Immune Responses Potentially Involved in the Gestational Complications of Brucella Infection
Abstract
1. Introduction
2. Epidemiology of Brucella-Induced Pregnancy Complications
3. Brucella Vaccines and Gestational Complications in Animals
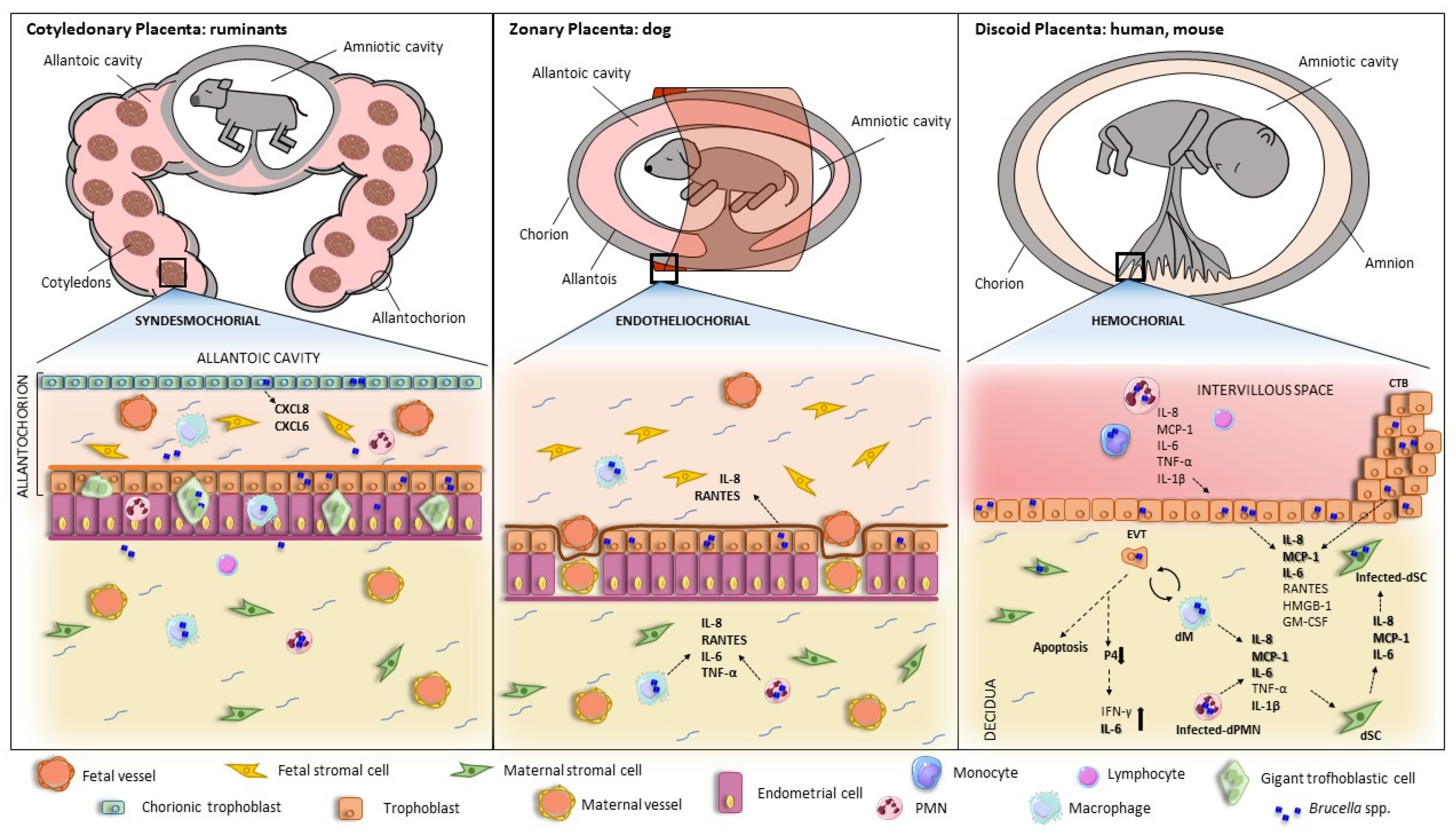
4. Pathological Findings in the Infected Placenta
5. Brucella Infection and Replication in Placental Cells
6. Brucella-Induced Inflammatory Responses in Trophoblasts and Other Cells from the Maternal—Fetal Unit
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pappas, G.; Papadimitriou, P.; Akritidis, N.; Christou, L.; Tsianos, E.V. The New Global Map of Human Brucellosis. Lancet Infect. Dis. 2006, 6, 91–99. [Google Scholar] [CrossRef]
- Laine, C.G.; Johnson, V.E.; Scott, H.M.; Arenas-Gamboa, A.M. Global Estimate of Human Brucellosis Incidence. Emerg. Infect. Dis. 2023, 29, 1789–1797. [Google Scholar] [CrossRef]
- Franco, M.P.; Mulder, M.; Gilman, R.H.; Smits, H.L. Human Brucellosis. Lancet Infect. Dis. 2007, 7, 775–786. [Google Scholar] [CrossRef]
- Bosilkovski, M.; Arapović, J.; Keramat, F. Human Brucellosis in Pregnancy—An Overview. Bosn. J. Basic Med. Sci. 2020, 20, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Aldo, P.; Alvero, A.B. The Unique Immunological and Microbial Aspects of Pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, L.; Nguyen, T.; Mccomb, S. From Mice to Women: The Conundrum of Immunity to Infection during Pregnancy. J. Reprod. Immunol. 2013, 97, 62–73. [Google Scholar] [CrossRef]
- Tsai, A.Y.; Byndloss, M.X.; Seyffert, N.; Winter, M.G.; Young, B.M.; Tsolis, R.M. Tumor Necrosis Factor Alpha Contributes to Inflammatory Pathology in the Placenta during Brucella abortus Infection. Infect. Immun. 2022, 90, e00013-22. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.G.; Ferrero, M.C.; Hielpos, M.S.; Fossati, C.A.; Baldi, P.C. Proinflammatory Response of Human Trophoblastic Cells to Brucella abortus Infection and upon Interactions with Infected Phagocytes. Biol. Reprod. 2016, 94, 131706. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Hou, H.; Zhao, W.; Wang, J.; Peng, Q. Administration of Exogenous Progesterone Protects Against Brucella abortus Infection-Induced Inflammation in Pregnant Mice. J. Infect. Dis. 2021, 224, 532–543. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, M.; Guo, X.; Zeng, H.; Shuai, X.; Guo, J.; Huang, Q.; Chu, Y.; Zhou, B.; Wen, J.; et al. Inflammatory Mechanism of Brucella Infection in Placental Trophoblast Cells. Int. J. Mol. Sci. 2022, 23, 13417. [Google Scholar] [CrossRef]
- Rossetti, C.A.; Maurizio, E.; Rossi, U.A. Comparative Review of Brucellosis in Small Domestic Ruminants. Front. Vet. Sci. 2022, 9, 7671. [Google Scholar] [CrossRef]
- Wanke, M.M. Canine Brucellosis. Anim. Reprod. Sci. 2004, 82–83, 195–207. [Google Scholar] [CrossRef]
- Olsen, S.; Tatum, F. Swine Brucellosis: Current Perspectives. Vet. Med. 2016, 8, 1–12. [Google Scholar] [CrossRef]
- Grilló, M.J.; Marín, C.M.; Barberán, M.; Blasco, J.M. Experimental Brucella ovis Infection in Pregnant Ewes. Vet. Rec. 1999, 144, 555–558. [Google Scholar] [CrossRef]
- Ris, D.R. The Bacteriology and Serology of Ewes Inoculated with Viable Brucella ovis Organisms. N. Z. Vet. J. 1970, 18, 2–7. [Google Scholar] [CrossRef]
- Khan, M.Y.; Mah, M.W.; Memish, Z.A. Brucellosis in Pregnant Women. Clin. Infect. Dis. 2001, 32, 1172–1177. [Google Scholar] [CrossRef]
- Elshamy, M.; Ahmed, A.I. The Effects of Maternal Brucellosis on Pregnancy Outcome. J. Infect. Dev. Ctries. 2008, 2, 230–234. [Google Scholar] [CrossRef]
- Kurdoglu, M.; Adali, E.; Kurdoglu, Z.; Karahocagil, M.K.; Kolusari, A.; Yildizhan, R.; Kucukaydin, Z.; Sahin, H.G.; Kamaci, M.; Akdeniz, H. Brucellosis in Pregnancy: A 6-Year Clinical Analysis. Arch. Gynecol. Obstet. 2010, 281, 201–206. [Google Scholar] [CrossRef]
- Makala, R.; Majigo, M.V.; Bwire, G.M.; Kibwana, U.; Mirambo, M.M.; Joachim, A. Seroprevalence of Brucella Infection and Associated Factors among Pregnant Women Receiving Antenatal Care around Human, Wildlife and Livestock Interface in Ngorongoro Ecosystem, Northern Tanzania. A Cross-Sectional Study. BMC Infect. Dis. 2020, 20, 152. [Google Scholar] [CrossRef]
- Ali, S.; Akhter, S.; Neubauer, H.; Scherag, A.; Kesselmeier, M.; Melzer, F.; Khan, I.; El-Adawy, H.; Azam, A.; Qadeer, S.; et al. Brucellosis in Pregnant Women from Pakistan: An Observational Study. BMC Infect. Dis. 2016, 16, 468. [Google Scholar] [CrossRef]
- Kledmanee, K.; Liabsuetrakul, T.; Sretrirutchai, S. Seropositivities against Brucellosis, Coxiellosis, and Toxoplasmosis and Associated Factors in Pregnant Women with Adverse Pregnancy Outcomes: A Cross-Sectional Study. PLoS ONE 2019, 14, e0216652. [Google Scholar] [CrossRef]
- Te-Chaniyom, T.; Geater, A.F.; Kongkaew, W.; Chethanond, U.; Chongsuvivatwong, V. Goat Farm Management and Brucella Serological Test among Goat Keepers and Livestock Officers, 2011–2012, Nakhon Si Thammarat Province, Southern Thailand. One Health 2016, 2, 126–130. [Google Scholar] [CrossRef]
- Vilchez, G.; Espinoza, M.; D’Onadio, G.; Saona, P.; Gotuzzo, E. Brucellosis in Pregnancy: Clinical Aspects and Obstetric Outcomes. Int. J. Infect. Dis. 2015, 38, 95–100. [Google Scholar] [CrossRef][Green Version]
- Al-Tawfiq, A.J.; Memish, A.Z. Pregnancy Associated Brucellosis. Recent Pat. Anti-Infect. Drug Discov. 2013, 8, 47–50. [Google Scholar] [CrossRef]
- Poester, F.P.; Samartino, L.E.; Santos, R.I. Pathogenesis and Pathobiology of Brucellosis in Livestock. Rev. Sci. Tech. 2013, 32, 105–115. [Google Scholar] [CrossRef]
- Gulsun, S.; Aslan, S.; Satici, O.; Gul, T. Brucellosis in Pregnancy. Trop. Dr. 2011, 41, 82–84. [Google Scholar] [CrossRef]
- Seoud, M.; Saade, G.; Awar, G.; Uwaydah, M. Brucellosis in Pregnancy. J. Reprod. Med. Obstet. Gynecol. 1991, 36, 441–445. [Google Scholar]
- Madkour, M.M. Pregnancy and Brucellosis. In Madkour’s Brucellosis; Springer: Berlin/Heidelberg, Germany, 2001; pp. 187–192. [Google Scholar]
- Makhseed, M.; Harouny, A.; Araj, G.; Moussa, M.A.A.; Sharma, P. Obstetric and Gynecologic Implication of Brucellosis in Kuwait. J. Perinatol. 1998, 18, 196–199. [Google Scholar]
- Inan, A.; Erdem, H.; Elaldi, N.; Gulsun, S.; Karahocagil, M.K.; Pekok, A.U.; Ulug, M.; Tekin, R.; Bosilkovski, M.; Kaya, S.; et al. Brucellosis in Pregnancy: Results of Multicenter ID-IRI Study. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1261–1268. [Google Scholar] [CrossRef]
- Bosilkovski, M.; Stojovski, M.; Siskova, D.; Ridov, A.; Kostoska, E.; Krstevski, K. Brucellosis in Pregnancy: Case Reports with Different Outcomes in an Endemic Region. Acta Clin. Croat. 2020, 59, 338–343. [Google Scholar] [CrossRef]
- Roushan, M.R.H.; Baiani, M.; Asnafi, N.; Saedi, F. Outcomes of 19 Pregnant Women with Brucellosis in Babol, Northern Iran. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 540–542. [Google Scholar] [CrossRef]
- Yumuk, Z.; O’Callaghan, D. Brucellosis in Turkey—An Overview. Int. J. Infect. Dis. 2012, 16, e228–e235. [Google Scholar] [CrossRef]
- Erdem, H.; Akova, M. Leading Infectious Diseases Problems in Turkey. Clin. Microbiol. Infect. 2012, 18, 1056–1067. [Google Scholar] [CrossRef]
- Mujuni, F.; Andrew, V.; Mngumi, E.B.; Chibwe, E.; Mshana, S.E.; Mirambo, M.M. Predominance of Brucella abortus Antibodies among Women with Spontaneous Abortion in the City of Mwanza: Unrecognized Link or Coincidence? BMC Res. Notes 2018, 11, 792. [Google Scholar] [CrossRef]
- Guerrier, G.; Daronat, J.M.; Morisse, L.; Yvon, J.F.; Pappas, G. Epidemiological and Clinical Aspects of Human Brucella suis Infection in Polynesia. Epidemiol. Infect. 2011, 139, 1621–1625. [Google Scholar] [CrossRef]
- Alsaif, M.; Dabelah, K.; Girim, H.; Featherstone, R.; Robinson, J.L. Congenital Brucellosis: A Systematic Review of the Literature. Vector-Borne Zoonotic Dis. 2018, 18, 393–403. [Google Scholar] [CrossRef]
- De Souza, T.D.; De Carvalho, T.F.; Mol, J.P.D.S.; Lopes, J.V.M.; Silva, M.F.; Da Paixão, T.A.; Santos, R.L. Tissue Distribution and Cell Tropism of Brucella canis in Naturally Infected Canine Foetuses and Neonates. Sci. Rep. 2018, 8, 7203. [Google Scholar] [CrossRef]
- Hashino, M.; Kim, S.; Tachibana, M.; Shimizu, T.; Watarai, M. Vertical Transmission of Brucella abortus Causes Sterility in Pregnant Mice. J. Vet. Med. Sci. 2012, 74, 1075–1077. [Google Scholar] [CrossRef]
- Alton, G.G. Control of Brucella melitensis Infection in Sheep and Goats-a Review. Trop. Anim. Health Prod. 1987, 19, 65–74. [Google Scholar] [CrossRef]
- Zundel, E.; Verger, J.M.; Grayon, M.; Michel, R. Conjunctival Vaccination of Pregnant Ewes and Goats with Brucella melitensis Rev 1 Vaccine: Safety and Serological Responses. Ann. Rech. Vet. 1992, 23, 177–188. [Google Scholar]
- Jiménez de Bagués, M.P.; Marin, C.M.; Barberán, M.; Blasco, J.M. Responses of Ewes to B. Melitensis Rev1 Vaccine Administered by Subcutaneous or Conjunctival Routes at Different Stages of Pregnancy. Ann. Rech. Vet. 1989, 20, 205–213. [Google Scholar]
- Hensel, M.E.; Garcia-Gonzalez, D.G.; Chaki, S.P.; Hartwig, A.; Gordy, P.W.; Bowen, R.; Ficht, T.A.; Arenas-Gamboa, A.M. Vaccine Candidate Brucella melitensis 16M ΔvjbR Is Safe in a Pregnant Sheep Model and Confers Protection. mSphere 2020, 5, e00120-20. [Google Scholar] [CrossRef]
- Sangari, F.J.; García-Lobo, J.M.; Agüero, J. The Brucella abortus Vaccine Strain B19 Carries a Deletion in the Erythritol Catabolic Genes. FEMS Microbiol. Lett. 1994, 121, 337–342. [Google Scholar] [CrossRef][Green Version]
- Schurig, G.G.; Roop, R.M.; Bagchi, T.; Boyle, S.; Buhrman, D.; Sriranganathan, N. Biological Properties of RB51; A Stable Rough Strain of Brucella abortus. Vet. Microbiol. 1991, 28, 171–188. [Google Scholar] [CrossRef]
- de Oliveira, M.M.; Pereira, C.R.; de Oliveira, I.R.C.; Godfroid, J.; Lage, A.P.; Dorneles, E.M.S. Efficacy of Brucella abortus S19 and RB51 Vaccine Strains: A Systematic Review and Meta-Analysis. Transbound. Emerg. Dis. 2022, 69, e32–e51. [Google Scholar] [CrossRef]
- Schurig, G.G.; Sriranganathan, N.; Corbel, M.J. Brucellosis Vaccines: Past, Present and Future. Vet. Microbiol. 2002, 90, 479–496. [Google Scholar] [CrossRef]
- Zriba, S.; Garcia-Gonzalez, D.G.; Khalaf, O.H.; Wheeler, L.; Chaki, S.P.; Rice-Ficht, A.; Ficht, T.A.; Arenas-Gamboa, A.M. Vaccine Safety Studies of Brucella abortus S19 and S19Δ VjbR in Pregnant Swine. Vaccine X 2019, 3, 100041. [Google Scholar] [CrossRef]
- Zabalza-Baranguá, A.; Poveda-Urkixo, I.; Mena-Bueno, S.; Ramírez, G.A.; De Bolle, X.; Grilló, M.J. Vaccine Properties of Brucella melitensis 16MΔwzm and Reactivation of Placental Infection in Pregnant Sheep. Vaccine 2023, 41, 1554–1566. [Google Scholar] [CrossRef]
- Kudi, A.C.; Kalla, D.J.U.; Kudi, M.C.; Kapio, G.I. Brucellosis in Camels. J. Arid Environ. 1997, 37, 413–417. [Google Scholar] [CrossRef]
- Miller, W.G.; Adams, L.G.; Ficht, T.A.; Cheville, N.F.; Payeur, J.P.; Harley, D.R.; House, C.; Ridgway, S.H. Brucella-Induced Abortions and Infection in Bottlenose Dolphins (Tursiops truncatus). J. Zoo Wildl. Med. 1999, 30, 100–110. [Google Scholar]
- Mackie, J.T.; Blyde, D.; Harris, L.; Roe, W.D.; Keyburn, A.L. Brucellosis Associated with Stillbirth in a Bottlenose Dolphin in Australia. Aust. Vet. J. 2020, 98, 92–95. [Google Scholar] [CrossRef]
- Rebollada-Merino, A.; García-Seco, T.; Pérez-Sancho, M.; Domínguez, L.; Rodríguez-Bertos, A. Histopathologic and Immunohistochemical Findings in the Placentas and Fetuses of Domestic Swine Naturally Infected with Brucella suis Biovar 2. J. Vet. Diagn. Investig. 2023, 35, 258–265. [Google Scholar] [CrossRef]
- Carmichael, L.E.; Kenney, R.M. Canine Abortion Caused by Brucella canis. J. Am. Vet. Med. Assoc. 1968, 152, 605–616. [Google Scholar] [PubMed]
- Gyuranecz, M.; Szeredi, L.; Rónai, Z.; Dénes, B.; Dencso, L.; Dán, Á.; Pálmai, N.; Hauser, Z.; Lami, E.; Makrai, L.; et al. Detection of Brucella canis-Induced Reproductive Diseases in a Kennel. J. Vet. Diagn. Investig. 2011, 23, 143–147. [Google Scholar] [CrossRef]
- Payne, J.M. Changes in the Rat Placenta and Foetus Following Experimental Infection with Various Species of Bacteria. J. Pathol. Bacteriol. 1958, 75, 367–385. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.M. The Pathogenesis of Experimental Brucellosis in the Pregnant Cow. J. Pathol. Bacteriol. 1959, 78, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.D.; Meador, V.P.; Cheville, N.F. Pathogenesis of Placentitis in the Goat Inoculated with Brucella abortus. I. Gross and Histologic Lesions. Vet. Pathol. 1986, 23, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Siddiqur, R.M.; Kirl, B.B. Clinical and Pathological Findings in Experimental Brucellosis in Pregnant Rats. J. Infect. Dev. Ctries. 2008, 2, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.D.; Cheville, N.F. Ultrastructural Morphometric Analysis of Brucella abortus-Infected Trophoblasts in Experimental Placentitis. Bacterial Replication Occurs in Rough Endoplasmic Reticulum. Am. J. Pathol. 1986, 124, 226. [Google Scholar]
- Meador, V.P.; Deyoe, B.L. Intracellular Localization of Brucella abortus in Bovine Placenta. Vet. Pathol. 1989, 26, 513–515. [Google Scholar] [CrossRef]
- Carvalho Neta, A.V.; Stynen, A.P.R.; Paixão, T.A.; Miranda, K.L.; Silva, F.L.; Roux, C.M.; Tsolis, R.M.; Everts, R.E.; Lewin, H.A.; Adams, L.G.; et al. Modulation of the Bovine Trophoblastic Innate Immune Response by Brucella abortus. Infect. Immun. 2008, 76, 1897–1907. [Google Scholar] [CrossRef]
- Carvalho Neta, A.V.; Mol, J.P.S.; Xavier, M.N.; Paixão, T.A.; Lage, A.P.; Santos, R.L. Pathogenesis of Bovine Brucellosis. Vet. J. 2010, 184, 146–155. [Google Scholar] [CrossRef]
- Bosseray, N. Brucella Infection and Immunity in Placenta. Ann. l’Institut Pasteur. Microbiol. 1987, 138, 110–113. [Google Scholar] [CrossRef]
- Bosseray, N. Colonization of Mouse Placentas by Brucella abortus Inoculated during Pregnancy. Br. J. Exp. Pathol. 1980, 61, 361–368. [Google Scholar]
- Poveda-Urkixo, I.; Ramírez, G.A.; Grilló, M.J. Kinetics of Placental Infection by Different Smooth Brucella Strains in Mice. Pathogens 2022, 11, 279. [Google Scholar] [CrossRef]
- Keestra-Gounder, A.M.; Byndloss, M.X.; Seyffert, N.; Young, B.M.; Chávez-Arroyo, A.; Tsai, A.Y.; Cevallos, S.A.; Winter, M.G.; Pham, O.H.; Tiffany, C.R.; et al. NOD1 and NOD2 Signalling Links ER Stress with Inflammation. Nature 2016, 532, 394–397. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, X.; Duan, K.; Peng, Q. Research Progress on Brucellosis. Curr. Med. Chem. 2018, 26, 5598–5608. [Google Scholar] [CrossRef]
- Santos, R.L.; Silva, T.M.A.; Costa, E.A.; Paixo, T.A.; Tsolis, R.M. Laboratory Animal Models for Brucellosis Research. J. Biomed. Biotechnol. 2011, 2011, 518323. [Google Scholar]
- Robbins, J.R.; Bakardjiev, A.I. Pathogens and the Placental Fortress. Curr. Opin. Microbiol. 2012, 15, 36–43. [Google Scholar] [CrossRef]
- Starr, T.; Ng, T.W.; Wehrly, T.D.; Knodler, L.A.; Celli, J. Brucella Intracellular Replication Requires Trafficking through the Late Endosomal/Lysosomal Compartment. Traffic 2008, 9, 678–694. [Google Scholar] [CrossRef]
- Comerci, D.J.; Martínez-Lorenzo, M.J.; Sieira, R.; Gorvel, J.P.; Ugalde, R.A. Essential Role of the VirB Machinery in the Maturation of the Brucella abortus-Containing Vacuole. Cell. Microbiol. 2001, 3, 159–168. [Google Scholar] [CrossRef]
- Delrue, R.M.; Martinez-Lorenzo, M.; Lestrate, P.; Danese, I.; Bielarz, V.; Mertens, P.; De Bolle, X.; Tibor, A.; Gorvel, J.P.; Letesson, J.J. Identification of Brucella Spp. Genes Involved in Intracellular Trafficking. Cell. Microbiol. 2001, 3, 487–497. [Google Scholar] [CrossRef]
- Brumell, J.H. Brucella “Hitches a Ride” with Autophagy. Cell Host Microbe 2012, 11, 2–4. [Google Scholar] [CrossRef][Green Version]
- Starr, T.; Child, R.; Wehrly, T.D.; Hansen, B.; Hwang, S.; López-Otin, C.; Virgin, H.W.; Celli, J. Selective Subversion of Autophagy Complexes Facilitates Completion of the Brucella Intracellular Cycle. Cell Host Microbe 2012, 11, 33–45. [Google Scholar] [CrossRef]
- Hanwei, J.; Nie, X.; Zhu, H.; Li, B.; Pang, F.; Yang, X.; Cao, R.; Yang, X.; Zhu, S.; Peng, D.; et al. MiR-146b-5p Plays a Critical Role in the Regulation of Autophagy in ∆per Brucella Melitensis-Infected RAW264.7 Cells. BioMed Res. Int. 2020, 2020, 3242. [Google Scholar] [CrossRef]
- Verbeke, J.; Fayt, Y.; Martin, L.; Yilmaz, O.; Sedzicki, J.; Reboul, A.; Jadot, M.; Renard, P.; Dehio, C.; Renard, H.; et al. Host Cell Egress of Brucella abortus Requires BNIP3L-Mediated Mitophagy. EMBO J. 2023, 42, 2817. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, S.P.; Chevrier, N.; Lacerda, T.L.S.; Ben Amara, A.; Gerart, S.; Gorvel, V.A.; De Chastellier, C.; Blasco, J.M.; Mege, J.L.; Gorvel, J.P. Pathogenic Brucellae Replicate in Human Trophoblasts. J. Infect. Dis. 2013, 207, 1075–1083. [Google Scholar] [CrossRef]
- García-Méndez, K.B.; Hielpos, S.M.; Soler-Llorens, P.F.; Arce-Gorvel, V.; Hale, C.; Gorvel, J.P.; O’Callaghan, D.; Keriel, A. Infection by Brucella melitensis or Brucella papionis Modifies Essential Physiological Functions of Human Trophoblasts. Cell. Microbiol. 2019, 21, 13019. [Google Scholar] [CrossRef]
- Alexander, B.; Schnurrenberger, P.R.; Brown, R.R. Numbers of Brucella abortus in the Placenta, Umbilicus and Fetal Fluid of Two Naturally Infected Cows. Vet. Rec. 1981, 108, 500. [Google Scholar] [CrossRef] [PubMed]
- Samartino, L.E.; Enright, F.M. Pathogenesis of Abortion of Bovine Brucellosis. Comp. Immunol. Microbiol. Infect. Dis. 1993, 16, 95–101. [Google Scholar] [CrossRef]
- Samartino, L.E.; Truax, R.E.; Enright, F.M. Invasion and Replication of Brucella abortus in Three Different Trophoblastic Cell Lines. Zentralblatt Veterinarmedizin. Reihe B. J. Vet. Med. Ser. B 1994, 41, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Letesson, J.J.; Barbier, T.; Zúñiga-Ripa, A.; Godfroid, J.; De Bolle, X.; Moriyón, I. Brucella Genital Tropism: What’s on the Menu. Front. Microbiol. 2017, 8, 506. [Google Scholar] [CrossRef]
- Smith, H.; Williams, A.E.; Pearce, J.H.; Keppie, J.; Harris-Smith, P.W.; Fitz-George, R.B.; Witt, K. Foetal Erythritol: A Cause of the Localization of Brucella abortus in Bovine Contagious Abortion. Nature 1962, 193, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Barbier, T.; Machelart, A.; Zúñiga-Ripa, A.; Plovier, H.; Hougardy, C.; Lobet, E.; Willemart, K.; Muraille, E.; De Bolle, X.; Van Schaftingen, E.; et al. Erythritol Availability in Bovine, Murine and Human Models Highlights a Potential Role for the Host Aldose Reductase during Brucella Infection. Front. Microbiol. 2017, 8, 1088. [Google Scholar] [CrossRef] [PubMed]
- Byndloss, M.X.; Tsai, A.Y.; Walker, G.T.; Miller, C.N.; Young, B.M.; English, B.C.; Seyffert, N.; Kerrinnes, T.; de Jong, M.F.; Atluri, V.L.; et al. Brucella abortus Infection of Placental Trophoblasts Triggers Endoplasmic Reticulum Stress-Mediated Cell Death and Fetal Loss via Type Iv Secretion System-Dependent Activation of CHOP. mBio 2019, 10, e01538-19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, M.; Li, Z.; Shi, J.; Zhang, Y.; Deng, X.; Liu, L.; Wang, Z.; Qi, Y.; Zhang, H. Deletion of the Type IV Secretion System Effector VceA Promotes Autophagy and Inhibits Apoptosis in Brucella-Infected Human Trophoblast Cells. Curr. Microbiol. 2019, 76, 510–519. [Google Scholar] [CrossRef]
- Zavattieri, L.; Ferrero, M.C.; Alonso Paiva, I.M.; Sotelo, A.D.; Canellada, A.M.; Baldi, P.C. Brucella abortus Proliferates in Decidualized and Non-Decidualized Human Endometrial Cells Inducing a Proinflammatory Response. Pathogens 2020, 9, 369. [Google Scholar] [CrossRef]
- Fernández, A.G.; Hielpos, M.S.; Ferrero, M.C.; Fossati, C.A.; Baldi, P.C. Proinflammatory Response of Canine Trophoblasts to Brucella canis Infection. PLoS ONE 2017, 12, e0186561. [Google Scholar] [CrossRef]
- Brennan, S.J.; Ngeleka, M.; Philibert, H.M.; Forbes, L.B.; Allen, A.L. Canine Brucellosis in a Saskatchewan Kennel. Can. Vet. J. 2008, 49, 703–708. [Google Scholar]
- Mol, J.P.S.; Costa, E.A.; Carvalho, A.F.; Sun, Y.H.; Tsolis, R.M.; Paixão, T.A.; Santos, R.L. Early Transcriptional Responses of Bovine Chorioallantoic Membrane Explants to Wild Type, ΔvirB2 or ΔbtpB Brucella abortus Infection. PLoS ONE 2014, 9, e0108606. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, M.; Wu, J.; Wang, J.; Peng, Q. HMGB1 Release from Trophoblasts Contributes to Inflammation during Brucella melitensis Infection. Cell. Microbiol. 2019, 21, 13080. [Google Scholar] [CrossRef] [PubMed]
- Mol, J.P.S.; Pires, S.F.; Chapeaurouge, A.D.; Perales, J.; Santos, R.L.; Andrade, H.M.; Lage, A.P. Proteomic Profile of Brucella abortus-Infected Bovine Chorioallantoic Membrane Explants. PLoS ONE 2016, 11, e0154209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.; Zhang, Y.; Li, Z.; Liu, J.; Shao, X.; Wu, C.; Wang, Y.; Wang, K.; Li, T.; Liu, L.; et al. Outer Membrane Protein 25 of Brucella Activates Mitogen-Activated Protein Kinase Signal Pathway in Human Trophoblast Cells. Front. Vet. Sci. 2017, 4, 197. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.L.; Kelly, R.W.; Critchley, H.O.D. Decidualization of the Human Endometrial Stromal Cell: An Enigmatic Transformation. Reprod. Biomed. Online 2003, 7, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Vigliani, M.B.; Bakardjiev, A.I. Intracellular Organisms as Placental Invaders. Fetal Matern. Med. Rev. 2014, 25, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Dong, S.L.; Watanabe, K.; Furuoka, H.; Suzuki, H.; Watarai, M. Interferon-Gamma Promotes Abortion Due to Brucella Infection in Pregnant Mice. BMC Microbiol. 2005, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Iwai, N.; Tachibana, M.; Furuoka, H.; Suzuki, H.; Watarai, M. Regulated upon Activation Normal T-Cell Expressed and Secreted (RANTES) Contributes to Abortion Caused by Brucella abortus Infection in Pregnant Mice. J. Vet. Med. Sci. 2008, 70, 681–686. [Google Scholar] [CrossRef][Green Version]
- Haider, S.; Knöfler, M. Human Tumour Necrosis Factor: Physiological and Pathological Roles in Placenta and Endometrium. Placenta 2009, 30, 111–123. [Google Scholar] [CrossRef]
- Adetunji, S.A.; Faustman, D.L.; Adams, L.G.; Garcia-Gonzalez, D.G.; Hensel, M.E.; Khalaf, O.H.; Arenas-Gamboa, A.M. Brucella abortus and Pregnancy in Mice: Impact of Chronic Infection on Fertility and the Role of Regulatory T Cells in Tissue Colonization. Infect. Immun. 2020, 88, e00257-20. [Google Scholar] [CrossRef]
- Gravett, M.G.; Adams, K.M.; Sadowsky, D.W.; Grosvenor, A.R.; Witkin, S.S.; Axthelm, M.K.; Novy, M.J. Immunomodulators plus Antibiotics Delay Preterm Delivery after Experimental Intraamniotic Infection in a Nonhuman Primate Model. Am. J. Obstet. Gynecol. 2007, 197, 518.e1–518.e8. [Google Scholar] [CrossRef]
| Brucella Species | Hosts | Gestational Manifestations | Vertical Transmission | Contagion Source |
|---|---|---|---|---|
| B. melitensis | Small ruminants | Abortion, weak offspring, reduced milk yields | + | Contaminated placenta or aborted fetus. Milk |
| B. abortus | Bovines | Abortion, weak offspring, reduced milk yields | + | Contaminated placenta or aborted fetus. Milk |
| B. suis (biovars 1, 2, 3) | Swine | Abortion, weak offspring | + | Contaminated placenta or aborted fetus. Milk. Contaminated semen |
| B. canis | Canines | Abortion, weak offspring | + | Contaminated placenta or aborted fetus. Milk. Contaminated semen |
| B. ovis | Sheep | Abortion, weak offspring (rare) | Not reported | Close contact or mating with infected rams. |
| B. melitensis, B. abortus, B. suis | Humans | Abortion, preterm birth, intrauterine fetal death, neonatal or maternal death | + | Contaminated milk and dairy products. Tissues or secretions from infected animals. Contaminated aerosols. |
| Cell Type (Line/Primary) | Brucella Species | Intracellular Replication | Ref. |
|---|---|---|---|
| CTB (BeWo) | B. abortus | Typical BCVs | [68] |
| CTB (BeWo) | B. melitensis, B. papionis | VirB-dependent | [69] |
| CTB (Primary) | B. abortus | Typical BCV | [68] |
| EVT (JEG-3) | B. abortus, B. suis | LAMP+/calnexin- inclusions | [68] |
| EVT (JEG-3) | B. melitensis | Typical BCV, VirB-dependent | [68,69] |
| EVT (Swan-71) | B. abortus | VirB-dependent | [8] |
| EVT (Primary) | B. abortus, B. suis | LAMP+/calnexin- inclusions | [68] |
| SYN (Fused BeWo) | B. melitensis, B. papionis, B. abortus | BCV (B. abortus) | [68,69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavattieri, L.; Muñoz González, F.; Ferrero, M.C.; Baldi, P.C. Immune Responses Potentially Involved in the Gestational Complications of Brucella Infection. Pathogens 2023, 12, 1450. https://doi.org/10.3390/pathogens12121450
Zavattieri L, Muñoz González F, Ferrero MC, Baldi PC. Immune Responses Potentially Involved in the Gestational Complications of Brucella Infection. Pathogens. 2023; 12(12):1450. https://doi.org/10.3390/pathogens12121450
Chicago/Turabian StyleZavattieri, Lucía, Florencia Muñoz González, Mariana C. Ferrero, and Pablo C. Baldi. 2023. "Immune Responses Potentially Involved in the Gestational Complications of Brucella Infection" Pathogens 12, no. 12: 1450. https://doi.org/10.3390/pathogens12121450
APA StyleZavattieri, L., Muñoz González, F., Ferrero, M. C., & Baldi, P. C. (2023). Immune Responses Potentially Involved in the Gestational Complications of Brucella Infection. Pathogens, 12(12), 1450. https://doi.org/10.3390/pathogens12121450








