Abstract
A cysticercosis model of Taenia crassiceps ORF strain in susceptible BALB/c mice revealed a Th2 response after 4 weeks, allowing for the growth of the parasite, whereas resistant C57BL/6 mice developed a sustained Th1 response, limiting parasitic growth. However, little is known about how cysticerci respond to an immunological environment in resistant mice. Here, we show that the Th1 response, during infection in resistant C57BL/6 mice, lasted up to 8 weeks and kept parasitemia low. Proteomics analysis of parasites during this Th1 environment showed an average of 128 expressed proteins; we chose 15 proteins whose differential expression varied between 70 and 100%. A total of 11 proteins were identified that formed a group whose expression increased at 4 weeks and decreased at 8 weeks, and another group with proteins whose expression was high at 2 weeks and decreased at 8 weeks. These identified proteins participate in tissue repair, immunoregulation and parasite establishment. This suggests that T. crassiceps cysticerci in mice resistant under the Th1 environment express proteins that control damage and help to establish a parasite in the host. These proteins could be targets for drugs or vaccine development.
1. Introduction
Taenia solium seriously affects human health in many Latin American, Asian, and African countries. It causes neurocysticercosis, which is the principal causes of epilepsy associated with infectious disease worldwide [1].
Because of the close phylogenetic relationship between Taenia crassiceps and T. solium and their antigenic and genomic similarity, the murine cysticercosis model for T. crassiceps has been widely used to study several physiological aspects of cysticercosis [2,3,4]. The resistance and susceptibility to T. crassiceps infections have been associated with the endocrine, genetic, and immune response in mice [5,6,7]. The susceptible BALB/c mouse favors a Th2 response and parasites’ growth with high levels of interleukin 4 (IL-4), IL-10 IL-13, IL-9, and transforming growth factor-beta (TGF-β), whereas the resistant C57BL/6 mouse develops a predominant Th1 response that limits the parasitic growth [8,9,10,11].
On the other hand, the immune response is influenced by parasite molecules. Cysticerci secretion/excretion (E/S) products promote a Th2 response with alternatively activated macrophages and T regulatory (Treg) cells in BALB/c mice [7,12,13,14]. Likewise, other parasite molecules drive toward the Th1 response with high levels of gamma interferon (IFN-γ) and IL-2, such as the cysticerci peptides KETc-1, KETc-2, and the enzyme glutathione transferases (SGSTF) [15,16]. In addition, antioxidant enzymes such as Cu,Zn superoxide dismutase, thioredoxin1, 2-Cys peroxiredoxin, and Glutathione transferases from Taenia participate in the protection and regulation of immune response. These antigens and enzymes have been proposed as vaccine candidates [17,18]. However, little is known about the protein expression when cysticerci are in a resistant host with a Th1 environment.
In the present study, we used 2-dimensional electrophoresis (2DE) and MALDI-TOF-mass spectrometry to determine the protein expression profile of cysticerci during an infection of C57BL/6 mice in a Th1 environment. In addition, we identified those proteins involved in repair, establishment, and defensive roles.
2. Materials and Methods
2.1. Mice
Female mice C57BL/6 and BALB/c strains were kept in the animal facilities at the Instituto de Investigaciones Biomedicas, UNAM, under controlled conditions of temperature (22 °C), a pathogen-free environment, relative humidity of 50–60%, 12-h dark-light cycles, and free access to food and water. The Institutional Ethics Committee approved all animal protocols (permission no. 2015–175).
2.2. Resistant Mice Infection with the T. crassiceps Cysticerci ORF Strain
C57BL/6 mice were intraperitoneally infected with ten cysticerci of 2–3 mm in 100 µL of sterile PBS; cysticerci were obtained from a female BALB/c with 6 months of infection. Infected mice were euthanized at 2 weeks, 4 weeks, and 8 weeks of infection in a CO2 chamber. The cysticerci were extracted from the peritoneal cavity, counted, washed 3-times with PBS in the presence of a protease inhibitor (Halt™ Protease Inhibitor Cocktail, Thermo Scientific, Waltham, MA, USA), and stored at −70 °C until used.
2.3. Spleen Cells Isolation and Cytokine Detection
Spleens from infected or non-infected mice were surgically removed after euthanasia under sterile conditions and splenocytes were isolated as previously reported [16]. Spleen cells (2 × 106/mL) of infected and non-infected mice were stimulated with 5 µg/mL of T. crassiceps crude extract and cultured during 72 h at 37 °C and 5% CO2. Tumor necrosis factor (TNF), IL-12, IFN-γ, IL-5, IL-6, and IL-4 were quantified in the culture supernatants by ELISA, according to the manufacturer’s instructions (Mini ELISA Development Kit PEPROTECH). Assays were performed in triplicate.
2.4. Two-Dimensional Gel Electrophoresis (2DE)
Protein extract was prepared with the frozen cysticerci, as previously reported [19], in 2DE buffer (8 M urea, 50 mM DTT, 2% CHAPS, 2% Ampholine®, pH 3–10) (Bio-Rad, Hercules, CA, USA) and protease inhibitor (Halt™ Protease Inhibitor Cocktail). The protein concentration was measured using the Bradford method [20]. A total of 100 µg of protein from each sample was applied to IPG strips (GE HealthCare, Chicago, IL, USA) in an immobilized pH gradient from 3 to 10 by isoelectric focusing at 10,000 V over 8 h. Next, the strips were equilibrated with one wash for 10 min with 8 M urea, 0.375 M Tris, pH 8.8, 2% SDS, 20% glycerol, and 2% (w/v) DTT, and another wash for 10 min with 8 M urea, 0.375 M Tris pH 8.8, 2% SDS, 20% glycerol, and 2.5% (w/v) iodoacetamide. The proteins were separated in a second dimension by SDS-PAGE in a buffer of 25 mM Tris, pH 8.3, 250 mM glycine, and 0.01% SDS.
2.5. Proteomics Analysis
Next, 2DE gels were digitized on an HP Scanjet-G4050 scanner with a resolution of 300 DPI and analyzed using PDQuest™ 2DE Software (Bio-Rad) to determine expression levels. Master images were created from 3 repetitions of each 2DE image from 2-, 4-, and 8-weeks infection time of 2 separate experiments. The coordinates of each spot were calculated according to isoelectric point markers from 2DE standards (Bio-Rad). Data were normalized with triosephosphate isomerase enzyme (TPI), which depicted a constant level of expression during infection.
2.6. Statistical Analysis
One or two-way analysis of variance (ANOVA) with Tukey’s multiple comparison test was used for the statistical analyses of collected data, and p values < 0.05 were considered significant.
2.7. MALDI-TOF Mass Spectrometry Protein-Identification
Proteins with up- or down-expression levels between 70 and 100% were selected and excised manually using a clean razor blade. Samples were prepared for mass spectrum analysis using a slight modification of a previously described procedure [19,21]. Peptides were desalted using a C18 ZipTip (Millipore, Bedford, MA, USA), according to manufacturer’s instructions. Analyses were performed using a Bruker Daltonics Autoflex (Bruker Daltonics, Bellerica, MA, USA). The peptides mixture was analyzed using a saturated solution of α-cyano-4-hydroxycinnamic acid (HCCA) in 50% acetonitrile/0.1% trifluoroacetic acid. Lists of masses from tryptic peptides were generated and compared to the helminths databases in NCBI (http://www.ncbi.nlm.nih.gov/, accessed on 21 April 2021), using the MASCOT search program (Matrix Science, Ltd., UK) with the following parameters: one missed cleavage allowed, carbamidomethyl cysteine as the fixed modification, and oxidation of methionine as the variable modification, mass tolerance 200 ppm. Proteins with a score higher than 67 and a threshold of significance of p < 0.05 for extensive homology were accepted as positive. Additionally, peptide sequences were analyzed using BLASTP against the Taenia solium Genome BioProject PRJNA170813 (http://www.ncbi.nlm.nih.gov/bioproject/PRJNA170813, accessed on 28 April 2021 and https://parasite.wormbase.org/Taenia_solium_prjna170813/Info/Index/, accessed on 26 October 2022) databases. Classification of proteins was based on Gene Ontology Biological Process information available from the UniProtKB database (http://www.uniprot.org/, accessed on 5 June 2021).
3. Results
3.1. Immune Response in the T. crassiceps Cysticercosis Model in C57BL/6 Mouse
IL-12 and IFN-γ showed similar levels in both infected and non-infected groups at 2 and 4 weeks (Figure 1A). However, at 8 weeks, both cytokines showed a statistically significant increase in the infected group. TNF levels in the non-infected and infected group at 2 and 8 weeks were higher than the levels at week 4, although the differences were not statistically significant in the control group. Whereas, in the infected group, a significant increase in TNF production was observed in the 8-week mice. The amount of IL-6 was similar in the non-infected control group at weeks 2, 4, and 8. In the infected group, a statistically significant increase was detected at week 4, as well as a statistically significant decrease at 8 weeks. IL-5 presented a baseline level in the non-infected group.
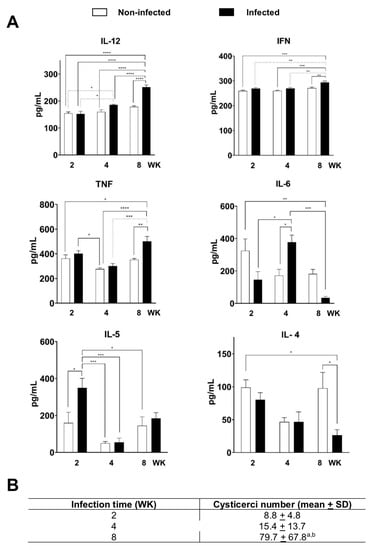
Figure 1.
Cytokine levels in C57/BL/6 mice with cysticercosis at 2, 4, and 8 weeks (WK) after stimulation with T. crassiceps crude extract. (A) Levels of IL-12, INF-γ, TNF, IL-6, IL5, and IL-4 in the supernatants of cultured splenocytes determined with ELISA, significance of * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001. (B) Average number of cysticerci in infected mice at 2, 4, and 8 weeks (WK). Mean for each experiment was measured for n = 18 (2 WK), n = 8 (4 WK), and n = 5 (8 WK). a p < 0.05 vs. 2 weeks; b p < 0.05 vs. 4 weeks. One-way ANOVA and Tukey’s multiple comparisons test.
In contrast, in infected groups, a significant increase at 2 weeks was observed, which gradually decreased its levels by half at week 8, whereas IL-4 maintained basal levels at 2 and 4 weeks in non-infected and infected groups, but its levels decreased by half at week 8 in the infected group. Figure 1B shows the average number of cysticerci increased during infection. In week 2, an average of 8.8 cysticerci, in week 4 of 15.4, and in week 8 of 79.9, were recorded.
3.2. Proteomics Analysis and Mass Spectrometry Protein-Identification
Figure 2 shows master gel images constructed from 2DE gels with cysticerci extracts from 2-, 4-, and 8-weeks infected mice. Differences in intensities of expression were noticeable, and the proteomics analyses detected 132 spots at 2 weeks, 137 spots at 4 weeks, and 116 spots at 8 weeks of infection. Most spots were distributed within the proteomics map in a molecular weight range of 5 to 150 kDa and a pH range of 4.0 to 9.4.
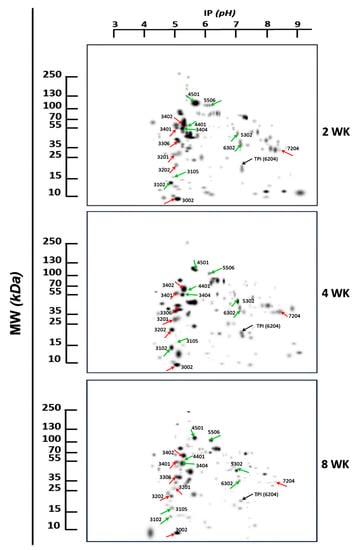
Figure 2.
Representative 2DE proteomics profiles of T. crassiceps cysticerci in infected mice at 2, 4, and 8 weeks (WK). Molecular weight (MW) and isoelectric point (IP) are indicated in kDa and pH, respectively. The arrows and numbers indicate the spots of proteins with a differential expression between 70% and 100% and sequenced by MALDI-TOF MS. TPI was used as a constitutive protein (black arrow). Red arrows show group 1, and green arrows show spots of group 2.
Figure 3 shows the differential expression of 15 spots randomly chosen, expressed in optical density after normalization with TPI. These spots were classified into two different groups, according to the protein differential expression. In group 1, 7 proteins with low expression at week 2 significantly increased their expression at week 4 and returned to low level at week 8. In group 2, 8 proteins were found in which expression was high at week 2, and gradually decreased at 8 weeks.
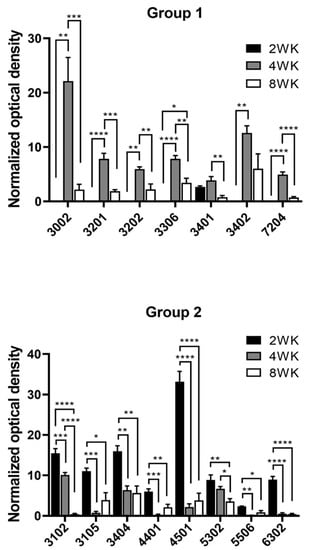
Figure 3.
Profiles of the differential expression of proteins by T. crassiceps cysticerci at 2, 4, and 8 weeks (WK) post-infection. Two groups were identified: Group (1) low expression at 2 weeks that increased at the 4th week and decreased on the 8th week, and Group (2) high expression at 2 weeks that decreased at 4 and 8 weeks, the last being the lowest. Significance of * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
Table 1 reveals the identification of 11 of the 15 proteins of T. crassiceps cysticerci. Results of the MASCOT analysis showed that these proteins match those of cestodes with a sequence coverage of 19 at 58%. In addition, all sequences matched the T. solium genome, obtaining the contig-ubication and the ID of the gene.

Table 1.
Protein identification from Taenia crassiceps ORF cysticerci by amino acid sequence and BLAST analysis with the Mascot and Taenia solium database genome. Gene Ontology program was used to identify process and molecular function. The theoretical Ip and MW were calculated using the sequence of each protein.
In group 1, the 14-3-3 protein zeta/delta (spot-3201, KCIP 1) was identified, which is an isoform of family adapter proteins [22]. In parasites, it recognizes and binds specifically to certain sites of phosphorylated proteins [23] that are involved in cell cycle and physiology regulation, apoptosis, control of gene transcription [24,25,26], evasion mechanisms, and establishment of infection; therefore, they have been considered as antigens to develop a vaccine for Echinococcus multilocularis [27,28].
Myosin regulatory light chainγ (spot-3202, MRLCγ) belongs to a large family of proteins involved in the contraction of the smooth muscle [29,30]. In parasites, these proteins play a critical role for migration because vigorous muscular movement is necessary for the penetration and tissue migration. Moreover, they are considered promising vaccinal antigens because they lead to the reduction in the trematodes load, such as Schistosoma mansoni, or the production of eggs. When they are phosphorylated, they generate a calcium influx, leading to parasite death due to the action of eosinophils [31]. In addition, activated platelets produce myosin light chain 9 networks (Myl9 nets), which recruit CD69-expressing inflammatory cells to inflamed tissues, leading to exacerbation of inflammation [32].
Annexin B7 (spot-3306, AnxB7) belongs to a family of proteins that can bind membranes via the Ca2+ ion, and negatively charged phospholipids are considered scaffolding proteins that participate in membrane dynamics. AnxB7 is involved in cell motility, endocytosis, fibrinolysis, ion channel formation, cell matrix interactions, apoptosis, angiogenesis, and anticoagulation [33,34]. In the genome of T. solium, 13 genes coding for the annexin family have been identified. AnxB7 forms a complex with members of the endosomal sorting complex required for transport (ESCRT-III), which helps to excise and repair the damaged region of the plasma membrane, including lipid peroxidation damage [35,36].
Tubulin β 2C chain (spot 3401), β-tubulin, and α-tubulin are components of microtubules and are important in cell division and intracellular trafficking. They are related to cysticerci plasticity, motility, dynamicity, and shape [37,38,39,40].
The 60-kDa heat-shock protein (spot-3402, HSP60) is expressed by cells exposed to stress or immune activation and during inflammation. HSP60 inhibits T cell chemotaxis and stimulates the production of IL-10 in T cells, shifting the profile toward Th2. Inside the cell, HSP60 is a chaperone [41,42]. The Schistosoma japonicum HSP60 promotes immunosuppressive Tregs by inducing TGF-β and IL-10 production and reducing mouse liver immunopathology [43].
In group 2, proteins such as 14-3-3 epsilon protein (spot 3102) were found, which is a regulator of the TrkB/PI3K/Akt pathway [44]. The 14-3-3 epsilon protein has been identified as a molecule recruited by the TNRF2 forming a complex that inhibits NF-kB and promotes M2 response [45].
ATP synthase subunit beta (spot 3404) is involved in ATP synthesis-coupled proton transport [46]. ATP synthase subunit beta has been identified in T. solium and in Trichinella britovi as an antigen of adult worm in early infection [47,48]. It is a protein that stimulates the production of antibodies, in addition to being a signaling protein [49].
Protein disulfide isomerase (spot 4401, PDI) is a ubiquitous REDOX and multifunction enzyme, belonging to the thioredoxin family, which catalyzes dithiol–disulfide exchange reactions and displays chaperone activity. The increase in PDI is involved in pathogen infection processes and plays an important virulence role during host infection [50,51]. Specific PDI inhibitors abolished the enzymatic activity and markedly affected parasite growth [52,53,54].
Paramyosin (spot 4501, Pmy). In T. solium, Pmy is a component of the musculature but has also been found associated with the tegumentary cytons and is released through the tegument. T. solium Pmy induces significant levels of protection in cysticercosis by T. crassiceps and schistosomiasis inducing a Th1-like immune response [55,56,57]. Moreover, passive immunization with a Pmy monoclonal antibody reduces the parasite load. In addition, Pmy plays a role in immune evasion by interfering with complement activation binding to C1q, C8, and C9 proteins [58,59]. Therefore, it has been considered a target for the creation of vaccines for diverse helminths.
Enolase 3 (spot 5302, Eno3, 2-phospho-D-glycerate hydrolase) is a glycolytic enzyme that catalyzes the conversion of 2-phosphoglycerate to phosphoenolpyruvate, it is excreted/secreted in helminths, acting as a plasminogen-binding protein and favoring the fibrin degradation to facilitate establishment of parasites in their hosts [60,61,62,63,64,65,66]. In T. solium, four enolase isoforms TsEno1, TsEno2, TsEno3, and TsEno4 have been identified. The last one lacks the plasminogen-binding motif [65]. Moreover, enolase has also been used as a diagnostic and vaccine target [49].
Fasciclin-1 (spot 5506, Fas-1). Three isoforms, Fas-1, 2, and 3 exist in T. solium cysticerci. These proteins are constitutively expressed in cysticerci and adult stages, preferentially located in the scolex and the vesicular wall, and are biomarkers for chronic neurocysticercosis (NCC) [67,68,69]. It has been reported that Fas-1 and 2 of T. solium cysticerci bind to plasminogen to convert it to plasmin, inhibiting the complement pathway and favoring immune evasion [65,70,71].
Some proteins from both groups were not identified, but their differential expression was very interesting (Figure 3). In group 1, spots 3002 and 7204, whereas in group 2, were spots 3105 and 6302.
4. Discussion
Taenia solium and T. crassiceps cysticerci cause cysticercosis in humans, pigs, and mice. Their establishment and survival depend on the environment encountered in hosts [5,6,7]. It has been demonstrated that BALB/c mice are more susceptible to parasite infection than C57BL/6 mice [5]. In the T. crassiceps cysticercosis model with susceptible BALB/c mice, cysticerci products shift to a Th2 response after 4 weeks, allowing for parasite growth, whereas resistant C57BL/6 mice develop a strong Th1 response that limits parasitic growth [9]. Despite the well-documented information about the immune response in both mice strains, there is limited information on how cysticerci respond to the immune response environment in resistant mice.
In this study, we developed a Th1 environment in the T. crassiceps cysticerci model with resistant C57BL/6 mice, which is confirmed by the sustained increase in IL-12, INF-γ, and TNF and a low parasitic load during 8 weeks of infection. This contrasts with previous results with susceptible BALB/c mice where the Th1 response is lost at week 4 of infection and replaced by a Th2 response with exponential parasites growth [19,71,72].
This sustained Th1 response and low parasite burden are consistent with previous reports on the control of parasite growth [73,74,75]. A prolonged Th1 environment does not prevent infection but slows the ability of cysticerci to reproduce [7,14]. Likewise, proteomics analysis allowed us to determine the differential protein expression profile in T. crassiceps cysticerci obtained under a Th1 environment in C57BL/6 mice. We succeeded in identifying 11 proteins; their differential expression allowed for the formation of 2 groups: The expression of proteins in group 1 was upregulated at week 4 and showed a significant downregulation at weeks 2 and 8. Group 2 proteins were highly expressed at week 2 and the expression was gradually downregulated until week 8.
It is noteworthy that high paramyosin levels correlate with high IL-5 levels during the second week of infection. This is consistent with previous reports where individuals resistant to reinfection by S. mansoni present high levels of anti-paramyosin antibodies and high levels of IL-2 and IL-5. In addition, it is known that IL-5 and IL-6 participate in the recruitment of eosinophils, which are considered important effectors in the destruction of parasites in porcine cysticercosis [76,77].
In addition, Eno3 is differentially expressed from week 2. It has been found to be expressed in cells with Th1/Th17 immune responses during the acute phase of experimental neurocysticercosis in T. crassiceps, so these responses could be present in resistant mice [78,79].
Of note, both BALB/c and C57BL/6 mice express proteins involved in establishment, reparation, and defensive roles that prevent the host’s immune attack, such as inhibition of complement and maintenance of the vesicular wall structure of cysticerci, for example Pmy, Eno, Fas-1, and Anxs, which are shared in both mice strains [19,38,39,46,50,51,65,69,80,81,82].
The downregulated expression of all proteins at week 8 in C57BL/6 mice could be indicating adaptation to the Th1 environment by cysticerci. It agrees with a previous report for C57BL/6 mice, where it controls T. crassiceps cysticerci growth, but the infection persists, and parasites reproduce slowly [9].
On the other hand, the data presented here demonstrate that the immune response against T. crassiceps differs between susceptible and resistant mice. C57BL/6 mice developed a sustained and strong Th1 response up to 8 weeks. This shows that the genetic background of this mouse strain is important to mounting a sustained Th1 response. For example, the expression of a nonclassical class I major histocompatibility complex (MHC) Qa-2 antigen in C57BL/6 mice is related to T. crassiceps cysticercosis resistance, whereas non-expression in BALB/c mice makes them susceptible [5].
A Th2 response and parasite regulatory factors are important for the survival of both the parasite and the host, but other factors, like hormones, could also be involved, for example, testosterone and 17-beta estradiol stimulate the reproduction and infectivity of the cysticercus while prostaglandin E2 favors the Th2 response, by downregulating IL-12 and IL-2 [83,84,85].
5. Conclusions
The 2DE and MALDI-TOF mass spectrometry methods allowed us to determine the protein expression profile of cysticerci during an infection of C57BL/6 mice in a Th1 environment. In this environment, parasites express proteins that help their establishment, repair of their vesicular walls, and protection against the immune responses of mice. Furthermore, our data support that the genetic background of the mice strains and a sustained Th1 response during infection with T. crassiceps cysticerci, control cysticercosis. Finally, these proteins could be targets for the development of vaccines or drugs against helminths.
Author Contributions
Conceptualization, A.L. and P.O.-S.; methodology, M.D.-Z., L.J., M.H., R.H.-Á., S.E.-G. and L.N.; software, M.H. and L.N.; formal analysis, A.L., M.D.-Z. and L.J.; investigation, L.J., M.D.-Z. and A.L.; resources, A.L. and P.O.-S.; writing—original draft preparation, L.J., M.D.-Z., A.L. and L.N.; writing—review and editing, L.J., M.D.-Z. and A.L.; supervision, A.L.; project administration, A.L. and P.O.-S.; funding acquisition, A.L. and P.O.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Dirección General de Asuntos del Personal Académico, UNAM (DGAPA-PAPIIT contract IN217419 and DGAPA-PAPIIT contract IN205422). The postdoctoral scholarship of Díaz-Zaragoza Mariana was supported by Programa de Becas Posdoctorales of UNAM.
Institutional Review Board Statement
The Institutional Ethics Committee of the Instituto de Investigaciones Biomedicas de la Universidad Nacional Autónoma de México approved all animal protocols (permission no. 2015–175), which were carried out in strict accordance with the Official Mexican Norm for the Production, Care, and Use of Laboratory Animals (NOM-062-ZOO-1999) and the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, USA.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Alicia Ochoa Sanchez for her valuable technical support, and the Immunology and Proteomics Laboratory of the Children’s Hospital “Federico Gómez”, for the acquisition of peptide mass spectra, which was carried out by means of the equipment UltrafleXtreme Bruker Daltonics (Bellerica, MA, USA).
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Working to Overcome the Global Impact of Neglected Tropical Diseases: First WHO Report on Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2010; Available online: https://apps.who.int/iris/handle/10665/44440 (accessed on 1 January 2023).
- Vaz, A.J.; Nunes, C.M.; Piazza, R.M.; Livramento, J.A.; Da Silva, M.V.; Nakamura, P.M.; Ferreira, A.W. Immunoblot with cerebrospinal fluid from patients with neuro-cysticercosis using antigen from cysticerci of Taenia solium and Taenia crassiceps. Am. J. Trop. Med. Hyg. 1997, 57, 354–357. [Google Scholar] [CrossRef]
- Willms, R.; Zurabian, R. Taenia crassiceps: In vivo and in vitro models. Para-sitology 2010, 137, 335–346. [Google Scholar] [CrossRef]
- Bobes, R.J.; Estrada, K.; Rios-Valencia, D.G.; Calderón-Gallegos, A.; de la Torre, P.; Carrero, J.C.; Sanchez-Flores, A.; Laclette, J.P. The genomes of two strains of Taenia crassiceps the animal model for the study of human cysticercosis. Front. Cell. Infect. Microbiol. 2022, 12, 876839. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, G.; Lamoyi, E.; Mellor, A.; Lomelí, C.; Hernández, M.; Sciutto, E. Increased resistance to Taenia crassiceps murine cysticercosis in Qa-2 transgenic mice. Infect. Immun. 1998, 66, 760–764. [Google Scholar] [CrossRef]
- Togno-Peirce, C.; Nava-Castro, K.; Terrazas, L.I.; Morales-Montor, J. Sex-associated expression of co-stimulatory molecules CD80, CD86, and accessory molecules, PDL-1, PDL-2 and MHC-II, in F480+ macrophages during murine cysticercosis. BioMed Res. Int. 2013, 2013, 570158. [Google Scholar] [CrossRef] [PubMed]
- Adalid-Peralta, L.; Lopez-Roblero, A.; Camacho-Vázquez, C.; Nájera-Ocampo, M.; Guevara-Salinas, A.; Ruiz-Monroy, N.; Melo-Salas, M.; Morales-Ruiz, V.; López-Recinos, D.; Ortiz-Hernández, E.; et al. Regulatory T cells as an escape mecanismo to the inmune response in Taenia crassiceps infección. Front. Cell. Infect. Microbiol. 2021, 11, 630583. [Google Scholar] [CrossRef]
- Dissanayake, S.; Amith, R.S.; Shahin, A. Taenia crassiceps carbohydrates stimulate IL-6 expression in naïve murine macrophages via Toll-like receptors (TLRs). Mol. Immunol. 2004, 41, 391–398. [Google Scholar] [CrossRef]
- Reyes, J.L.; Terrazas, C.A.; Vera-Arias, L.; Terrazas, L.I. Differential response of antigen presenting cells from susceptible and resistant strains of mice to Taenia crassiceps infection. Infect. Genet. Evol. 2009, 9, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.A.; Moura, V.B.L.; Gonçalves, S.F.; Rodrigues, A.A.; Félix, R.M.; Soares, T.P.; Irusta, V.C.R.; Vinaud, M.C.; Oliveira, M.A.P.; Lino-Junior, R.S. Kinetics of the inflammatory response in subcutaneous cysticercosis induced in mice by Taenia crassiceps. J. Comp. Pathol. 2012, 147, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, E.B.; Sakai, Y.I.; De Gaspari, E. A mouse air pouch model for evaluating the immune response to Taenia crassiceps infection. Exp. Parasitol. 2014, 137, 66–73. [Google Scholar] [CrossRef]
- Spolski, R.S.; Corson, J.; Thomas, P.G.; Kuhn, R.E. Parasite-secreted products regulate the host response to larval Taenia crassiceps. Parasite Immunol. 2000, 22, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Gómez-García, L.; Rivera-Montoya, I.; Rodríguez-Sosa, M.; Terrazas, L.I. Carbohydrate components of Taenia crassiceps metacestodes display Th2-adjuvant and anti-inflammatory properties when co-injected with bystander antigen. Parasitol. Res. 2006, 99, 440–448. [Google Scholar] [CrossRef]
- Peón, A.N.; Espinoza-Jiménez, A.; Terrazas, L.I. Immunoregulation by Taenia crassiceps and its antigens. BioMed Res. Int. 2013, 2013, 498583. [Google Scholar] [CrossRef]
- Toledo, A.; Fragoso, G.; Rosas, G.; Hernández, M.; Gevorkian, G.; López-Casillas, F.; Hernández, B.; Acero, G.; Huerta, M.; Larralde, C.; et al. Two epitopes shared by Taenia crassiceps and Taenia solium confer protection against murine T. crassiceps cysticercosis along with a prominent T1 response. Infect. Immun. 2001, 69, 1766–1773. [Google Scholar] [CrossRef]
- Vega-Angeles, V.; Terrazas, L.; Ledesma-Soto, Y.; Jiménez, L.; Landa, A. Taenia solium glutathione transferase fraction activates macrophages and favors the development of Th1-type response. Biosci. Rep. 2019, 39, BSR20181132. [Google Scholar] [CrossRef] [PubMed]
- Vaca-Paniagua, F.; Torres-Rivera, A.; Parra-Unda, R.; Landa, A. Taenia solium: Antioxidant metabolism enzymes as targets for cestocidal drugs and vaccines. Curr. Top. Med. Chem. 2008, 8, 393–399. [Google Scholar] [CrossRef]
- Vibanco-Pérez, N.; Jiménez, L.; Mendoza-Hernández, G.; Landa, A. Characterization of a recombinant mu-class glutathione S-transferase from Taenia solium. Parasitol. Res. 2022, 88, 398–404. [Google Scholar]
- Díaz-Zaragoza, M.; Jiménez, L.; Hernández, M.; Hernández-Ávila, R.; Navarro, L.; Ochoa-Sánchez, A.; Encarnación-Guevara, S.; Ostoa-Saloma, P.; Landa, A. Protein expression profile of Taenia crassiceps cysticerci related to Th1- and Th2-type responses in the mouse cysticercosis model. Acta Trop. 2020, 212, 105696. [Google Scholar] [CrossRef] [PubMed]
- Kruger, N.J. The Bradford method for protein quantitation. In The Protein Protocols Handbook. Springer Protocols Handbooks; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Villarreal, J.M.; Becerra-Lobato, N.; Rebollar-Flores, J.E.; Medina-Aparicio, L.; Carbajal-Gómez, E.; Zavala-García, M.L.; Vázquez, A.; Gutiérrez-Ríos, R.M.; Olvera, L.; Encarnación, S.; et al. The Salmonella enterica serovar Typhi ltrR-ompR-ompC-ompF genes are involved in resistance to the bile salt sodium deoxycholate and in bacterial transformation. Mol. Microbiol. 2014, 92, 1005–1024. [Google Scholar] [CrossRef]
- Aitken, A.; Collinge, D.B.; van Heusden, B.P.; Isobe, T.; Roseboom, P.H.; Rosenfeld, G.; Soll, J. 14-3-3 proteins: A highly conserved, widespread family of eukaryotic proteins. Trends Biochem. Sci. 1992, 17, 498–501. [Google Scholar] [CrossRef]
- Jones, D.H.; Ley, S.; Aitken, A. Isoforms of 14-3-3 protein can form homo- and heterodimers in vivo and in vitro: Implications for function as adapter proteins. FEBS Lett. 1995, 368, 55. [Google Scholar] [CrossRef] [PubMed]
- McGonigle SMelissa JBeall, M.J.; Pearce, E.J. Eukaryotic initiation factor 2a subunit associates with TGFb receptors and 14-3-31 and acts as a modulator of the TGFb response. Biochemistry 2002, 41, 579587. [Google Scholar] [CrossRef]
- Obsilova, V.; Silhan, J.; Boura, E.; Teisinger, J.; Obsil, T. 14-3-3 proteins: A family of versatile molecular regulators. Physiol. Res. 2008, 57 (Suppl. S3), S11–S21. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, Y.; Cai, X.; Liu, L.; Wu, K.; Yu, M. Down-regulation of 14-3-3 Zeta inhibits TGF-β1–induced actomyosin contraction in human trabecular meshwork cells through RhoA signaling pathway. Investig. Ophthalmol. Vis. Sci. 2016, 57, 719–730. [Google Scholar] [CrossRef]
- Siles-Lucas, M.; Merli, M.; Mackenstedt, U.; Gottstein, B. The Echinococcus multilocularis 14-3-3 protein protects mice against primary but not secondary alveolar Echinococcosis. Vaccine 2003, 21, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Virginio, V.G.; Monteiro, K.M.; Drumond, F.; de Carvalho, M.O.; Vargas, D.M.; Zaha, A.; Ferreira, H.B. Excretory/secretory products from in vitro-cultured Echinococcus granulosus protoscoleces. Mol. Biochem. Parasitol. 2012, 183, 15–22. [Google Scholar] [CrossRef]
- Park, I.; Han, C.; Jin, S.; Lee, B.; Choi, H.; Kwon, J.T.; Kim, D.; Kim, J.; Lifirsu, E.; Park, W.J.; et al. Myosin regulatory light chains are required to maintain the stability of myosin II and cellular integrity. Biochem. J. 2011, 434, 171–180. [Google Scholar] [CrossRef]
- Buffoni, L.; Piva, M.M.; Baska, P.; Januszkiewicz, K.; Norbury, L.J.; Prior, K.C.; Dezen, D.; Silva, A.S.; Wedrychowicz, H.; Mendes, R.E. Immunization with the recombinant myosin regulatory light chain (FhrMRLC) in Adjuplex® adjuvant elicits a Th1-biased immune response and a reduction of parasite burden in Fasciola hepatica infected rats. Parasitol. Int. 2020, 75, 102037. [Google Scholar] [CrossRef]
- Gnanasekar, M.; Salunkhe, A.M.; Mallia, A.K.; He, Y.X.; Kalyanasundaram, R. Praziquantel affects the regulatory myosin light chain of Schistosoma mansoni. Antimicrob. Agents Chemother 2009, 53, 1054–1060. [Google Scholar] [CrossRef]
- Yokoyama, M.; Kimura, M.Y.; Ito, T.; Hayashizaki, K.; Endo, Y.; Wang, Y.; Yagi, R.; Nakagawa, T.; Kato, N.; Matsubara, H.; et al. Myosin Light Chain 9/12 regulates the pathogenesis of inflammatory Bowel disease. Front. Immunol. 2021, 11, 594297. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, M.; Guo, Q.; Li, R.; Li, G.; Tan, S.; Li, X.; Wei, Y.; Wu, M. Annexin A2 binds to endosomes and negatively regulates TLR4-triggered inflammatory responses via the TRAM-TRIF pathway. Sci. Rep. 2015, 5, 15859. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, Y.; Chung, J.Y.; Li, Y.; Yu, Z.; Kim, J.W.; Lok, J.M.; Whalen, M.J.; Wang, X. Annexin A2 deficiency exacerbates neuroinflammation and long-term neurological deficits after traumatic brain injury in mice. Int. J. Mol. Sci. 2019, 20, 6125. [Google Scholar] [CrossRef] [PubMed]
- Sønder, S.L.; Boye, T.L.; Tölle, R.; Dengjel, J.; Maeda, K.; Jäättelä, M.; Simonsen, A.C.; Jaiswal, J.K.; Nylandsted, J. Annexin A7 is required for ESCRT III-mediated plasma membrane repair. Sci. Rep. 2019, 9, 6726. [Google Scholar] [CrossRef] [PubMed]
- Ammendolia, D.A.; Bement, W.M.; Brumell, J.H. Plasma membrane integrity: Implications for health and disease. BMC Biol. 2021, 19, 71. [Google Scholar] [CrossRef]
- Hammond, J.W.; Cai, D.; Verhey, K.J. Tubulin modifications and their cellular functions. Curr. Opin. Cell Biol. 2008, 20, 71–76. [Google Scholar] [CrossRef]
- Márquez-Navarro, A.; Pérez-Reyes, A.; Zepeda-Rodríguez, A.; Reynoso-Ducoing, O.; Hernández-Campos, A.; Hernández-Luis, F.; Castillo, R.; Yépez-Mulia, L.; Ambrosio, J.R. RCB20, an experimental benzimidazole derivative, affects tubulin expression and induces gross anatomical changes in Taenia crassiceps cysticerci. Parasitol. Res. 2013, 112, 2215–2226. [Google Scholar] [CrossRef]
- Reynoso-Ducoing, O.; Valverde-Islas, L.; Paredes-Salomon, C.; Pérez-Reyes, A.; Landa, A.; Robert, L.; Mendoza, G.; Ambrosio, J.R. Analysis of the expression of cytoskeletal proteins of Taenia crassiceps ORF strain cysticerci (Cestoda). Parasitol. Res. 2014, 113, 1955–1969. [Google Scholar] [CrossRef]
- De Lima, N.F.; Picanço, G.A.; Valencia, D.G.R.; Villegas, E.O.L.; Mellado, M.D.R.E.; Ambrosio, J.R.; Vinaud, M.C. Alterations in Taenia crassiceps cysticerci cytoskeleton induced by nitazoxanide and flubendazole. Acta Trop. 2021, 221, 106027. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, A.; Surcel, H.M.; Halttunen, M.; Tiitinen, A.; Morrison, R.P.; Morrison, S.G.; Koskela, P.; Lehtinen, M.; Paavonen, J. Chlamydia trachomatis heat shock protein-60 induced interferon-gamma and interleukin-10 production in infertile women: Chlamydial HSP60-induced cytokines in infertile women. Clin. Exp. Immunol. 2003, 131, 299–303. [Google Scholar] [CrossRef]
- Zanin-Zhorov, A.; Bruck, R.; Tal, G.; Oren, S.; Aeed, H.; Hershkoviz, R.; Cohen, I.R.; Lider, O. Heat shock protein 60 inhibits Th1-mediated hepatitis model via innate regulation of Th1/Th2 transcription factors and cytokines. J. Immunol. 2005, 174, 3227–3236. [Google Scholar] [CrossRef]
- Zhou, S.; Jin, X.; Chen, X.; Zhu, J.; Xu, Z.; Wang, X.; Liu, F.; Hu, W.; Zhou, L.; Su, C. Heat shock protein 60 in eggs specifically induces Tregs and reduces liver Immunopathology in mice with Schistosomiasis Japonica. PLoS ONE 2015, 10, e0139133. [Google Scholar] [CrossRef]
- Zhao, Y.; Coulson, E.J.; Su, X.; Zhang, J.; Sha, B.; Xu, H.; Deng, Y.; Chen, Y.; Cao, J.; Wang, Y.; et al. Identification of 14-3-3 epsilon as a regulator of the neural apoptotic pathway for chronic-stress-induced depression. iScience 2021, 24, 102043. [Google Scholar] [CrossRef]
- Fu, W.; Hu, W.; Yi, Y.S.; Hettinghouse, A.; Sun, G.; Bi, Y.; He, W.; Zhang, L.; Gao, G.; Liu, J.; et al. TNFR2/14-3-3ε signaling complex instructs macrophage plasticity in inflammation and autoimmunity. J. Clin. Investig. 2021, 131, e144016. [Google Scholar] [CrossRef] [PubMed]
- Köhnke, R.; Mei, J.; Park, M.; York, D.A.; Erlanson-Albertsson, C. Fatty acids and glucose in high concentration down-regulates ATP synthase beta-subunit protein expression in INS-1 cells. Nutr. Neurosci. 2007, 10, 273–278. [Google Scholar] [CrossRef]
- Santivañez, S.J.; Hernández-González, A.; Chile, N.; Oleaga, A.; Arana, Y.; Palma, S.; Verastegui, M.; Gonzalez, A.E.; Gilman, R.; Garcia, H.H.; et al. Proteomic study of activated Taenia solium oncospheres. Mol. Biochem. Parasitol. 2010, 171, 32–39. [Google Scholar] [CrossRef]
- Grzelak, S.; Stachyra, A.; Bień-Kalinowska, J. The first analysis of Trichinella spiralis and Trichinella britovi adult worm excretory-secretory proteins by two-dimensional electrophoresis coupled with LC-MS/MS. Vet. Parasitol. 2021, 297, 109096. [Google Scholar] [CrossRef]
- Reamtong, O.; Rujimongkon, K.; Sookrung, N.; Saeung, A.; Thiangtrongjit, T.; Sakol-varee, Y.; Thammapalo, S.; Loymek, S.; Chaicumpa, W. Immunome and immune complex-forming components of Brugia malayi identified by microfilaremic human sera. Exp. Parasitol. 2019, 200, 92–98. [Google Scholar] [CrossRef]
- Stolf, B.S.; Smyrnias, I.; Lopes, L.R.; Vendramin, A.; Goto, H.; Laurindo, F.R.M.; Shah, A.M.; Santos, C.X.C. Protein disulfide isomerase and host-pathogen interaction. Sci. World J. 2011, 11, 1749–1761. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Q.; Zhang, M.; Gu, B.; Huang, G.; Wang, Q.; Shan, W. The protein disulfide isomerase 1 of Phytophthora parasitica (PpPDI1) is associated with the haustoria-like structures and contributes to plant infection. Front. Plant Sci. 2015, 6, 632. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.M.; Söling, H.D. The protein disulphide-isomerase family: Unravelling a string of folds. Biochem. J. 1999, 339 Pt 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Benhnini, F.; Chenik, M.; Laouini, D.; Louzir, H.; Cazenave, P.A.; Dellagi, K. Comparative evaluation of two vaccine candidates against experimental leishmaniasis due to Leishmania major infection in four inbred mouse strains. Clin. Vaccine Immunoly 2009, 16, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Chen, D.; Ji, S.; Yang, L.T.; Huang, Q.; Guan, I.; Chang, K.; Li, D.; Yuan, R.; et al. Dust-mite-derived protein disulfide isomerase suppresses airway allergy by inducing tolerogenic dendritic cells. J. Biol. Chem. 2021, 296, 100585. [Google Scholar] [CrossRef]
- Kalinna, B.H.; McManus, D.P. A vaccine against the Asian schistosome, Schistosoma japonicum: An update on paramyosin as a target of protective immunity. Int. J. Parasitol. 1997, 27, 1213–1219. [Google Scholar] [CrossRef]
- Vázquez-Talavera, J.; Solís, C.F.; Terrazas, L.I.; Laclette, J.P. Characterization and protective potential of the immune response to Taenia solium paramyosin in a murine model of cysticercosis. Infect. Immun. 2001, 69, 5412–5416. [Google Scholar] [CrossRef]
- Hao, C.; Wang, W.; Zhan, B.; Wang, Z.; Huang, J.; Sun, X.; Zhu, X. Trichinella spiralis Paramyosin Induces Colonic Regulatory T Cells to Mitigate Inflammatory Bowel Disease. Front. Cell Dev. Biol. 2021, 9, 695015. [Google Scholar] [CrossRef] [PubMed]
- Laclette, J.P.; Shoemaker, C.B.; Richter, D.; Arcos, L.; Pante, N.; Cohen, C.; Bing, D.; Nicholson-Weller, A. Paramyosin inhibits complement C1. J. Immunol. 1992, 148, 124–128. [Google Scholar] [CrossRef]
- Sun, R.; Zhao, X.; Wang, Z.; Yang, J.; Zhao, L.; Zhan, B.; Zhu, X. Trichinella spiralis paramyosin binds human complement C1q and inhibits classical complement activation. PLoS Negl. Trop. Dis. 2015, 9, e0004310. [Google Scholar] [CrossRef]
- Pancholi, V. Multifunctional α-enolase: Its role in diseases. Cell. Mol. Life Sci. 2001, 58, 902–920. [Google Scholar] [CrossRef] [PubMed]
- Jolodar, A.; Fischer, P.; Bergmann, S.; Buttner, D.W.; Hammerschmidt, S.; Brattig, N.W. Molecular cloning of an alpha-enolase from the human filarial parasite Onchocerca volvulus that binds human plasminogen. Biochim. Biophys. Acta 2003, 1627, 111–120. [Google Scholar] [CrossRef]
- Bernal, D.; de la Rubia, J.E.; Carrasco-Abad, A.M.; Toledo, R.; Mas-Coma, S.; Marcilla, A. Identification of enolase as a plasminogen-binding protein in excretory-secretory products of Fasciola hepatica. FEBS Lett. 2004, 563, 203–206. [Google Scholar] [CrossRef]
- Gan, W.; Zhao, G.; Xu, H.; Wu, W.; Du, W.; Huang, J.; Yu, X.; Hu, X. Reverse vaccinology approach identifies an Echinococcus granulosus tegumental membrane protein enolase as vaccine candidate. Parasitol. Res. 2010, 106, 873–882. [Google Scholar] [CrossRef]
- Wongkamchai, S.; Chiangjong, W.; Sinchaikul, S.; Chen, S.T.; Choochote, W.; Thongboonkerd, V. Identification of Brugia malayi immunogens by an immunoproteomics approach. J. Proteom. 2011, 74, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Ayón-Núñez, D.A.; Fragoso, G.; Bobes, R.J.; Laclette, J.P. Plasminogen-binding proteins as an evasion mechanism of the host’s innate immunity in infectious diseases. Biosci. Rep. 2018, 38, BSR20180705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; You, Y.; Luo, X.; Zheng, Y.; Cai, X. Molecular and biochemical characterization of Taenia solium α-enolase. Vet. Parasitol. 2018, 254, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Yeom, J.; Wang, H.; Kim, S.; Ahn, C.; Kim, J.; Yang, H.; Kong, Y. Taenia solium metacestode fasciclin-like protein is reactive with sera of chronic neurocysticercosis. Trop. Med. Int. Health 2014, 19, 719–725. [Google Scholar] [CrossRef]
- Ahn, C.; Kim, J.; Bae, Y.; Kim, S.; Shin, J.; Yang, Y.; Kang, I.; Kong, Y. Fasciclin calcareous corpuscle binary complex mediated protein-protein interactions in Taenia solium metacestode. Parasites Vectors 2017, 10, 438. [Google Scholar] [CrossRef]
- Ahn, C.; Kim, J.; Huh, S.; Kang, I.; Kong, Y. Advances in serological diagnosis of Taenia solium neurocysticercosis in Korea. Genom. Inf. 2019, 17, e7. [Google Scholar] [CrossRef]
- Barthel, D.; Schindler, S.; Zipfel, P.F. Plasminogen is a complement inhibitor. J. Biol. Chem. 2012, 287, 18831–18842. [Google Scholar] [CrossRef]
- Espíndola, N.; De Gaspari, E.; Nakamura, P.; Vaz, A. Cross-reactivity of anti-Taenia crassiceps cysticerci immune antibodies with Taenia solium antigens. Vet. Parasitol. 2000, 89, 321–326. [Google Scholar] [CrossRef]
- Rodríguez-Sosa, M.; Rosas, L.E.; David, J.R.; Bojalil, R.; Satoskar, A.R.; Terrazas, L.I. Macrophage migration inhibitory factor plays a critical role in mediating protection against the helminth parasite Taenia crassiceps. Infect. Immun. 2003, 71, 1247–1254. [Google Scholar] [CrossRef]
- Sciutto, E.; Fragoso, G.; Baca, M.; De la Cruz, V.; Lemus, L.; Lamoyi, E. Depressed T-cell proliferation associated with susceptibility to experimental Taenia crassiceps infection. Infect. Immun. 1995, 63, 2277–2281. [Google Scholar] [CrossRef]
- Terrazas, L.I.; Bojalilt, R.; Govezensky, T.; Larralde, C. Shift from an Early Protective TH1-Type Immune Response to a Late Permissive TH2-Type Response in Murine Cysticercosis (Taenia crassiceps). J. Parasitol. 1998, 84, 74–81. [Google Scholar] [CrossRef]
- Toenjes, S.A.; Kuhn, R.E. The initial immune response during experimental cysticercosis is of the mixed Thl/Th2 type. Parasitol. Res. 2003, 89, 407–413. [Google Scholar] [CrossRef]
- Al-Sherbiny, M.; Osman, A.; Barakat, R.; El Morshedy, H.; Bergquist, R.; Olds, R. In vitro cellular and humoral responses to Schistosoma mansoni vaccine candidate antigens. Acta Trop. 2003, 88, 117–130. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Londono, D.P.; Alvarez, A.L.; Trujillo, J.; Jaramillo, M.M.; Restrepo, B.I. Granuloma formation and parasite disintegration in porcine cysticercosis: Comparison with human neurocysticercosis. J. Comp. Pathol. 2002, 127, 186–193. [Google Scholar] [CrossRef]
- Mohammad, I.; Nousiainen, K.; Bhosale, S.D.; Starskaia, I.; Moulder, R.; Rokka, A.; Cheng, F.; Mohanasundaram, P.; Eriksson, J.E.; Goodlett, D.R.; et al. Quantitative proteomic characterization and comparison of T helper 17 and induced regulatory T cells. PLoS Biol. 2018, 16, e2004194. [Google Scholar] [CrossRef]
- Moura, V.B.; Lima, S.B.; Matos-Silva, H.; Vinaud, M.C.; Loyola, P.R.; Lino, R.S. Cellular immune response in intraventricular experimental neurocysticercosis. Parasitology 2016, 143, 334–342. [Google Scholar] [CrossRef]
- Vargas-Parada, L.; Laclette, J.P. Gene structure of Taenia solium paramyosin. Parasitol. Res. 2003, 89, 375–378. [Google Scholar] [CrossRef]
- Soleyman, N.M.; Darnhofer, B.; Birner-Gruenberger, R.; Abnous, K.; Borji, H. Proteomic analysis of soluble protein extract of adult Toxocara cati. Comparative Immunology. Microbiol. Infect. Dis. 2020, 73, 101528. [Google Scholar] [CrossRef]
- Dallacasagrande, V.; Hajjar, K.A. Annexin A2 in inflammation and host defense. Cells 2020, 9, 1499. [Google Scholar] [CrossRef]
- Larralde, C.; Morales, J.; Terrazas, L.I.; Govezensky, T.; Romano, M. Sex hormone changes induced by the parasite lead to feminization of the male host in murine Taenia crassiceps cysticercosis. J. Steroid Biochem. Mol. Biol. 1995, 52, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, G.; Larralde, C.; Chavarria, A.; Cerbón, M.A.; Morales-Montor, J. Molecular mechanisms involved in the differential effects of sex steroids on the reproductions and infectivity of Taenia crassiceps. J. Parasitol. 2004, 90, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Terrazas, L.I.; Bojalil, R.; Rodríguez-Sosa, M.; Govesenky, T.; Larralde, C. Taenia crassiceps cysticercosis: A role for prostaglandin E2 in susceptibility. Parastol. Res. 1999, 85, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).