Systematic Review of the Key Factors Influencing the Indoor Airborne Spread of SARS-CoV-2
Abstract
1. Introduction
2. Materials and Methods
- ((SARS-CoV-2) and (COVID-19)) and ((Indoor) or (Inside)) and ((CO2) or (carbon dioxide))
- ((SARS-CoV-2) and (COVID-19)) and ((Indoor) or (Inside)) and ((airborne transmission) or (aerosol transmission))
- ((SARS-CoV-2) and (COVID-19)) and ((hvac) or (air quality control) or (air conditioning))
- ((SARS-CoV-2) and (COVID-19)) and ((Indoor) or (Inside)) and ((Temperature) or (Humidity))
- ((SARS-CoV-2) and (COVID-19)) and ((Indoor) or (Inside)) and ((Fine particles) or (Fine Particulate matter) or (PM))
- ((SARS-CoV-2) and (COVID-19)) and ((Indoor) or (Inside)) and ((aerosol) or (bioaerosol) or (airborne))
- ((SARS-CoV-2) and (COVID-19)) and ((Indoor) or (Inside)) and (air) and ((mitigation control) or (mitigation measures) or (mitigation))
3. Results and Discussion
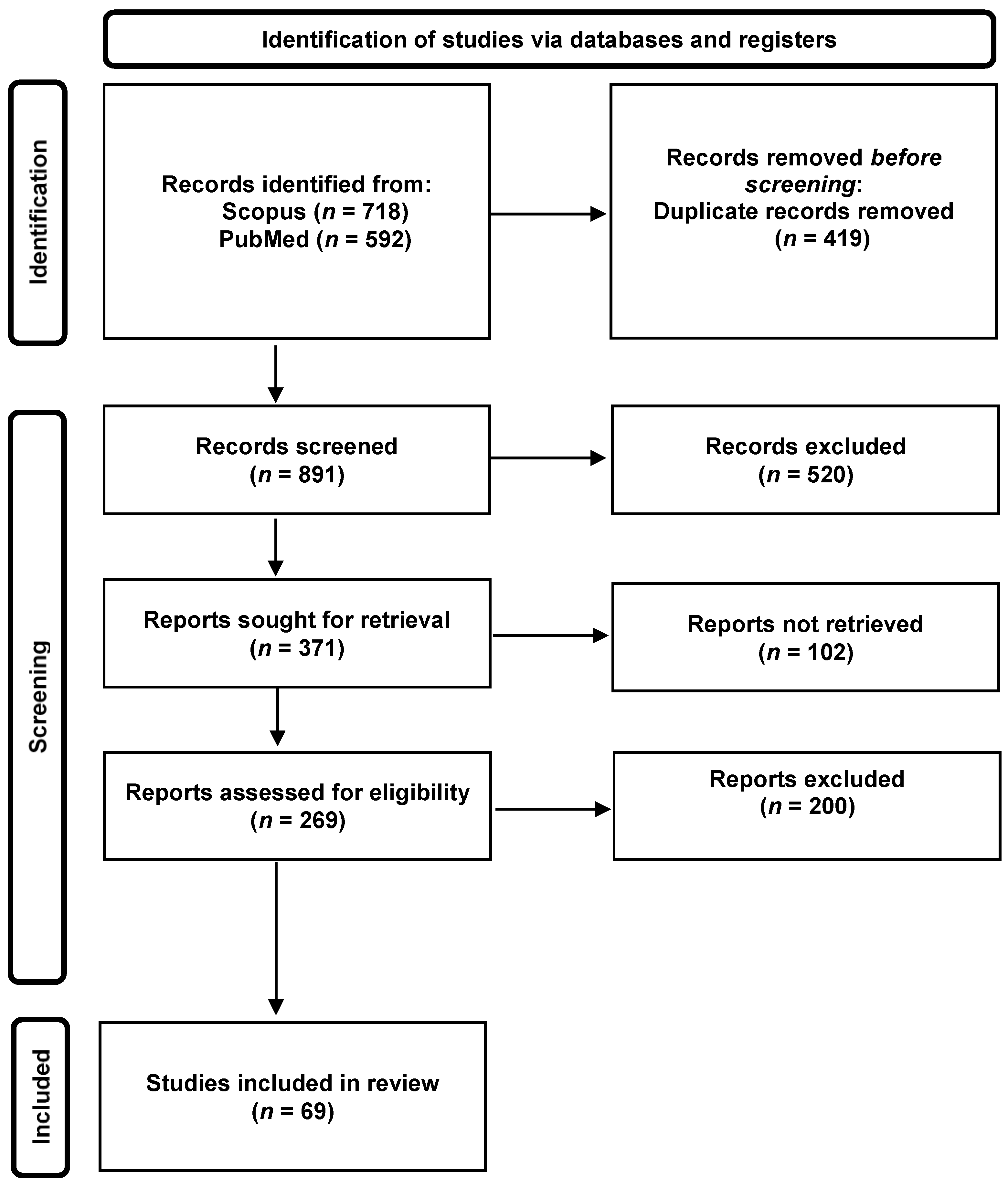
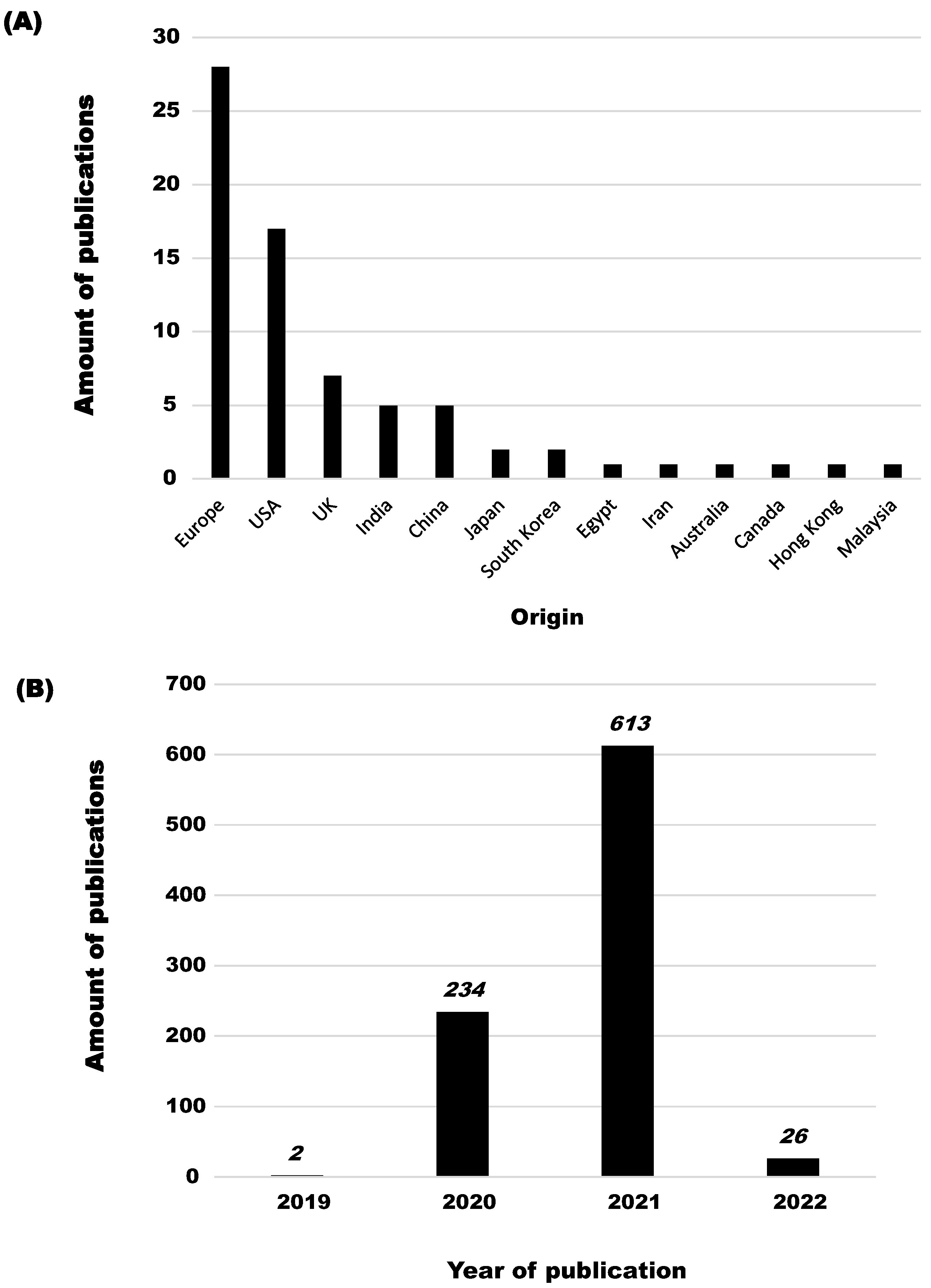
3.1. Selection of Publications Related to the Indoor Airborne Spread of SARS-CoV-2
3.2. Description of Aerosol and Droplet Transmission
3.3. The Wells Riley Model and Its Successive Improvements
3.4. Quantum of Infection and Quantum Generation Rate
3.5. Risk Factor Assessment
3.6. Influence of Temperature and Humidity on Airborne Spread
3.7. CO2 as an Indicator of the Room Ventilation
3.8. Heating, Ventilation and Air Conditioning (Indoor Air Quality Control Systems)
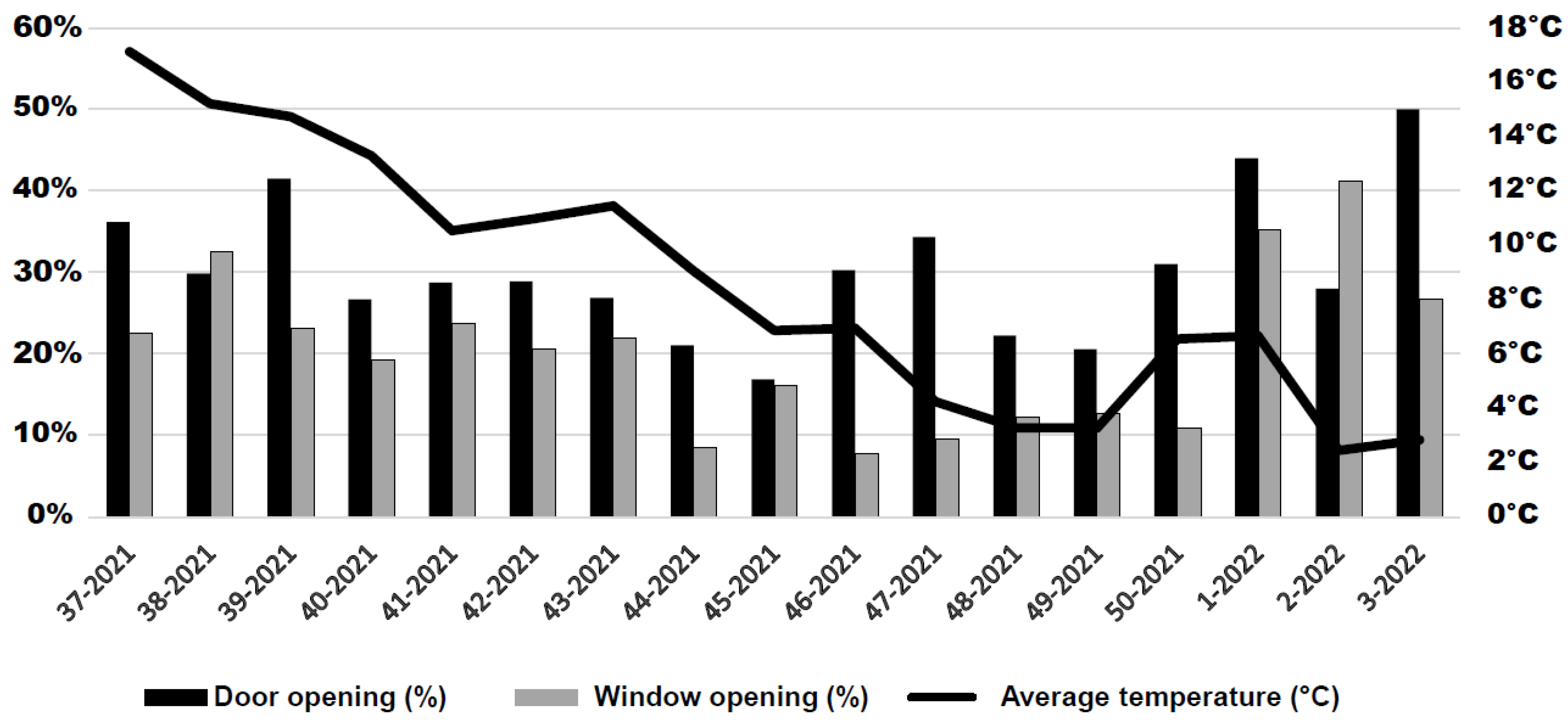
3.9. Natural Ventilation and Manual Operation of Doors and Windows in Enclosed Spaces
3.10. Ultra Violet Radiation, Photocatalytic Filters and Other Germicidal Compounds
3.11. Mitigation Measures
3.12. Seasonality of the SARS-CoV-2 Virus
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | 1 |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | 2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | 2–4 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | 4 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | 4–5 |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists, and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | 4–5 |
| Search strategy | 7 | Present the full search strategies for all databases, registers, and websites, including any filters and limits used. | 4–5 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | 4–5 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | 4–5 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, and analyses), and if not, the methods used to decide which results to collect. | 5–6 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, and funding sources). Describe any assumptions made about any missing or unclear information. | 8 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study, and whether they worked independently, and if applicable, details of automation tools used in the process. | 6 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio and mean difference) used in the synthesis or presentation of results. | 8 (Table 2) |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | 5–6 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | Not appropriate | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | 8 (Table 2) | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | 5–6 No meta-analysis | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis and meta-regression). | Not appropriate | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | No appropriate | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | 6–20 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | 6–20 |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | 4–6 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | 4–6 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | 6 Appendix B |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | 6–20 |
| Results of individual studies | 19 | For all outcomes, present for each study: (a) summary statistics for each group (where appropriate), and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | 5–9 |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies. | 6–20 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | 6–20 No meta-analysis | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | 6–20 | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | Not appropriate | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | Not appropriate |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | 6–20 |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | 6–20 |
| 23b | Discuss any limitations of the evidence included in the review. | 6–20 | |
| 23c | Discuss any limitations of the review processes used. | 6–20 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | 15–20 | |
| OTHER INFORMATION | - | ||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | Not registered |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | − | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | − | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | 21 |
| Competing interests | 26 | Declare any competing interests of review authors. | 21 |
| Availability of data, code, and other materials | 27 | Report which of the following are publicly available and where they can be found template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | 21 |
Appendix B
| Reference (Number) | Author (Year) | Parameter(s) Described * |
| 1 | WHO (2021) | − |
| 2 | WHO (2022) | − |
| 3 | Thanh Le et al. (2020) | − |
| 4 | So et al. (2020) | − |
| 5 | Mathieu et al. (2021) | − |
| 6 | Moriyama et al. (2020) | T, RH |
| 7 | Kohanski et al. (2020) | λ |
| 8 | Wang et al. (2021) | P, q, Q, Cq, N, Ni |
| 9 | Tang et al. (2021) | η |
| 10 | Chirico et al. (2020) | T, RH, λ |
| 11 | da Silva et al. (2021) | T, RH |
| 12 | Burridge et al. (2021) | Δ, N, Ni, λ, q, Q, Cq, k |
| 13 | Jones et al. (2020) | λ, V, N, Ni, Q, η |
| 14 | Lelieveld et al. (2020) | λ, q, Q, Cq, V, η |
| 15 | Azuma et al. (2020) | T, RH, P, q, Q, Cq, N, Ni, λ |
| 16 | Bazant et al. (2021) | λ, P, q, Q, Cq, N, Ni, η |
| 17 | Stabile et al. (2021) | Δ, P, N, Ni, λ, q, Q, Cq, k |
| 18 | Xie et al. (2021) | − |
| 19 | Santurtún et al. (2021) | − |
| 20 | Aganovic et al. (2021) | T, RH, N, Ni, λ, q, Q, Cq |
| 21 | Smieszek et al. (2019) | P, λ |
| 22 | Page et al. (2021) | − |
| 23 | Chatterjee et al. (2021) | T, RH |
| 24 | Netz et al. (2020) | T, RH, λ |
| 25 | Srinivasan et al. (2021) | T, RH, λ |
| 26 | Bazant et al. (2021) | Δ, P, N, Ni, λ, q, Q, Cq, k, ε, η |
| 27 | Delikhoon et al. (2021) | T, RH, λ |
| 28 | Pal et al. (2021) | T, RH |
| 29 | Coleman et al. (2021) | q, Q, Cq |
| 30 | Trancossi et al. (2021) | q, Q, Cq, T, RH, λ |
| 31 | Spena et al. (2020) | T, RH |
| 32 | Riley et al. (1978) | T, RH, λ |
| 33 | Shen et al. (2021) | λ, P, q, Q, Cq, N, Ni, η |
| 34 | Buonanno et al. (2020) | q, Q, Cq, λ, P, T, RH |
| 35 | Dai et al. (2020) | λ, P, q, Q, Cq, N, Ni, η |
| 36 | Miller et al. (2021) | q, Q, Cq, λ |
| 37 | Kurnitski et al. (2021) | λ, P, q, Q, Cq, N, Ni, η, V |
| 38 | Shen et al. (2021) | λ, P, q, Q, Cq, N, Ni, η, V |
| 39 | Beggs et al. (2021) | T, RH |
| 40 | Quraishi et al. (2020) | T, RH |
| 41 | Biryukov et al. (2020) | T, RH |
| 42 | Elsaid et al. (2021) | T, RH, λ |
| 43 | Bu et al. (2021) | T, RH, λ, P |
| 44 | Vassella et al. (2021) | Δ, λ, k, T, RH |
| 45 | Peng et al. (2021) | Δ, λ, k, ε, P |
| 46 | Vouriot et al. (2021) | Δ, λ, k, q, Q, Cq, P |
| 47 | Chillon et al. (2021) | Δ, λ, T, RH |
| 48 | Lepore et al. (2021) | Δ, λ |
| 49 | Morawska et al. (2020) | λ |
| 50 | Lung et al. (2021) | λ |
| 51 | Lee et al. (2021) | λ, V, ε |
| 52 | Aguilar et al. (2021) | Δ, λ, T, RH, V |
| 53 | Rodriguez et al. (2021) | λ |
| 54 | Bono et al. (2021) | λ |
| 55 | Garcia de Abajo et al. (2020) | λ |
| 56 | ASHRAE (2019) | − |
| 57 | Melikov et al. (2020) | λ, N, Ni, V |
| 58 | Park et al. (2021) | λ, P |
| 59 | Rencken et al. (2021) | λ, Q, η |
| 60 | Singer et al. (2022) | λ, Q |
| 61 | Ascione et al. (2021) | λ, T, RH |
| 62 | Duill et al. (2021) | Δ, λ, Q, V |
| 63 | Gil-Baez et al. (2021) | Δ, λ |
| 64 | Kulo et al. (2021) | Δ, λ, T, RH |
| 65 | Nazarenko et al. (2020) | λ |
| 66 | de Almeida et al. (2020) | λ |
| 67 | de Almeida et al. (2021) | λ |
| 68 | Lendvay et al. (2022) | λ |
| 69 | Kwon et al. (2021) | T, RH |
| * The nomenclature list of different abbreviations of parameters are described in Table 2. | ||
Appendix C
References
- WHO. World Health Organization—Convened Global Study of Origins of SARS-CoV-2: China Part. Joint WHO-China Study (14 January–10 February 2021). 2021. Available online: https://www.who.int/publications/i/item/who-convened-global-study-of-origins-of-sars-cov-2-china-part (accessed on 16 April 2022).
- WHO. World Health Organization Coronavirus (COVID-19) Dashboard. WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. 2022. Available online: https://covid19.who.int/ (accessed on 1 October 2022).
- Thanh Le, T.; Andreadakis, Z.; Kumar, A.; Román, R.G.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef]
- So, A.D.; Woo, J. Special Paper: Reserving coronavirus disease 2019 vaccines for global access: Cross sectional analysis. BMJ 2020, 371, m4750. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, E.; Ritchie, H.; Ortiz-Ospina, E.; Roser, M.; Hasell, J.; Appel, C.; Giattino, C.; Rodés-Guirao, G. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021, 5, 947–953. [Google Scholar] [CrossRef]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of Respiratory Viral Infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Lo, L.J.; Waring, M.S. Review of indoor aerosol generation, transport, and control in the context of COVID-19. Int. Forum Allergy Rhinol. 2020, 10, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne transmission of respiratory viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.; Bahnfleth, W.P.; Bluyssen, P.M.; Buonanno, G.; Jimenez, J.L.; Kurnitski, J.; Li, Y.; Miller, S.; Sekhar, C.; Morawska, L.; et al. Dismantling myths on the airborne transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J. Hosp. Infect. 2021, 110, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Chirico, F.; Sacco, A.; Bragazzi, N.L.; Magnavita, N. Can Air-Conditioning Systems Contribute to the Spread of SARS/MERS/COVID-19 Infection? Insights from a Rapid Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 6052. [Google Scholar] [CrossRef]
- Da Silva, P.G.; Nascimento, M.S.J.; Soares, R.R.G.; Sousa, S.I.V.; Mesquita, J.R. Airborne spread of infectious SARS-CoV-2: Moving forward using lessons from SARS-CoV and MERS-CoV. Sci. Total Environ. 2021, 76, e142802. [Google Scholar] [CrossRef]
- Burridge, H.C.; Fan, S.; Jones, R.L.; Noakes, C.J.; Linden, P.F. Predictive and retrospective modelling of airborne infection risk using monitored carbon dioxide. Indoor Built Environ. 2021, 31, 1363–1380. [Google Scholar] [CrossRef]
- Jones, B.; Sharpe, P.; Iddon, C.; Hathway, E.A.; Noakes, C.J.; Fitzgeralde, S. Modelling uncertainty in the relative risk of exposure to the SARS-CoV-2 virus by airborne aerosol transmission in well mixed indoor air. Build. Environ. 2020, 191, e107617. [Google Scholar] [CrossRef] [PubMed]
- Lelieveld, J.; Helleis, F.; Borrman, S.; Cheng, Y.; Drewnick, F.; Haug, G.; Klimach, T.; Sciare, J.; Su, H.; Pösch, U. Model Calculations of Aerosol Transmission and Infection Risk of COVID-19 in Indoor Environments. Int. J. Environ. Res. Public Health 2020, 17, 8114. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Yanagi, U.; Kagi, N.; Kim, H.; Ogata, M.; Hayashi, M. Environmental factors involved in SARS-CoV-2 transmission: Effect and role of indoor environmental quality in the strategy for COVID-19 infection control. Environ. Health Prev. Med. 2020, 25, 66. [Google Scholar] [CrossRef] [PubMed]
- Bazant, M.Z.; Bush, J.W.M. A guideline to limit indoor airborne transmission of COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2018995118. [Google Scholar] [CrossRef]
- Stabile, L.; Pacitto, A.; Mikszewski, A.; Morawska, L.; Buonanno, G. Ventilation procedures to minimize the airborne transmission of viruses in classrooms. Build. Environ. 2021, 202, e108042. [Google Scholar] [CrossRef]
- Xie, W.; Yanpeng Li, Y.; Bai, W.; Hou, J.; Ma, T.; Zeng, X.; Zhang, L.; An, T. The source and transport of bioaerosols in the air: A review. Front. Environ. Sci. Eng. 2021, 15, 44. [Google Scholar] [CrossRef]
- Santurtún, A.; Colom, M.L.; Fdez-Arroyabe, P.; Del Real, P.; Fernández-Olmo Zarrabeitia, M.T. Exposure to particulate matter: Direct and indirect role in the COVID-19 pandemic. Environ. Res. 2021, 206, e112261. [Google Scholar] [CrossRef]
- Aganovic, A.; Bi, Y.; Cao, G.; Drangsholt, F.; Kurnitski, J.; Wargocki, P. Estimating the impact of indoor relative humidity on SARS-CoV-2 airborne transmission risk using a new modification of the Wells-Riley model. Build. Environ. 2021, 205, e108278. [Google Scholar] [CrossRef]
- Smieszek, T.; Lazzari, G.; Salathé, M. Assessing the Dynamics and Control of Droplet- and Aerosol-Transmitted Influenza Using an Indoor Positioning System. Sci. Rep. 2019, 9, 2185. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 2021, 372, 71. [Google Scholar] [CrossRef]
- Chatterjee, S.; Murallidharan, J.S.; Agrawal, A.; Bhardwaj, R. How coronavirus survives for hours in aerosols. Phys. Fluids 2021, 33, 081708. [Google Scholar] [CrossRef] [PubMed]
- Netz, R.R. Mechanisms of Airborne Infection via Evaporating and Sedimenting Droplets Produced by Speaking. J. Phys. Chem. B 2020, 124, 7093–7101. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Krishan, J.; Bathula, S.; Mayya, Y.S. Modeling the viral load dependence of residence times of virus-laden droplets from COVID-19-infected subjects in indoor environments. Indoor Air 2021, 31, 1786–1797. [Google Scholar] [CrossRef]
- Bazant, M.Z.; Martin, Z.; Bazant, M.Z.; Kodio, O.; Cohen, A.E.; Khan, K.; Gu, Z.; Bush, J.W.M. Monitoring carbon dioxide to quantify the risk of indoor airborne transmission of COVID-19. Flow 2021, 1, E10. [Google Scholar] [CrossRef]
- Delikhoon, M.; Guzman, M.I.; Nabizadeh, R.; Norouzian, E.; Baghani, A. Modes of Transmission of Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) and Factors Influencing on the Airborne Transmission: A Review. Int. J. Environ. Res. Public Health 2021, 18, 395. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Sarkar, S.; Mukhopadhyay, A. Influence of ambient conditions on evaporation and transport of respiratory droplets in indoor environment. Int. Commun. Heat Mass Transf. 2021, 129, e105750. [Google Scholar] [CrossRef]
- Coleman, K.; Tay, D.; Tan, K.; Ong, S.; Than, T.; Koh, M.; Chin, Y.; Nasir, H.; Mak, T.; Chu, J.; et al. Viral Load of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Respiratory Aerosols Emitted by Patients With Coronavirus Disease 2019 (COVID-19) While Breathing, Talking, and Singing. Clin. Infect. Dis. 2022, 74, 1722–1728. [Google Scholar] [CrossRef]
- Trancossi, M.; Carli, C.; Cannistraro, G.; Pascoa, J.; Sharma, S. Could thermodynamics and heat and mass transfer research produce a fundamental step advance toward and significant reduction of SARS-COV-2 spread? Int. J. Heat Mass Transf. 2021, 170, e120983. [Google Scholar] [CrossRef]
- Spena, A.; Palombi, L.; Corcione, M.; Carestia, M.; Spena, V.A. On the Optimal Indoor Air Conditions for SARS-CoV-2 Inactivation. An Enthalpy-Based Approach. Int. J. Environ. Res. Public Health 2020, 17, 6083. [Google Scholar] [CrossRef]
- Riley, E.C.; Murphy, G.; Riley, R.L. Airborne spread of measles in a suburban elementary school. Am. J. Epidemiol. 1978, 107, 421–432. [Google Scholar] [CrossRef]
- Shen, J.; Kong, M.; Dong, B.; Birnkrant, M.J.; Zhang, J. Airborne transmission of SARS-CoV-2 in indoor environments: A comprehensive review. Sci. Technol. Built Environ. 2021, 27, 1331–1336. [Google Scholar] [CrossRef]
- Buonanno, G.; Stabile, L.; Morawska, L. Estimation of airborne viral emission: Quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ. Int. 2020, 141, e105794. [Google Scholar] [CrossRef]
- Dai, H.; Zhao, B. Association of the infection probability of COVID-19 with ventilation rates in confined spaces. Build. Simul. 2020, 13, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L.; Nazaroff, W.W.N.; Jimenez, J.L.; Boerstra, A.; Buonanno, G.; Dancer, S.J.; Kurnitski, J.; Mar, L.C.; Morawska, L.; Noakes, K. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air 2021, 31, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Kurnitski, S.J.; Martin Kiil, M.; Wargocki, P.; Boersta, A.; Seppänen, O.; Olesen, B.; Morawska, L. Respiratory infection risk-based ventilation design method. Build. Environ. 2021, 206, e108387. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Kong, M.; Dong, B.; Birnkrant, M.J.; Zhang, J. A systematic approach to estimating the effectiveness of multi-scale IAQ strategies for reducing the risk of airborne infection of SARS-CoV-2. Build. Environ. 2021, 200, e107926. [Google Scholar] [CrossRef] [PubMed]
- Beggs, C.B.; Avital, E.J. A psychrometric model to assess the biological decay of the SARS-CoV-2 virus in aerosols. Peer J. 2021, 9, e11024. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.A.; Berra, L.; Nozari, A. Indoor temperature and relative humidity in hospitals: Workplace considerations during the novel coronavirus pandemic. Occup. Environ. Med. 2020, 77, 508. [Google Scholar] [CrossRef]
- Biryukov, J.; Boydston, J.A.; Dunning, R.A.; Yeager, J.J.; Wood, S.; Reese, A.L.; Ferris, A.; Miller, D.; Weaver, W.; Zeitouni, N.E.; et al. Increasing Temperature and Relative Humidity Accelerates Inactivation of SARS-CoV-2 on Surfaces. mSphere 2020, 5, e00441-20. [Google Scholar] [CrossRef]
- Elsaid, A.M.; Mohamed, H.A.; Abdelaziz, G.B.; Ahmed, M.S. A critical review of heating, ventilation, and air conditioning (HVAC) systems within the context of a global SARS-CoV-2 epidemic. Process Saf. Environ. Prot. 2021, 155, 230–261. [Google Scholar] [CrossRef]
- Bu, Y.; Ooka, R.; Kikumoto, H.; Oh, W. Recent research on expiratory particles in respiratory viral infection and control strategies: A review. Sustain. Cities Soc. 2021, 73, e103106. [Google Scholar] [CrossRef]
- Vassella, C.C.; Koch, J.; Henzi, A.; Jordan, A.; Waeber, R.; Iannaccone, R.; Charrière, R. From spontaneous to strategic natural window ventilation: Improving indoor air quality in Swiss schools. Int. J. Hyg. Environ. Health 2021, 234, e113746. [Google Scholar] [CrossRef]
- Peng, Z.; Jimenez, J.L. Exhaled CO2 as a COVID-19 infection risk proxy for different indoor environments and activities. Environ. Sci. Technol. Lett. 2021, 8, 392–397. [Google Scholar] [CrossRef]
- Vouriot, C.V.M.; Burridge, H.C.; Noakes, C.J.; Linden, P.F. Seasonal variation in airborne infection risk in schools due to changes in ventilation inferred from monitored carbon dioxide. Indoor Air 2021, 31, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Chillon, S.A.; Millan, M.; Aramendia, I.; Unai Fernandez-Gamiz, U.; Zulueta, E.; Mendaza-Sagastizabal, X. Natural Ventilation Characterization in a Classroom under Different Scenarios. Int. J. Environ. Res. Public Health 2021, 18, 5425. [Google Scholar] [CrossRef] [PubMed]
- Lepore, E.; Aguilera Benito, P.; Piña Ramírez, C.; Viccione, G. Indoors ventilation in times of confinement by SARS-CoV-2 epidemic: A comparative approach between Spain and Italy. Sustain. Cities Soc. 2021, 72, e103051. [Google Scholar] [CrossRef]
- Morawska, L.; Tang, J.W.; Bahnfleth, W.; Bluyssen, P.M.; Boerstra, A.; Buonanno, G.; Cao, J.; Dancer, S.; Floto, A.; Franchimon, F.; et al. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020, 142, e105832. [Google Scholar] [CrossRef]
- Lung, D.C.; Kwan, M.Y.W.; Chow, C.B. Airborne transmission of SARS-CoV-2: Ventilation improvement strategies in preparation for school re-opening. Hong Kong Med. J. 2021, 27, 328–329. [Google Scholar] [CrossRef]
- Lee, H.; Ahn, K.-H. Estimate of the critical exposure time based on 70 confirmed COVID-19 cases. J. Korean Phys. Soc. 2021, 79, 492–498. [Google Scholar] [CrossRef]
- Aguilar, A.J.; De La Hoz-Torres, M.L.; Martínez-Aires, M.D.; Ruiz, D.P. Monitoring and Assessment of Indoor Environmental Conditions after the Implementation of COVID-19-Based Ventilation Strategies in an Educational Building in Southern Spain. Sensors 2021, 21, 7223. [Google Scholar] [CrossRef]
- Rodríguez, M.; Palop, M.L.; Seseña, S.; Rodríguez, A. Are the Portable Air Cleaners (PAC) really effective to terminate airborne SARS-CoV-2? Sci. Total Environ. 2021, 785, e147300. [Google Scholar] [CrossRef] [PubMed]
- Bono, N.; Ponti, F.; Punta, C.; Candiani, G. Effect of UV irradiation and TiO2-photocatalysis on airborne bacteria and viruses: An overview. Materials 2021, 14, 1075. [Google Scholar] [CrossRef] [PubMed]
- García de Abajo, F.J.; Javier Hernández, R.; Kaminer, I.; Meyerhans, A.; Rosell-Llompart, J.; Sanchez-Elsner, T. Back to Normal: An Old Physics Route to Reduce SARS-CoV-2 Transmission in Indoor Spaces. ACS Nano 2020, 14, 7704–7713. [Google Scholar] [CrossRef] [PubMed]
- Standard 62.1-2019; Ventilation for Acceptable Indoor Air Quality. ASHRAE: Atlanta, GA, USA, 2019; 87p.
- Melikov, A.K.; Ai, Z.T.; Markov, D.G. Intermittent occupancy combined with ventilation: An efficient strategy for the reduction of airborne transmission indoors. Sci. Total Environ. 2020, 744, e140908. [Google Scholar] [CrossRef]
- Park, S.; Choi, Y.; Song, D.; Kim, E.K. Natural ventilation strategy and related issues to prevent coronavirus disease 2019 (COVID-19) airborne transmission in a school building. Sci. Total Environ. 2021, 789, e147764. [Google Scholar] [CrossRef]
- Rencken, G.K.; Rutherford, E.K.; Ghanta, N.; Kongoletos, J.; Glicksman, L. Patterns of SARS-CoV-2 aerosol spread in typical classrooms. Build. Environ. 2021, 204, e108167. [Google Scholar] [CrossRef]
- Singer, B.C.; Zhao, H.; Preble, C.V.; Delp, W.W.; Pantelic, J.; Sohn, M.D.; Kirchstetter, T.W. Measured influence of overhead on exposure to airborne contaminants from simulated speaking in a meeting and a classroom. Indoor Air 2022, 32, e12917. [Google Scholar] [CrossRef]
- Ascione, F.; De Masi, R.F.; Mastellone, M.; Vanoli, G.P. The design of safe classrooms of educational buildings for facing contagions and transmission of diseases: A novel approach combining audits, calibrated energy models, building performance (BPS) and computational fluid dynamic (CFD) simulations. Energy Build. 2021, 230, e110533. [Google Scholar] [CrossRef]
- Duill, F.F.; Florian Schulz, F.; Jain, A.; Krieger, L.; van Wachem, B.; Beyrau, F. The Impact of Large Mobile Air Purifiers on Aerosol Concentration in Classrooms and the Reduction of Airborne Transmission of SARS-CoV-2. Int. J. Environ. Res. Public Health 2021, 18, 11523. [Google Scholar] [CrossRef]
- Gil-Baez, M.; Lizana, J.; Becerra Villanueva, J.A.; Molina-Huelva, M.; Serrano-Jimenez, A.; Chacartegui, R. Natural ventilation in classrooms for healthy schools in the COVID era in Mediterranean climate. Build. Environ. 2021, 206, e108345. [Google Scholar] [CrossRef]
- Kulo, A.; Klarić, S.; Ćetković, A.; Blekić, A.; Kusturica, A.; Spahić, N.; Armin Šljivo, A.; Šečić, D. School children exposure to low indoor air quality in classrooms during covid-19 pandemic: Results of a pilot study. Psychiatr. Danub. 2021, 33, 83–94. [Google Scholar] [CrossRef]
- Nazarenko, Y. Air filtration and SARS-CoV-2. Epidemiol. Health 2020, 42, e2020049. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, D.S.; Droprinchinski Martins, L.; Curti Muniz, E.; Rudke, A.P.; Squizzato, R.; Beal, F.A.; de Souza, P.R.; Patrícia, D.; Bonfim, F.; Lopes Aguiar, M.; et al. Biodegradable CA/CPB electrospun nanofibers for efficient retention of airborne nanoparticles. Process Saf. Environ. Prot. 2020, 144, 177–185. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, D.S.; Scacchetti, F.A.P.; Santos, R.; Lopes Aguiar, M.; Beal, A.; Anderson, P.; Rudke, A.P.; de Souza Santana, M.H.; Valério Lisboa, A.M.; Bezerra, F.M.; et al. Evaluation of biocidal properties of biodegradable nanofiber filters and their use in face masks. Environ. Technol. 2021, 9, e1982020. [Google Scholar] [CrossRef] [PubMed]
- Lendvay, T.S.; Chen, J.; Harcourt, B.H.; Scholte, F.E.M.; Lin, Y.L.; Kilinc-Balci, F.S.; Lamb, M.M.; Homdayjanakul, K.; Cui, Y.; Price, A.; et al. Addressing personal protective equipment (PPE) decontamination: Methylene blue and light inactivates SARS-COV-2 on N95 respirators and medical masks with maintenance of integrity and fit. Infect. Control. Hosp. Epidemiol. 2022, 43, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.K.; Gaudreault, N.N.; Richt, J.A. Seasonal stability of SARS-CoV-2 in biological fluids. Pathogens 2021, 10, 540. [Google Scholar] [CrossRef] [PubMed]

| Airborne | Anything in the Air |
|---|---|
| Aerosol | Suspension (carried along with air currents) of particles in a gas |
| Droplet | Liquid particle that can potentially carry pathogens |
| Droplet Nuclei | Small particle (diameter less than 5 µm) that are the result of the desiccation of larger droplets |
| Bioaerosol | Aerosol composed of fungi, bacteria, and other micro-organisms and biological matter usually ranging from 1 nm to 0.1 mm |
| Particulate Matter | The sum of chemical and biogenic compounds, of natural and/or anthropogenic origin, whose size vary between 1 nm and 100 μm, and which are found in the air and can be diffused and transported even over long distance |
| Aerosol Transmission | Transmission of a pathogen either through large particles of respiratory fluids (droplets), or through smaller particles that can remain aerosolized (droplet nuclei). This transmission mode can occur over larger distances, and does not require close contact between the susceptible and infected individuals |
| Droplet Transmission | Short range, direct transmission of a pathogen over short distances (<3 m) through large droplets (diameter upper 5 µm) whose trajectories are dictated by gravitational settling |
| Code and Nomenclature | Unit | References | |
|---|---|---|---|
| P | probability of infection | − | [32] |
| N | number of occupants in the room | − | [16,26,33] |
| Ni | number of infectors | − | [16,26,33] |
| q | quantum generation rate | h−1 | [29,34] |
| Cq | concentration of infectious quanta in the exhaled air | m−3 | [34] |
| Q | pulmonary ventilation rate (breathing rate) | m3/h | [29,34] |
| RS | fraction of infectious particles penetrating through the mask of a susceptible individual | − | [16,26,33] |
| RI | fraction of infectious particles penetrating through the mask of an infector (infectious individual) | − | [16,26,33] |
| ε | risk factor | − | [16,26] |
| sr | transmissibility factor | − | [16,26] |
| V | volume of the room | m3 | |
| η | mask filtration efficiency | − | [33] |
| λ | particle loss rate | h−1 | [16,26] |
| λa | ventilation rate | h−1 | [16,26] |
| λv | viral deactivation rate | h−1 | [16,26] |
| λs | particle sedimentation rate | h−1 | [16,26] |
| λf | air filtration rate | h−1 | [16,26] |
| t | exposure time | H | [32] |
| k | concentration of CO2 in the exhaled air | ppm | [16,26] |
| Scenario | Exposure Time t (h) | Mask Wearing RS, RI | Breathing Flow Rate Q (m3/h) | Concentration of Infectious Quanta Cq (m−3) | Excess CO2 Level Δ (ppm) |
|---|---|---|---|---|---|
| Classroom (teacher is the infector) | 1.5 | RS = 0.15 RI = 1 | 1.6 | 100 | 106 |
| Classroom (student is the infector) | 1.5 | RS = 0.15 RI = 0.15 | 0.3 | 5 | 75,000 |
| Indoor sport activity (no masks) | 1 | RS = 1 RI = 1 | 3.0 | 300 | 4 |
| Meeting (with masks) | 1 | RS = 0.15 RI = 0.15 | 0.3 | 10 | 56,300 |
| Meeting (no masks) | 1 | RS = 1 RI = 1 | 0.3 | 10 | 1267 |
| Continuous Measures | |||||
|---|---|---|---|---|---|
| Factor Influencing Airborne Transmission | Mitigation Measures | Seasonal Influence on the Measures | Efficacy | Feasibility | Acceptability |
| Ventilation | (1) Room ventilation (doors and windows) | Yes | +++ | +++ | ++ |
| (2) Room ventilation (HVAC systems) | No | +++ | ++ | +++ | |
| Viral concentration | (3) Portable air cleaners | No | ++ | ++ | +++ |
| (4) Filters within fixed HVAC systems | No | ++ | + | +++ | |
| (5) Air quality monitoring | No | ++ | +++ | +++ | |
| (6) External UV-C lighting | No | + | + | + | |
| (7) Mask usage | No | +++ | +++ | ++ | |
| Room occupancy | (8) Reducing occupants | No | +++ | ++ | ++ |
| (9) Reducing time | No | ++ | ++ | + | |
| Temperature and humidity | (10) Temperature and humidity control (HVAC) | Yes | + | + | ++ |
| Measures Prior to Room Occupancy | |||||
| Factor Influencing Virus Transmission | Mitigation Measures | Seasonal Influence on the Measures | Efficacy | Feasibility | Acceptability |
| Number of infectors | (11) Refusing unvaccinated individuals | No | + | + | + |
| (12) Body temperature control | No | + | + | + | |
| (13) Refusing symptomatic individuals | Yes | ++ | ++ | ++ | |
| (14) Self-testing before access | No | ++ | + | + | |
| (15) Presentation of COVID-19 certificate | No | + | ++ | + | |
| Most Efficient | Most Feasible | Most Acceptable |
|---|---|---|
| Ventilation | Ventilation (doors and windows) | Ventilation (mechanical) |
| Mask wearing | Mask wearing | Air filters |
| Reducing room occupancy | Air quality monitoring | Air quality monitoring |
| Least Efficient | Least Feasible | Least Acceptable |
| External UV-C lighting | External UV-C lighting | External UV-C lighting |
| T and RH control | T and RH control | Reducing occupation time |
| Refusing access to certain individuals | Refusing access to certain individuals | Refusing access to certain individuals |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Crane D’Heysselaer, S.; Parisi, G.; Lisson, M.; Bruyère, O.; Donneau, A.-F.; Fontaine, S.; Gillet, L.; Bureau, F.; Darcis, G.; Thiry, E.; et al. Systematic Review of the Key Factors Influencing the Indoor Airborne Spread of SARS-CoV-2. Pathogens 2023, 12, 382. https://doi.org/10.3390/pathogens12030382
de Crane D’Heysselaer S, Parisi G, Lisson M, Bruyère O, Donneau A-F, Fontaine S, Gillet L, Bureau F, Darcis G, Thiry E, et al. Systematic Review of the Key Factors Influencing the Indoor Airborne Spread of SARS-CoV-2. Pathogens. 2023; 12(3):382. https://doi.org/10.3390/pathogens12030382
Chicago/Turabian Stylede Crane D’Heysselaer, Simon, Gianni Parisi, Maxime Lisson, Olivier Bruyère, Anne-Françoise Donneau, Sebastien Fontaine, Laurent Gillet, Fabrice Bureau, Gilles Darcis, Etienne Thiry, and et al. 2023. "Systematic Review of the Key Factors Influencing the Indoor Airborne Spread of SARS-CoV-2" Pathogens 12, no. 3: 382. https://doi.org/10.3390/pathogens12030382
APA Stylede Crane D’Heysselaer, S., Parisi, G., Lisson, M., Bruyère, O., Donneau, A.-F., Fontaine, S., Gillet, L., Bureau, F., Darcis, G., Thiry, E., Ducatez, M., Snoeck, C. J., Zientara, S., Haddad, N., Humblet, M.-F., Ludwig-Begall, L. F., Daube, G., Thiry, D., Misset, B., ... Haubruge, E. (2023). Systematic Review of the Key Factors Influencing the Indoor Airborne Spread of SARS-CoV-2. Pathogens, 12(3), 382. https://doi.org/10.3390/pathogens12030382








