Pathological Characteristics of Domestic Pigs Orally Infected with the Virus Strain Causing the First Reported African Swine Fever Outbreaks in Vietnam
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Virus Strain
2.3. Animals
2.4. Sample Collection
2.5. DNA Extraction and Real-Time PCR
2.6. Scoring of ASF Symptoms and Gross Pathology Findings
2.7. Statistical Analysis
3. Results
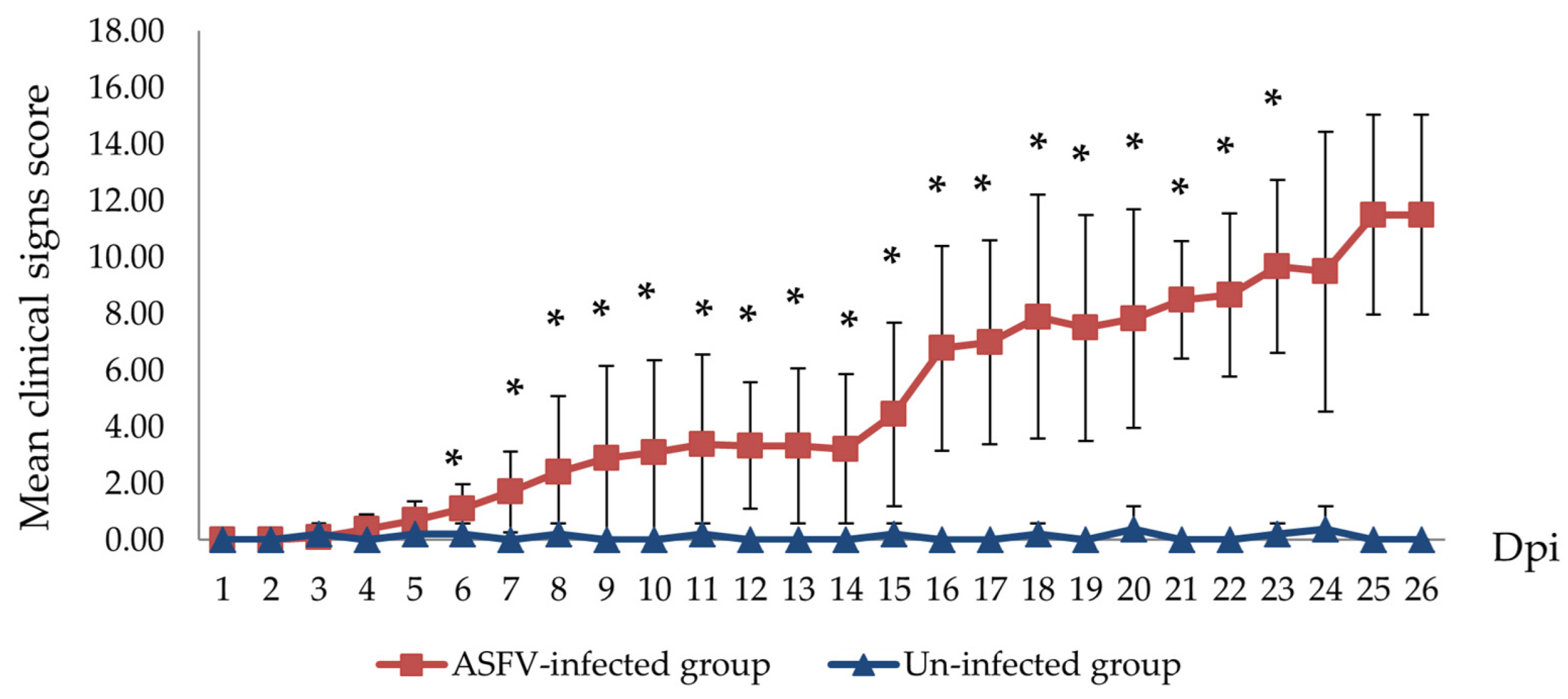
3.1. Clinical Signs
3.2. Blood and Oral Fluid Sample Analysis
3.3. Gross Pathological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Zhang, X.; Qi, W.; Yang, Y.; Liu, Z.; An, T.; Wu, X.; Chen, J. Prevention and Control Strategies of African Swine Fever and Progress on Pig Farm Repopulation in China. Viruses 2021, 13, 2552. [Google Scholar] [CrossRef]
- Dee, S.A.; Bauermann, F.V.; Niederwerder, M.C.; Singrey, A.; Clement, T.; de Lima, M.; Long, C.; Patterson, G.; Sheahan, M.A.; Stoian, A.M.M.; et al. Survival of Viral Pathogens in Animal Feed Ingredients under Transboundary Shipping Models. PLoS ONE 2018, 13, e0194509. [Google Scholar] [CrossRef]
- Urbano, A.C.; Ferreira, F. African Swine Fever Control and Prevention: An Update on Vaccine Development. Emerg. Microbes Infect. 2022, 11, 2021–2033. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, B.; Kang, H.E. Control Measures to African Swine Fever Outbreak: Active Response in South Korea, Preparation for the Future, and Cooperation. J. Vet. Sci. 2021, 22, e13. [Google Scholar] [CrossRef]
- Dixon, L.K.; Chapman, D.A.G.; Netherton, C.L.; Upton, C. African Swine Fever Virus Replication and Genomics. Virus Res. 2013, 173, 3–14. [Google Scholar] [CrossRef]
- Galindo, I.; Alonso, C. African Swine Fever Virus: A Review. Viruses 2017, 9, E103. [Google Scholar] [CrossRef]
- Quembo, C.J.; Jori, F.; Vosloo, W.; Heath, L. Genetic Characterization of African Swine Fever Virus Isolates from Soft Ticks at the Wildlife/Domestic Interface in Mozambique and Identification of a Novel Genotype. Transbound. Emerg. Dis. 2018, 65, 420–431. [Google Scholar] [CrossRef]
- Gallardo, C.; Fernández-Pinero, J.; Pelayo, V.; Gazaev, I.; Markowska-Daniel, I.; Pridotkas, G.; Nieto, R.; Fernández-Pacheco, P.; Bokhan, S.; Nevolko, O.; et al. Genetic Variation among African Swine Fever Genotype II Viruses, Eastern and Central Europe. Emerg. Infect. Dis. 2014, 20, 1544–1547. [Google Scholar] [CrossRef]
- Kim, H.-J.; Cho, K.-H.; Ryu, J.-H.; Jang, M.-K.; Chae, H.-G.; Choi, J.-D.; Nah, J.-J.; Kim, Y.-J.; Kang, H.-E. Isolation and Genetic Characterization of African Swine Fever Virus from Domestic Pig Farms in South Korea, 2019. Viruses 2020, 12, 1237. [Google Scholar] [CrossRef]
- Kovalenko, G.; Ducluzeau, A.-L.; Ishchenko, L.; Sushko, M.; Sapachova, M.; Rudova, N.; Solodiankin, O.; Gerilovych, A.; Dagdag, R.; Redlinger, M.; et al. Complete Genome Sequence of a Virulent African Swine Fever Virus from a Domestic Pig in Ukraine. Microbiol. Resour. Announc. 2019, 8, e00883-19. [Google Scholar] [CrossRef]
- Olesen, A.S.; Lohse, L.; Dalgaard, M.D.; Woźniakowski, G.; Belsham, G.J.; Bøtner, A.; Rasmussen, T.B. Complete Genome Sequence of an African Swine Fever Virus (ASFV POL/2015/Podlaskie) Determined Directly from Pig Erythrocyte-Associated Nucleic Acid. J. Virol. Methods 2018, 261, 14–16. [Google Scholar] [CrossRef]
- Sanna, G.; Dei Giudici, S.; Bacciu, D.; Angioi, P.P.; Giammarioli, M.; De Mia, G.M.; Oggiano, A. Improved Strategy for Molecular Characterization of African Swine Fever Viruses from Sardinia, Based on Analysis of P30, CD2V and I73R/I329L Variable Regions. Transbound. Emerg. Dis. 2017, 64, 1280–1286. [Google Scholar] [CrossRef]
- Sauter-Louis, C.; Forth, J.H.; Probst, C.; Staubach, C.; Hlinak, A.; Rudovsky, A.; Holland, D.; Schlieben, P.; Göldner, M.; Schatz, J.; et al. Joining the Club: First Detection of African Swine Fever in Wild Boar in Germany. Transbound. Emerg. Dis. 2021, 68, 1744–1752. [Google Scholar] [CrossRef]
- Mai, N.T.A.; Vu, X.D.; Nguyen, T.T.H.; Nguyen, V.T.; Trinh, T.B.N.; Kim, Y.J.; Kim, H.-J.; Cho, K.-H.; Nguyen, T.L.; Bui, T.T.N.; et al. Molecular Profile of African Swine Fever Virus (ASFV) Circulating in Vietnam during 2019-2020 Outbreaks. Arch. Virol. 2021, 166, 885–890. [Google Scholar] [CrossRef]
- Mazur-Panasiuk, N.; Walczak, M.; Juszkiewicz, M.; Woźniakowski, G. The Spillover of African Swine Fever in Western Poland Revealed Its Estimated Origin on the Basis of O174L, K145R, MGF 505-5R and IGR I73R/I329L Genomic Sequences. Viruses 2020, 12, 1094. [Google Scholar] [CrossRef]
- Mazur-Panasiuk, N.; Woźniakowski, G. The Unique Genetic Variation within the O174L Gene of Polish Strains of African Swine Fever Virus Facilitates Tracking Virus Origin. Arch. Virol. 2019, 164, 1667–1672. [Google Scholar] [CrossRef]
- Eustace Montgomery, R. On A Form of Swine Fever Occurring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Martínez-López, B. African Swine Fever (ASF): Five Years around Europe. Vet. Microbiol. 2013, 165, 45–50. [Google Scholar] [CrossRef]
- Revilla, Y.; Pérez-Núñez, D.; Richt, J.A. African Swine Fever Virus Biology and Vaccine Approaches. Adv. Virus Res. 2018, 100, 41–74. [Google Scholar]
- Ramirez-Medina, E.; O’Donnell, V.; Silva, E.; Espinoza, N.; Velazquez-Salinas, L.; Moran, K.; Daite, D.A.; Barrette, R.; Faburay, B.; Holland, R.; et al. Experimental Infection of Domestic Pigs with an African Swine Fever Virus Field Strain Isolated in 2021 from the Dominican Republic. Viruses 2022, 14, 1090. [Google Scholar] [CrossRef]
- Le, V.P.; Jeong, D.G.; Yoon, S.-W.; Kwon, H.-M.; Trinh, T.B.N.; Nguyen, T.L.; Bui, T.T.N.; Oh, J.; Kim, J.B.; Cheong, K.M.; et al. Outbreak of African Swine Fever, Vietnam, 2019. Emerg. Infect. Dis. 2019, 25, 1433–1435. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.; Huang, L.; Zhang, X.; Zhang, J.; Shen, D.; Zhang, Z.; Wang, Z.; Huo, H.; Wang, W.; Huangfu, H.; et al. Genotype I African Swine Fever Viruses Emerged in Domestic Pigs in China and Caused Chronic Infection. Emerg. Microbes Infect. 2021, 10, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Gomez-Villamandos, J.C.; Carrasco, L. An Update on the Epidemiology and Pathology of African Swine Fever. J. Comp. Pathol. 2015, 152, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Bui, V.N.; Dao, D.T.; Bui, N.A.; Le, T.D.; Kieu, M.A.; Nguyen, Q.H.; Tran, L.H.; Roh, J.-H.; So, K.-M.; et al. Pathogenicity of an African Swine Fever Virus Strain Isolated in Vietnam and Alternative Diagnostic Specimens for Early Detection of Viral Infection. Porc. Health Manag. 2021, 7, 36. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method Of Estimating Fifty Per Cent Endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Malmquist, W.A.; Hay, D. Hemadsorption and Cytopathic Effect Produced by African Swine Fever Virus in Swine Bone Marrow and Buffy Coat Cultures. Am. J. Vet. Res. 1960, 21, 104–108. [Google Scholar]
- Mur, L.; Gallardo, C.; Soler, A.; Zimmermman, J.; Pelayo, V.; Nieto, R.; Sánchez-Vizcaíno, J.M.; Arias, M. Potential Use of Oral Fluid Samples for Serological Diagnosis of African Swine Fever. Vet. Microbiol. 2013, 165, 135–139. [Google Scholar] [CrossRef]
- Galindo-Cardiel, I.; Ballester, M.; Solanes, D.; Nofrarías, M.; López-Soria, S.; Argilaguet, J.M.; Lacasta, A.; Accensi, F.; Rodríguez, F.; Segalés, J. Standardization of Pathological Investigations in the Framework of Experimental ASFV Infections. Virus Res. 2013, 173, 180–190. [Google Scholar] [CrossRef]
- Salguero, F.J. Comparative Pathology and Pathogenesis of African Swine Fever Infection in Swine. Front. Vet. Sci. 2020, 7, 282. [Google Scholar] [CrossRef]
- Nga, B.T.T.; Tran Anh Dao, B.; Nguyen Thi, L.; Osaki, M.; Kawashima, K.; Song, D.; Salguero, F.J.; Le, V.P. Clinical and Pathological Study of the First Outbreak Cases of African Swine Fever in Vietnam, 2019. Front. Vet. Sci. 2020, 7, 392. [Google Scholar] [CrossRef]
- Guinat, C.; Reis, A.L.; Netherton, C.L.; Goatley, L.; Pfeiffer, D.U.; Dixon, L. Dynamics of African Swine Fever Virus Shedding and Excretion in Domestic Pigs Infected by Intramuscular Inoculation and Contact Transmission. Vet. Res. 2014, 45, 93. [Google Scholar] [CrossRef]
- Lohse, L.; Nielsen, J.; Uttenthal, Å.; Olesen, A.S.; Strandbygaard, B.; Rasmussen, T.B.; Belsham, G.J.; Bøtner, A. Experimental Infections of Pigs with African Swine Fever Virus (Genotype II); Studies in Young Animals and Pregnant Sows. Viruses 2022, 14, 1387. [Google Scholar] [CrossRef]
- Tran, X.H.; Le, T.T.P.; Nguyen, Q.H.; Do, T.T.; Nguyen, V.D.; Gay, C.G.; Borca, M.V.; Gladue, D.P. African Swine Fever Virus Vaccine Candidate ASFV-G-ΔI177L Efficiently Protects European and Native Pig Breeds against Circulating Vietnamese Field Strain. Transbound. Emerg. Dis. 2021, 69, e497–e504. [Google Scholar]
- Post, J.; Weesendorp, E.; Montoya, M.; Loeffen, W.L. Influence of Age and Dose of African Swine Fever Virus Infections on Clinical Outcome and Blood Parameters in Pigs. Viral Immunol. 2017, 30, 58–69. [Google Scholar] [CrossRef]
- Niederwerder, M.C.; Stoian, A.M.M.; Rowland, R.R.R.; Dritz, S.S.; Petrovan, V.; Constance, L.A.; Gebhardt, J.T.; Olcha, M.; Jones, C.K.; Woodworth, J.C.; et al. Infectious Dose of African Swine Fever Virus When Consumed Naturally in Liquid or Feed. Emerg. Infect. Dis. 2019, 25, 891–897. [Google Scholar] [CrossRef]
- Howey, E.B.; O’Donnell, V.; de Carvalho Ferreira, H.C.; Borca, M.V.; Arzt, J. Pathogenesis of Highly Virulent African Swine Fever Virus in Domestic Pigs Exposed via Intraoropharyngeal, Intranasopharyngeal, and Intramuscular Inoculation, and by Direct Contact with Infected Pigs. Virus Res. 2013, 178, 328–339. [Google Scholar] [CrossRef]
- Cho, K.-H.; Hong, S.-K.; Jang, M.-K.; Ryu, J.-H.; Kim, H.-J.; Lee, Y.-R.; Roh, I.-S.; Sohn, H.-J.; Kang, H.-E.; Park, J.-Y. Comparison of the Virulence of Korean African Swine Fever Isolates from Pig Farms during 2019–2021. Viruses 2022, 14, 2512. [Google Scholar] [CrossRef]
- Walczak, M.; Żmudzki, J.; Mazur-Panasiuk, N.; Juszkiewicz, M.; Woźniakowski, G. Analysis of the Clinical Course of Experimental Infection with Highly Pathogenic African Swine Fever Strain, Isolated from an Outbreak in Poland. Aspects Related to the Disease Suspicion at the Farm Level. Pathogens 2020, 9, 237. [Google Scholar] [CrossRef]
- Kameyama, K.; Nishi, T.; Yamada, M.; Masujin, K.; Morioka, K.; Kokuho, T.; Fukai, K. Experimental Infection of Pigs with a Classical Swine Fever Virus Isolated in Japan for the First Time in 26 Years. J. Vet. Med. Sci. 2019, 81, 1277–1284. [Google Scholar] [CrossRef]
- Goonewardene, K.B.; Chung, C.J.; Goolia, M.; Blakemore, L.; Fabian, A.; Mohamed, F.; Nfon, C.; Clavijo, A.; Dodd, K.A.; Ambagala, A. Evaluation of Oral Fluid as an Aggregate Sample for Early Detection of African Swine Fever Virus Using Four Independent Pen-Based Experimental Studies. Transbound. Emerg. Dis. 2021, 68, 2867–2877. [Google Scholar] [CrossRef]





| No. | Date of Onset of Clinical Manifestations | Dead | Onset of Viremia | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Anorexia | Recumbency | Diarrhea | Cough | Lethargy | Fever | Skin Hemorrhages | |||
| 1 | 14 | 15 | 14 | 11 | 5 | 4 | 15 | 18 | 8 |
| 2 | 14 | 16 | - | - | 11 | 11 | - | 21 | 12 |
| 3 | 16 | 19 | 18 | 11 | 10 | 15 | - | 20 | 14 |
| 4 | 22 | 23 | - | - | 12 | 19 | - | 25 | 16 |
| 5 | 9 | - | - | 5 | 6 | 4 | 8 | 10 | 6 |
| 6 | 18 | 19 | - | - | 14 | 15 | - | 22 | 12 |
| 7 | 22 | 23 | - | - | 12 | 20 | 26 | 27 | 16 |
| 8 | 15 | - | - | 7 | 8 | 8 | - | 18 | 8 |
| 9 | 16 | 19 | 19 | - | 12 | 15 | - | 20 | 8 |
| 10 | 14 | 15 | - | 15 | 12 | 11 | 15 | 17 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.H.; Nguyen, V.T.; Le, P.N.; Mai, N.T.A.; Dong, V.H.; Bui, T.A.D.; Nguyen, T.L.; Ambagala, A.; Le, V.P. Pathological Characteristics of Domestic Pigs Orally Infected with the Virus Strain Causing the First Reported African Swine Fever Outbreaks in Vietnam. Pathogens 2023, 12, 393. https://doi.org/10.3390/pathogens12030393
Nguyen TTH, Nguyen VT, Le PN, Mai NTA, Dong VH, Bui TAD, Nguyen TL, Ambagala A, Le VP. Pathological Characteristics of Domestic Pigs Orally Infected with the Virus Strain Causing the First Reported African Swine Fever Outbreaks in Vietnam. Pathogens. 2023; 12(3):393. https://doi.org/10.3390/pathogens12030393
Chicago/Turabian StyleNguyen, Thi Thu Huyen, Van Tam Nguyen, Phuong Nam Le, Nguyen Tuan Anh Mai, Van Hieu Dong, Tran Anh Dao Bui, Thi Lan Nguyen, Aruna Ambagala, and Van Phan Le. 2023. "Pathological Characteristics of Domestic Pigs Orally Infected with the Virus Strain Causing the First Reported African Swine Fever Outbreaks in Vietnam" Pathogens 12, no. 3: 393. https://doi.org/10.3390/pathogens12030393
APA StyleNguyen, T. T. H., Nguyen, V. T., Le, P. N., Mai, N. T. A., Dong, V. H., Bui, T. A. D., Nguyen, T. L., Ambagala, A., & Le, V. P. (2023). Pathological Characteristics of Domestic Pigs Orally Infected with the Virus Strain Causing the First Reported African Swine Fever Outbreaks in Vietnam. Pathogens, 12(3), 393. https://doi.org/10.3390/pathogens12030393











