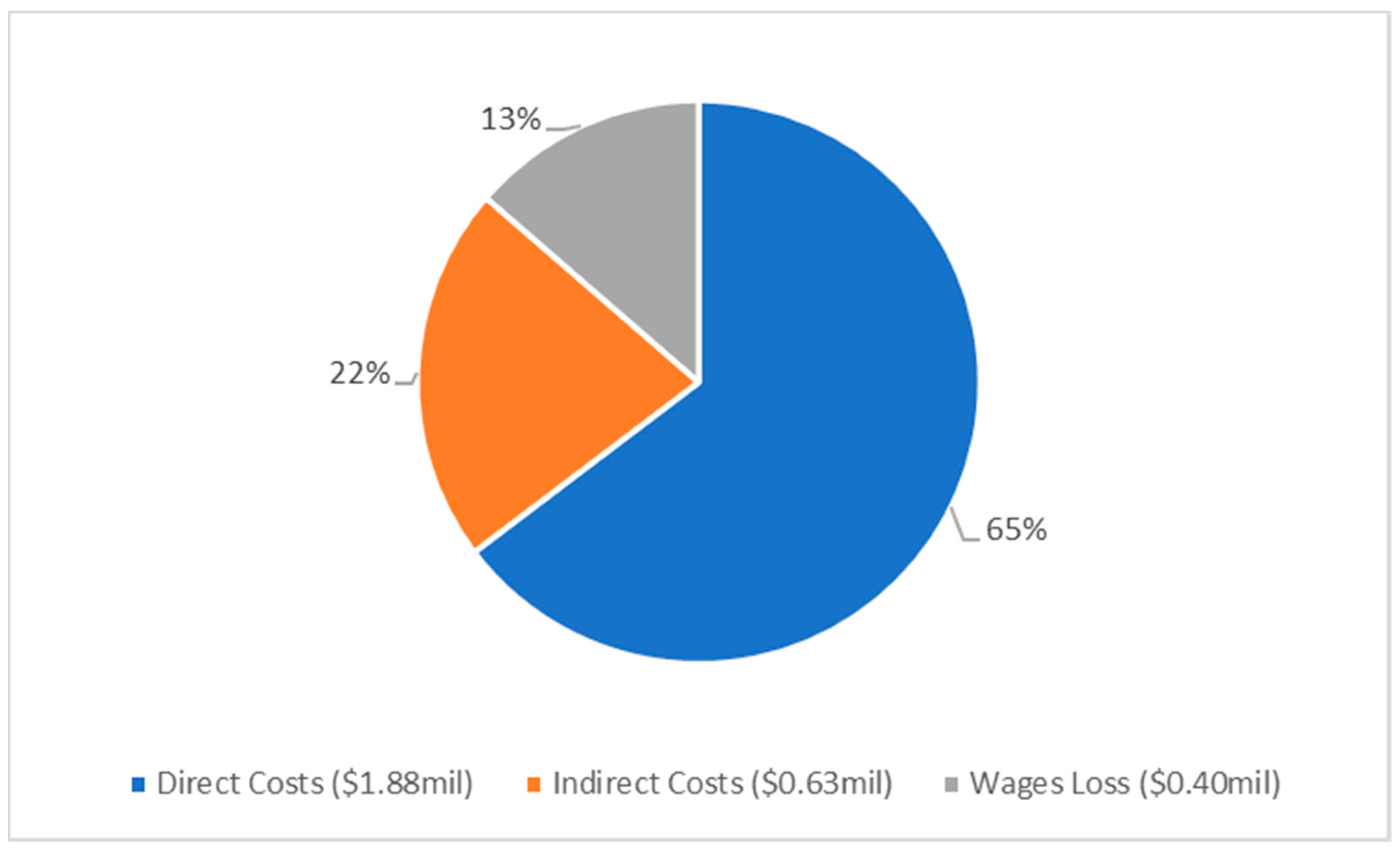
| Wages Loss | 2576 (1752) | 318,941 (216,880) | 966 (657) | 76,314 (51,894) |
| Total Costs Direct | 11,075 (7531) | 1766,130 (1200,968) | 1415 (962) | 111,793 (76,019) |
| Total Costs Indirect | 6810 (4631) | 919,320 (625,138) | 1419 (965) | 111,495 (75,817) |
| Total Costs | 18,003 (12,242) | 2685,450 (1,826,106) | 2839 (1931) | 223,288 (151,836) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daley, J.R.; Lee, M.K.; Wang, X.; Ly, M.; Samarawickrama, C. Epidemiology and Economic Cost Analysis of Microbial Keratitis from a Tertiary Referral Hospital in Australia. Pathogens 2023, 12, 413. https://doi.org/10.3390/pathogens12030413
Daley JR, Lee MK, Wang X, Ly M, Samarawickrama C. Epidemiology and Economic Cost Analysis of Microbial Keratitis from a Tertiary Referral Hospital in Australia. Pathogens. 2023; 12(3):413. https://doi.org/10.3390/pathogens12030413
Chicago/Turabian StyleDaley, Jason Richard, Matthew Kyu Lee, Xingdi Wang, Matin Ly, and Chameen Samarawickrama. 2023. "Epidemiology and Economic Cost Analysis of Microbial Keratitis from a Tertiary Referral Hospital in Australia" Pathogens 12, no. 3: 413. https://doi.org/10.3390/pathogens12030413
APA StyleDaley, J. R., Lee, M. K., Wang, X., Ly, M., & Samarawickrama, C. (2023). Epidemiology and Economic Cost Analysis of Microbial Keratitis from a Tertiary Referral Hospital in Australia. Pathogens, 12(3), 413. https://doi.org/10.3390/pathogens12030413





