Phenotypical Differences between Leishmania (Leishmania) amazonensis PH8 and LV79 Strains May Impact Survival in Mammal Host and in Phlebotomine Sand Flies
Abstract
1. Introduction
2. Material and Methods
2.1. Leishmania Promastigotes Culture
2.2. Macrophage Infection
2.3. SDS-PAGE and Western Blot
2.4. LPG Characterization
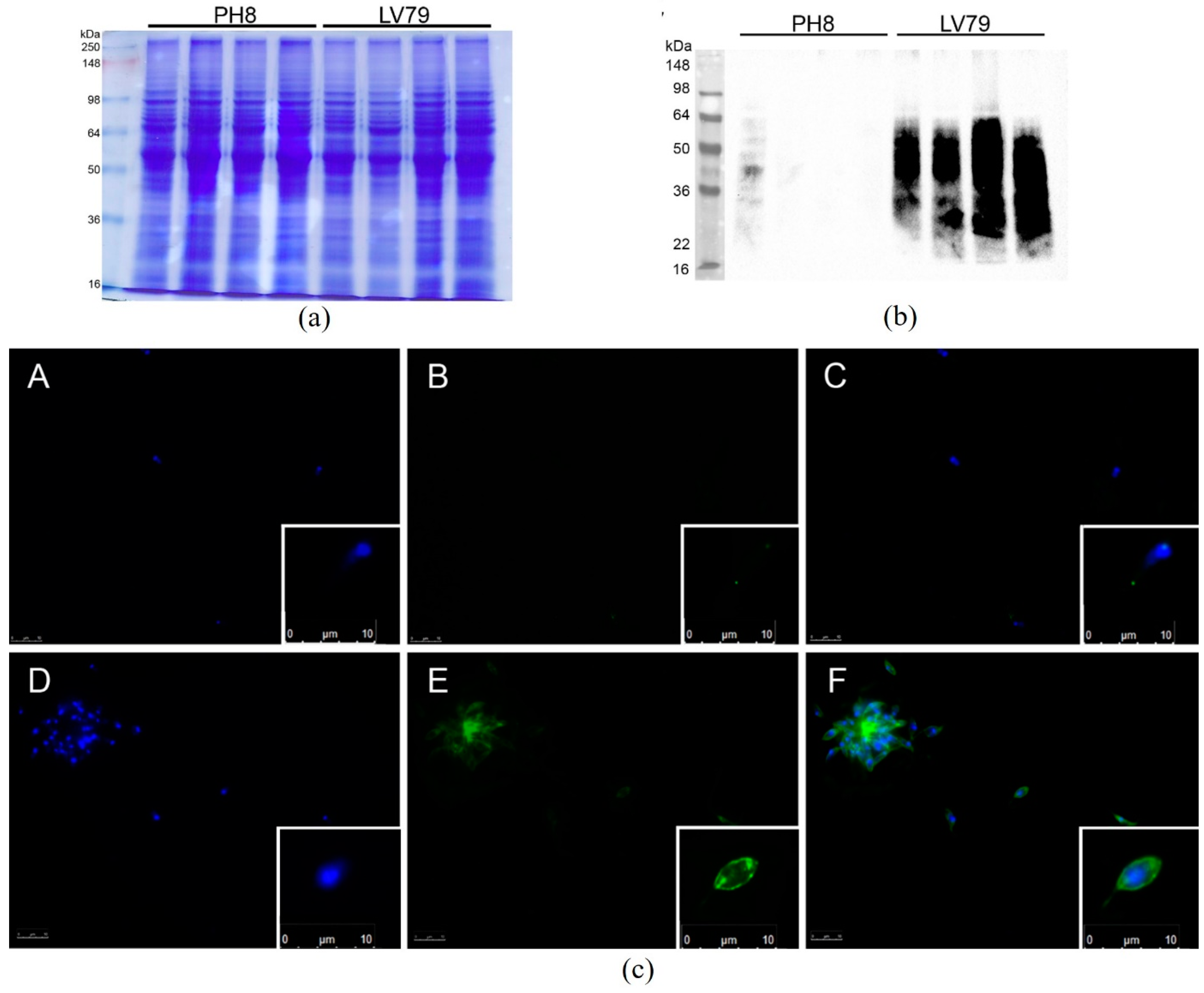
2.5. Immunofluorescence
2.6. Complement Lysis Assay
2.7. Insects
2.7.1. Lutzomyia longipalpis Artificial Infection
2.7.2. RNA Purification and cDNA Synthesis of Leishmania-Infected Sandflies
2.7.3. Quantification of Parasites in L. longipalpis
3. Results
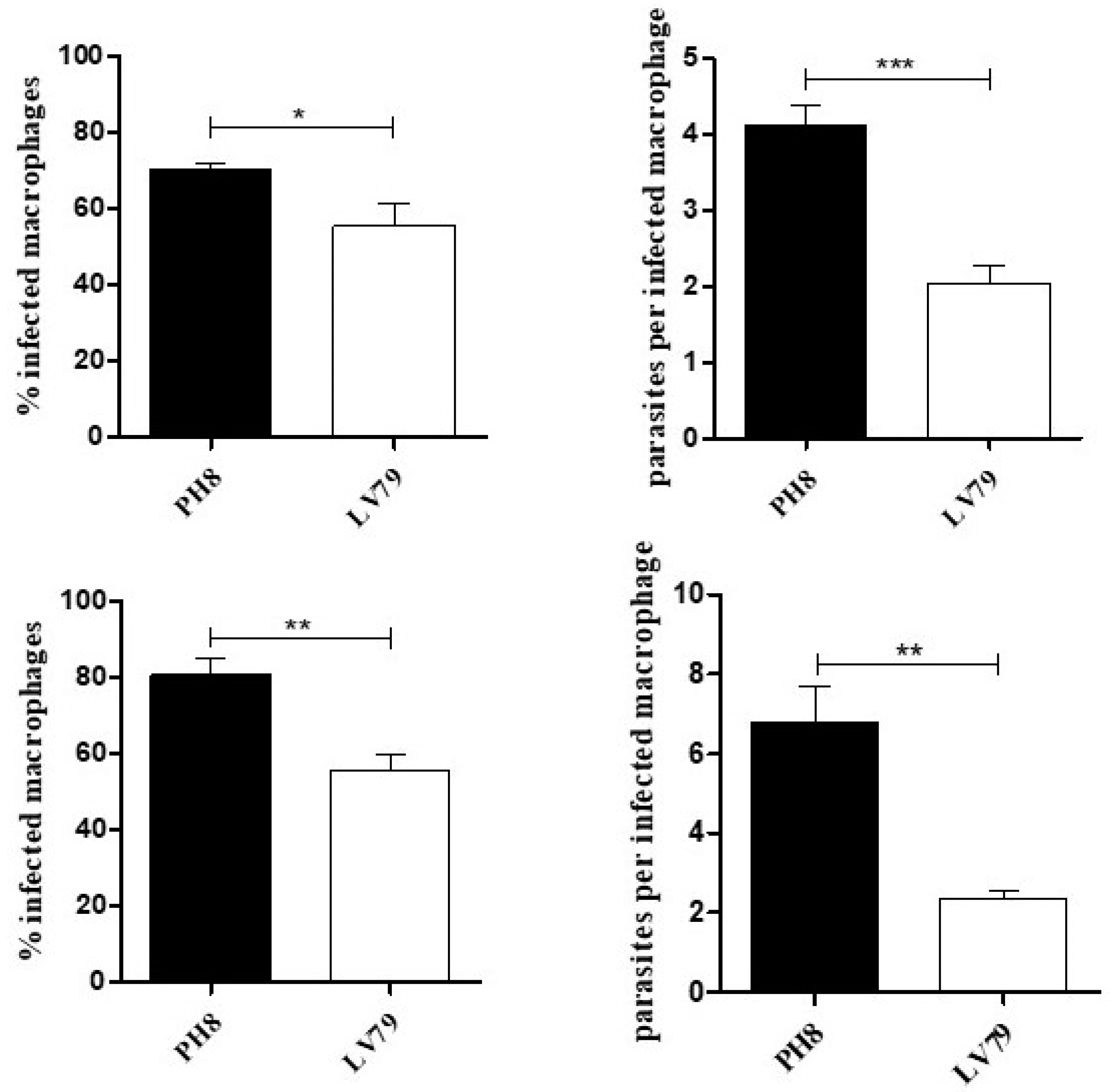
3.1. Macrophage Infection by PH8 and LV79 Promastigotes
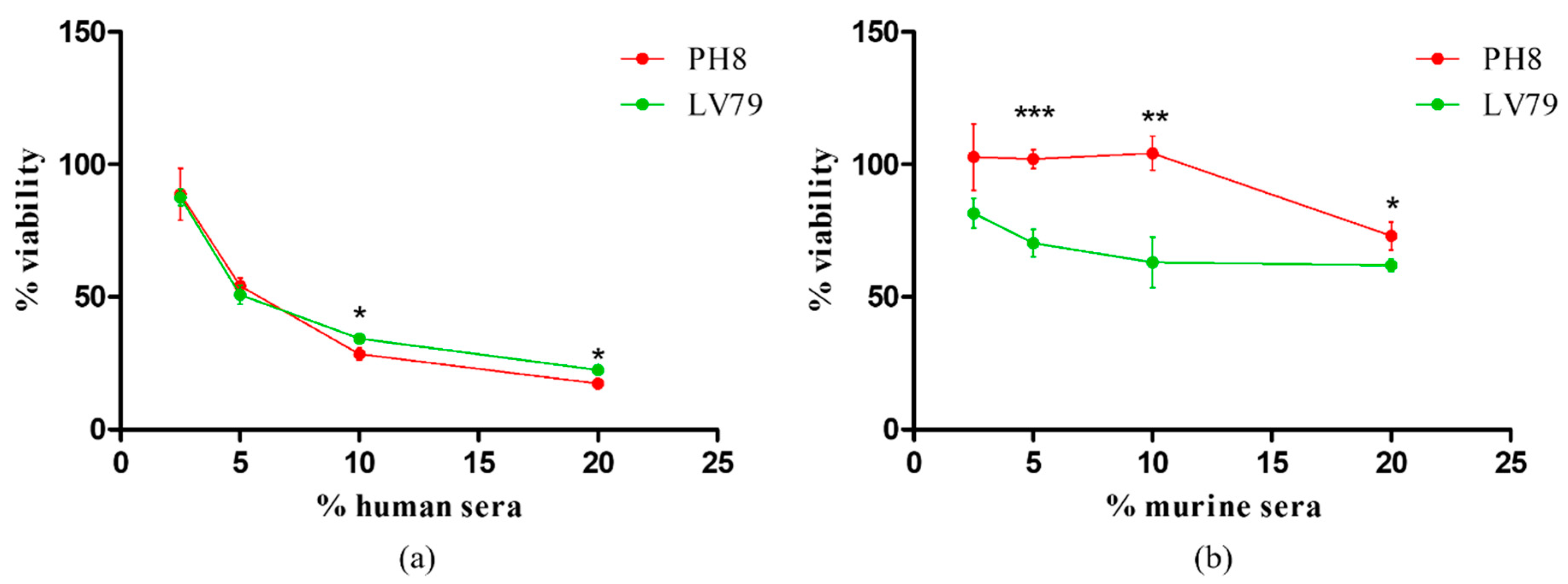
3.2. PH8 Promastigotes Are More Resistant to Complement Lysis
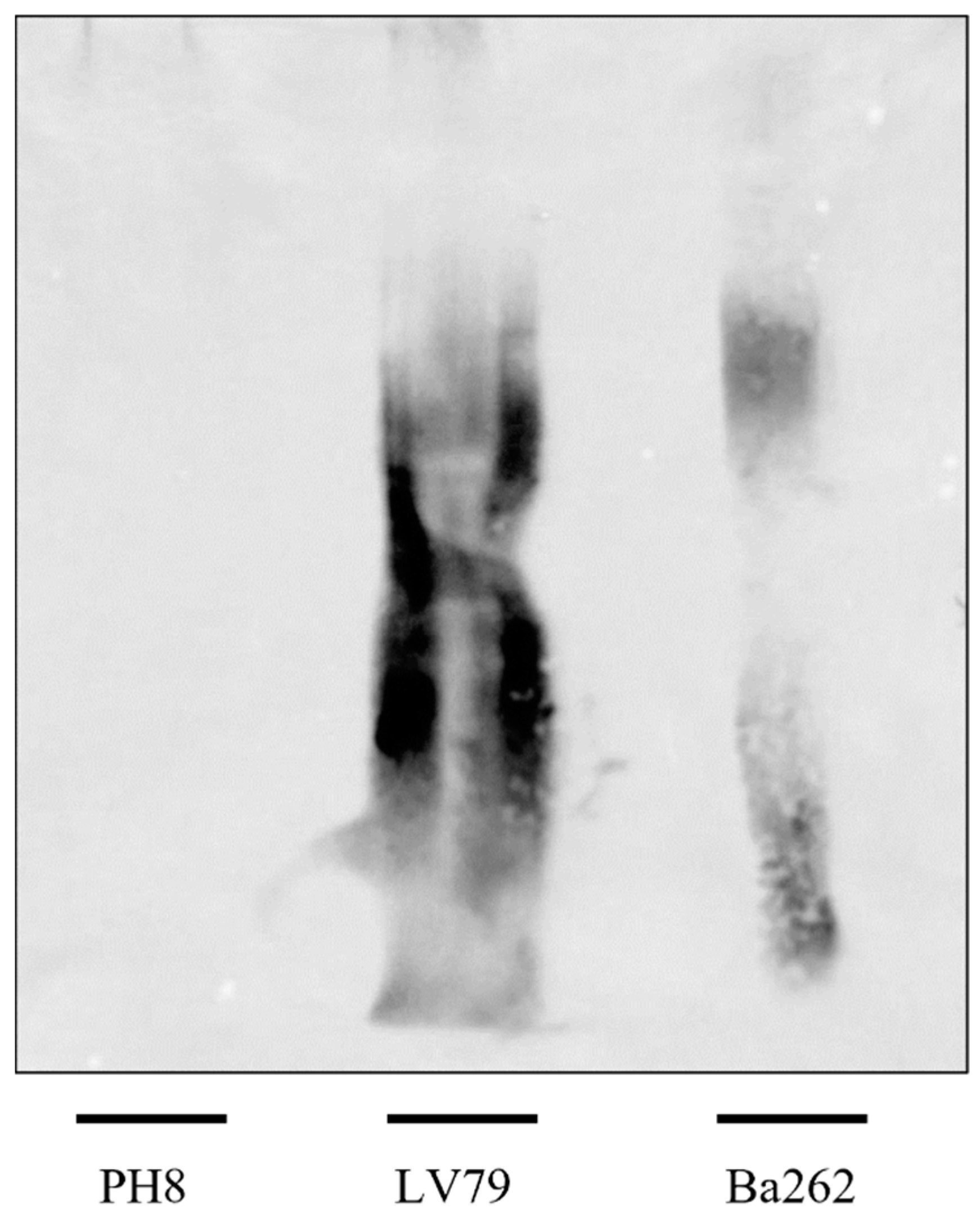
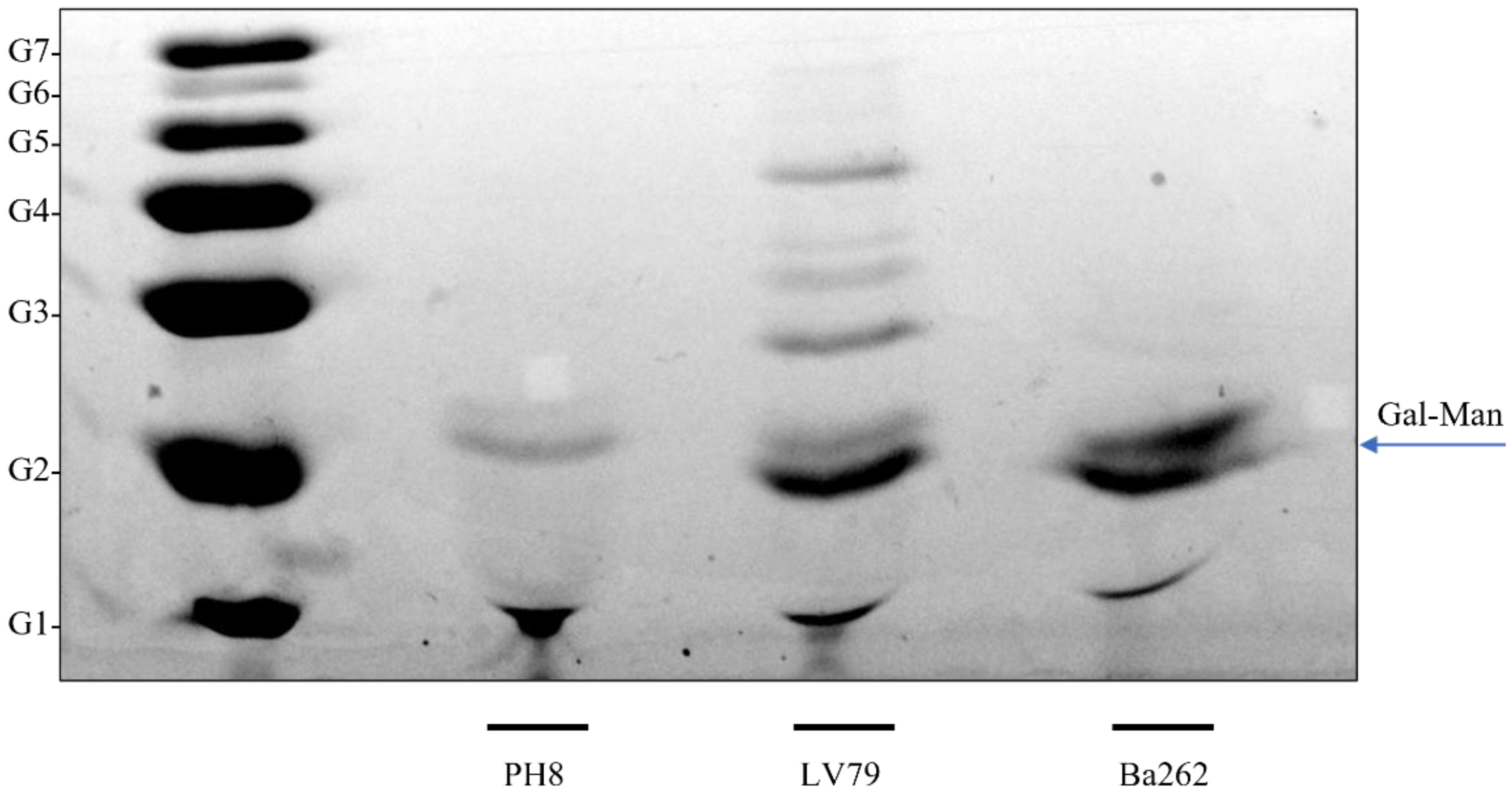
3.3. LPG Composition Differs between PH8 and LV79 Strains
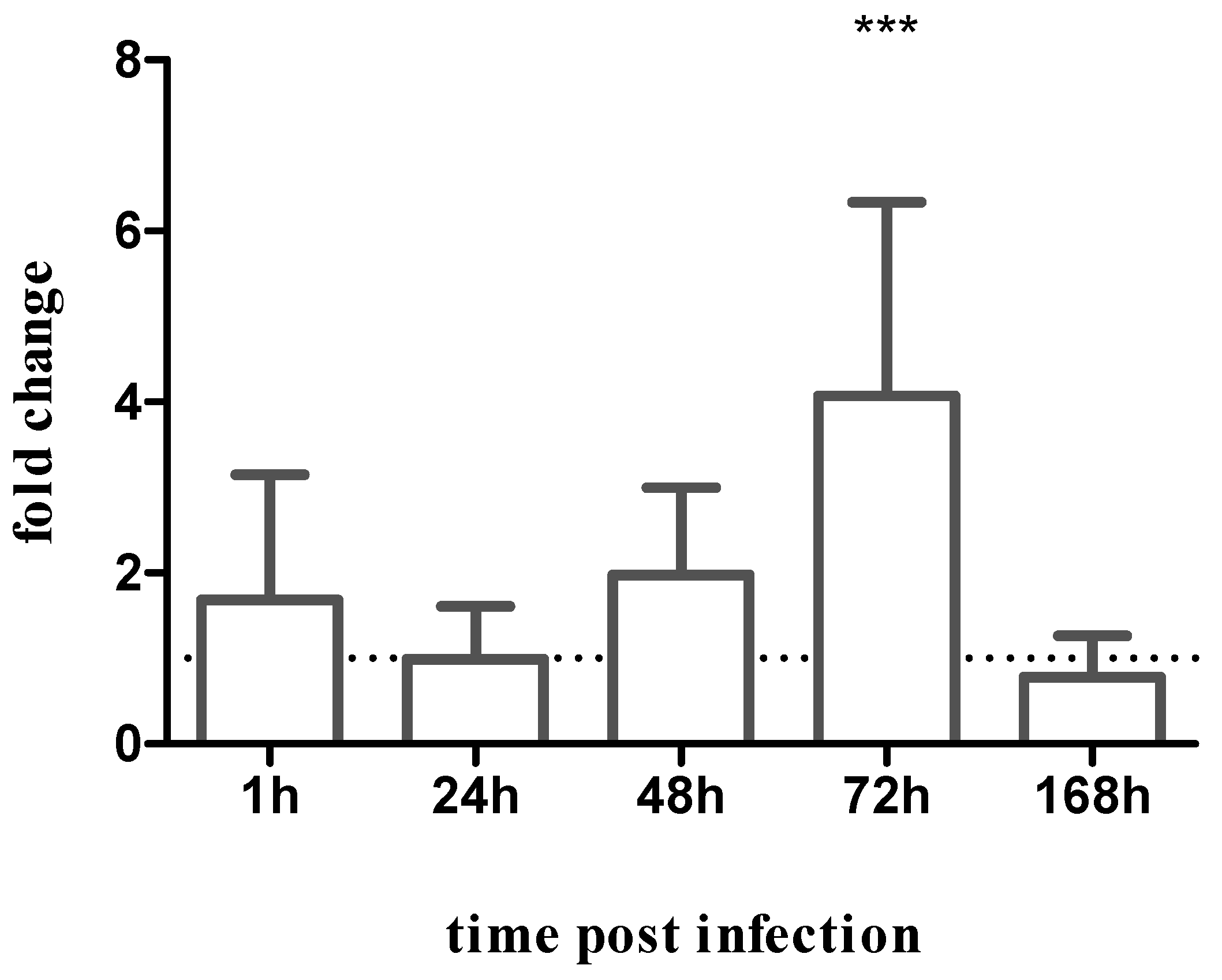
3.4. Phlebotomine Levels of Infection Differs between PH8 and LV79 Strains
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaye, P.M.; Cruz, I.; Picado, A.; Van Bocxlaer, K.; Croft, S.L. Leishmaniasis immunopathology—Impact on design and use of vaccines, diagnostics and drugs. Semin. Immunopathol. 2020, 42, 247–264. [Google Scholar] [CrossRef]
- Ruiz-Postigo, J.A.; Jain, S.; Mikhailov, A.; Maia-Elkhoury, A.N.; Valadas, S.; Warusavithana, S.; Osman, M.; Lin, Z.; Beshah, A.; Yajima, A.; et al. Global Leishmaniasis Surveillance: 2019–2020, a Baseline for the 2030 Roadmap; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Podinovskaia, M.; Descoteaux, A. Leishmania and the macrophage: A multifaceted interaction. Futur. Microbiol. 2015, 10, 111–129. [Google Scholar] [CrossRef]
- Velasquez, L.G.; Galuppo, M.K.; De Rezende, E.; Brandão, W.N.; Peron, J.P.; Uliana, S.R.B.; Duarte, M.I.; Stolf, B.S. Distinct courses of infection with Leishmania (L.) amazonensis are observed in BALB/c, BALB/c nude and C57BL/6 mice. Parasitology 2016, 143, 692–703. [Google Scholar] [CrossRef] [PubMed]
- de Rezende, E.; Kawahara, R.; Peña, M.S.; Palmisano, G.; Stolf, B.S. Quantitative proteomic analysis of amastigotes from Leishmania (L.) amazonensis LV79 and PH8 strains reveals molecular traits associated with the virulence phenotype. PLoS Negl. Trop. Dis. 2017, 11, e0006090. [Google Scholar] [CrossRef] [PubMed]
- Volpedo, G.; Pacheco-Fernandez, T.; Holcomb, E.A.; Cipriano, N.; Cox, B.; Satoskar, A.R. Mechanisms of Immunopathogenesis in Cutaneous Leishmaniasis and Post Kala-azar Dermal Leishmaniasis (PKDL). Front. Cell. Infect. Microbiol. 2021, 11, 685296. [Google Scholar] [CrossRef] [PubMed]
- Cupolillo, E.; Medina-Acosta, E.; Noyes, H.; Momen, H.; Grimaldi, G., Jr. A Revised Classification for Leishmania and Endotrypanum. Parasitol. Today 2000, 16, 142–144. [Google Scholar] [CrossRef]
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votypka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Negl. Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef]
- Espinosa, O.A.; Serrano, M.G.; Camargo, E.P.; Teixeira, M.M.G.; Shaw, J.J. An appraisal of the taxonomy and nomenclature of trypanosomatids presently classified as Leishmania and Endotrypanum. Parasitology 2018, 145, 430–442. [Google Scholar] [CrossRef]
- Kevric, I.; Cappel, M.A.; Keeling, J.H. New World and Old World Leishmania Infections: A Practical Review. Dermatol. Clin. 2015, 33, 579–593. [Google Scholar] [CrossRef]
- Dostálová, A.; Volf, P. Leishmania development in sand flies: Parasite-vector interactions overview. Parasites Vectors 2012, 5, 276. [Google Scholar] [CrossRef]
- Al-Khalaifah, H.S. Major Molecular Factors Related to Leishmania Pathogenicity. Front. Immunol. 2022, 13, 847797. [Google Scholar] [CrossRef]
- Brittingham, A.; Morrison, C.J.; McMaster, W.R.; McGwire, B.S.; Chang, K.P.; Mosser, D.M. Role of the Leishmania surface protease gp63 in complement fixation, cell adhesion, and resistance to complement-mediated lysis. J. Immunol. 1995, 155, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.L.; Modi, G.; Rowton, E.; Spath, G.; Epstein, L.; Turco, S.J.; Beverley, S.M. The role of phosphoglycans in Leishmania-sand fly interactions. Proc. Natl. Acad. Sci. USA 2000, 97, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-P.; Reed, S.G.; McGwire, B.S.; Soong, L. Leishmania model for microbial virulence: The relevance of parasite multiplication and pathoantigenicity. Acta Trop. 2003, 85, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Späth, G.F.; Garraway, L.A.; Turco, S.J.; Beverley, S.M. The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts. Proc. Natl. Acad. Sci. USA 2003, 100, 9536–9541. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Donelson, J.E.; Wilson, M.E. The major surface protease (MSP or GP63) of Leishmania sp. Biosynthesis, regulation of expression, and function. Mol. Biochem. Parasitol. 2003, 132, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Svárovská, A.; Ant, T.H.; Seblová, V.; Jecná, L.; Beverley, S.; Volf, P. Leishmania major Glycosylation Mutants Require Phosphoglycans (lpg2−) but Not Lipophosphoglycan (lpg1−) for Survival in Permissive Sand Fly Vectors. PLoS Negl. Trop. Dis. 2010, 4, e580. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edelson, P.J. The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leish-mania promastigotes. J. Immunol. 1985, 135, 2785–2789. [Google Scholar] [CrossRef] [PubMed]
- Atayde, V.D.; Hassani, K.; da Silva Lira Filho, A.; Borges, A.R.; Adhikari, A.; Martel, C.; Olivier, M. Leishmania exosomes and other virulence factors: Impact on innate immune response and macrophage functions. Cell. Immunol. 2016, 309, 7–18. [Google Scholar] [CrossRef] [PubMed]
- McGwire, B.S.; Chang, K.-P.; Engman, D.M. Migration through the Extracellular Matrix by the Parasitic Protozoan Leishmania Is Enhanced by Surface Metalloprotease gp63. Infect. Immun. 2003, 71, 1008–1010. [Google Scholar] [CrossRef]
- Puentes, S.M.; Sacks, D.L.; Da Silva, R.P.; Joiner, K. Complement binding by two developmental stages of Leishmania major promastigotes varying in expression of a surface lipophosphoglycan. J. Exp. Med. 1988, 167, 887–902. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.L.; Brodin, T.N.; Turco, S.J. Developmental modification of the lipophosphoglycan from Leishmania major promastigotes during metacyclogenesis. Mol. Biochem. Parasitol. 1990, 42, 225–233. [Google Scholar] [CrossRef]
- Talamas-Rohana, P.; Wright, S.D.; Lennartz, M.R.; Russell, D.G. Lipophosphoglycan from Leishmania mexicana promastigotes binds to members of the CR3, p150,95 and LFA-1 family of leukocyte integrins. J. Immunol. 1990, 144, 4817–4824. [Google Scholar] [CrossRef] [PubMed]
- Kamhawi, S.; Ramalho-Ortigao, M.; Pham, V.M.; Kumar, S.; Lawyer, P.G.; Turco, S.J.; Barillas-Mury, C.; Sacks, D.L.; Valenzuela, J.G. A role for insect galectins in parasite survival. Cell 2004, 119, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Coutinho-Abreu, I.V.; Oristian, J.; de Castro, W.; Wilson, T.R.; Meneses, C.; Soares, R.P.; Borges, V.M.; Descoteaux, A.; Kamhawi, S.; Valenzuela, J.G. Binding of Leishmania infantum Lipophosphoglycan to the Midgut Is Not Sufficient to Define Vector Competence in Lutzomyia longipalpis Sand Flies. Msphere 2020, 5, e00594-20. [Google Scholar] [CrossRef]
- Nogueira, P.M.; Assis, R.R.; Torrecilhas, A.C.; Saraiva, E.M.; Pessoa, N.L.; Campos, M.A.; Marialva, E.F.; Ríos-Velasquez, C.M.; Pessoa, F.A.; Secundino, N.F.; et al. Lipophosphoglycans from Leishmania amazonensis Strains Display Immunomodulatory Properties via TLR4 and Do Not Affect Sand Fly Infection. PLoS Negl. Trop. Dis. 2016, 10, e0004848. [Google Scholar] [CrossRef]
- Nogueira, P.M.; Guimarães, A.C.; Assis, R.R.; Sadlova, J.; Myskova, J.; Pruzinova, K.; Hlavackova, J.; Turco, S.J.; Torrecilhas, A.C.; Volf, P.; et al. Lipophosphoglycan polymorphisms do not affect Leishmania amazonensis development in the permissive vectors Lutzomyia migonei and Lutzomyia longipalpis. Parasites Vectors 2017, 10, 608. [Google Scholar] [CrossRef]
- Coelho-Finamore, J.; Freitas, V.; Assis, R.; Melo, M.; Novozhilova, N.; Secundino, N.; Pimenta, P.; Turco, S.; Soares, R. Leishmania infantum: Lipophosphoglycan intraspecific variation and interaction with vertebrate and invertebrate hosts. Int. J. Parasitol. 2010, 41, 333–342. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Nogueira, P.M.; Silva, S.D.O.; Sadlova, J.; Pruzinova, K.; Hlaváčová, J.; Melo, M.N.; Soares, R.P. Lower galactosylation levels of the Lipophosphoglycan from Leishmania (Leishmania) major-like strains affect interaction with Phlebotomus papatasi and Lutzomyia longipalpis. Mem. Inst. Oswaldo Cruz 2018, 113, e170333. [Google Scholar] [CrossRef]
- Scorza, B.M.; Carvalho, E.M.; Wilson, M.E. Cutaneous Manifestations of Human and Murine Leishmaniasis. Int. J. Mol. Sci. 2017, 18, 1296. [Google Scholar] [CrossRef]
- Silveira, F.T.; Lainson, R.; Corbett, C.E.P. Clinical and immunopathological spectrum of American cutaneous leishmaniasis with special reference to the disease in Amazonian Brazil: A review. Mem. Inst. Oswaldo Cruz 2004, 99, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, H.O.; Almeida, L.V.; Roatt, B.M.; Reis-Cunha, J.L.; Pereira, A.A.S.; Gontijo, C.; Fujiwara, R.T.; Reis, A.B.; Sanders, M.J.; Cotton, J.A.; et al. Comparative genomics of canine-isolated Leishmania (Leishmania) amazonensis from an endemic focus of visceral leishmaniasis in Governador Valadares, southeastern Brazil. Sci. Rep. 2017, 7, 40804. [Google Scholar] [CrossRef]
- Lainson, R.; Shaw, J.J.; Silveira, F.; De Souza, A.A.A.; Braga, R.R.; Ishikawa, E.A.Y. The dermal leishmaniases of Brazil, with special reference to the eco-epidemiology of the disease in Amazonia. Mem. Inst. Oswaldo Cruz 1994, 89, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Tano, F.T.; Barbosa, G.R.; de Rezende, E.; Souza, R.O.O.; Muxel, S.M.; Silber, A.M.; Palmisano, G.; Stolf, B.S. Proteome and morphological analysis show unexpected differences between promastigotes of Leishmania amazonensis PH8 and LV79 strains. PLoS ONE 2022, 17, e0271492. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.P.; Barron, T.; McCoy-Simandle, K.; Svobodova, M.; Warburg, A.; Turco, S.J. Leishmania tropica: Intraspecific polymorphisms in lipophosphoglycan correlate with transmission by different Phlebotomus species. Exp. Parasitol. 2004, 107, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Ueno, N.; Wilson, M.E. Receptor-mediated phagocytosis of Leishmania: Implications for intracellular survival. Trends Parasitol. 2012, 28, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, L.M.; Ozaki, M.; Donelson, J.E.; Beetham, J.K. Genetic complementation of Leishmania deficient in PSA (GP46) restores their resistance to lysis by complement. Mol. Biochem. Parasitol. 2004, 137, 185–189. [Google Scholar] [CrossRef]
- Rego, F.D.; Cardoso, C.D.A.; Moreira, P.O.L.; Nogueira, P.M.; Araujo, M.S.; Borges, V.M.; Laurenti, M.D.; Bartholomeu, D.C.; Reis, A.B.; Monte-Neto, R.L.D.; et al. Leishmania amazonensis from distinct clinical forms/hosts has polymorphisms in Lipophosphoglycans, displays variations in immunomodulatory properties and, susceptibility to antileishmanial drugs. Cell Biol. Int. 2022, 46, 1947–1958. [Google Scholar] [CrossRef]
- Telleria, E.L.; Martins-Da-Silva, A.; Tempone, A.J.; Traub-Csekö, Y.M. Leishmania, microbiota and sand fly immunity. Parasitology 2018, 145, 1336–1353. [Google Scholar] [CrossRef]
- Manzano, J.I.; Perea, A.; León-Guerrero, D.; Campos-Salinas, J.; Piacenza, L.; Castanys, S.; Gamarro, F. Leishmania LABCG1 and LABCG2 transporters are involved in virulence and oxidative stress: Functional linkage with autophagy. Parasites Vectors 2017, 10, 267. [Google Scholar] [CrossRef]
- Ong, G.L.; Mattes, M. Mouse strains with typical mammalian levels of complement activity. J. Immunol. Methods 1989, 125, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.A.; Araujo, G.V.; Sandoval, C.M.; Nogueira, P.M.; Zúniga, C.; Sosa-Ochoa, W.H.; Laurenti, M.D.; Soares, R.P. Lipophosphoglycans from dermotropic Leishmania infantum are more pro-inflammatory than those from viscerotropic strains. Mem. Inst. Oswaldo Cruz 2020, 115, e200140. [Google Scholar] [CrossRef]
- Assis, R.R.; Ibraim, I.C.; Noronha, F.S.; Turco, S.J.; Soares, R.P. Glycoinositolphospholipids from Leishmania braziliensis and L. infantum: Modulation of Innate Immune System and Variations in Carbohydrate Structure. PLoS Negl. Trop. Dis. 2012, 6, e1543. [Google Scholar] [CrossRef] [PubMed]
- Paranaíba, L.F.; de Assis, R.R.; Nogueira, P.M.; Torrecilhas, A.C.; Campos, J.H.; Silveira, A.C.D.O.; Martins-Filho, O.A.; Pessoa, N.L.; Campos, M.A.; Parreiras, P.M.; et al. Leishmania enriettii: Biochemical characterisation of lipophosphoglycans (LPGs) and glycoinositolphospholipids (GIPLs) and infectivity to Cavia porcellus. Parasites Vectors 2015, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Vieira, T.D.S.; Rugani, J.N.; Nogueira, P.M.; Torrecilhas, A.C.; Gontijo, C.M.F.; Descoteaux, A.; Soares, R.P. Intraspecies Polymorphisms in the Lipophosphoglycan of L. braziliensis Differentially Modulate Macrophage Activation via TLR4. Front. Cell. Infect. Microbiol. 2019, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, L.; Nikolaev, A.V.; Feng, G.J.; Wei, W.Q.; Ferguson, M.A.; Brimacombe, J.S.; Liew, F.Y. Regulation of the expression of nitric oxide synthase and leishmanicidal activity by glycoconjugates of Leishmania lipophosphoglycan in murine macrophages. Proc. Natl. Acad. Sci. USA 1996, 93, 10984–10989. [Google Scholar] [CrossRef]
- Telleria, E.L.; Azevedo-Brito, D.A.; Kykalová, B.; Tinoco-Nunes, B.; Pitaluga, A.N.; Volf, P.; Traub-Csekö, Y.M. Leishmania infantum Infection Modulates the Jak-STAT Pathway in Lutzomyia longipalpis LL5 Embryonic Cells and Adult Females, and Affects Parasite Growth in the Sand Fly. Front. Trop. Dis. 2021, 2, 747820. [Google Scholar] [CrossRef]
- Telleria, E.L.; Sant’Anna, M.R.; Ortigão-Farias, J.R.; Pitaluga, A.N.; Dillon, V.M.; Bates, P.A.; Traub-Csekö, Y.M.; Dillon, R.J. Caspar-like Gene Depletion Reduces Leishmania Infection in Sand Fly Host Lutzomyia longipalpis. J. Biol. Chem. 2012, 287, 12985–12993. [Google Scholar] [CrossRef]
- Di-Blasi, T.; Telleria, E.L.; Marques, C.; Couto, R.D.M.; Da Silva-Neves, M.; Jančářová, M.; Volf, P.; Tempone, A.J.; Traub-Csekö, Y.M. Lutzomyia longipalpis TGF-β Has a Role in Leishmania infantum chagasi Survival in the Vector. Front. Cell. Infect. Microbiol. 2019, 9, 71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tano, F.T.; Telleria, E.L.; Rêgo, F.D.; Coelho, F.S.; de Rezende, E.; Soares, R.P.; Traub-Cseko, Y.M.; Stolf, B.S. Phenotypical Differences between Leishmania (Leishmania) amazonensis PH8 and LV79 Strains May Impact Survival in Mammal Host and in Phlebotomine Sand Flies. Pathogens 2023, 12, 173. https://doi.org/10.3390/pathogens12020173
Tano FT, Telleria EL, Rêgo FD, Coelho FS, de Rezende E, Soares RP, Traub-Cseko YM, Stolf BS. Phenotypical Differences between Leishmania (Leishmania) amazonensis PH8 and LV79 Strains May Impact Survival in Mammal Host and in Phlebotomine Sand Flies. Pathogens. 2023; 12(2):173. https://doi.org/10.3390/pathogens12020173
Chicago/Turabian StyleTano, Fabia Tomie, Erich Loza Telleria, Felipe Dutra Rêgo, Felipe Soares Coelho, Eloiza de Rezende, Rodrigo Pedro Soares, Yara Maria Traub-Cseko, and Beatriz Simonsen Stolf. 2023. "Phenotypical Differences between Leishmania (Leishmania) amazonensis PH8 and LV79 Strains May Impact Survival in Mammal Host and in Phlebotomine Sand Flies" Pathogens 12, no. 2: 173. https://doi.org/10.3390/pathogens12020173
APA StyleTano, F. T., Telleria, E. L., Rêgo, F. D., Coelho, F. S., de Rezende, E., Soares, R. P., Traub-Cseko, Y. M., & Stolf, B. S. (2023). Phenotypical Differences between Leishmania (Leishmania) amazonensis PH8 and LV79 Strains May Impact Survival in Mammal Host and in Phlebotomine Sand Flies. Pathogens, 12(2), 173. https://doi.org/10.3390/pathogens12020173







