First Detection of West Nile Virus Lineage 2 in Mosquitoes in Switzerland, 2022
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Screening of Mosquito Pools and FTA Cards
2.3. Sequencing and Phylogenetic Analysis
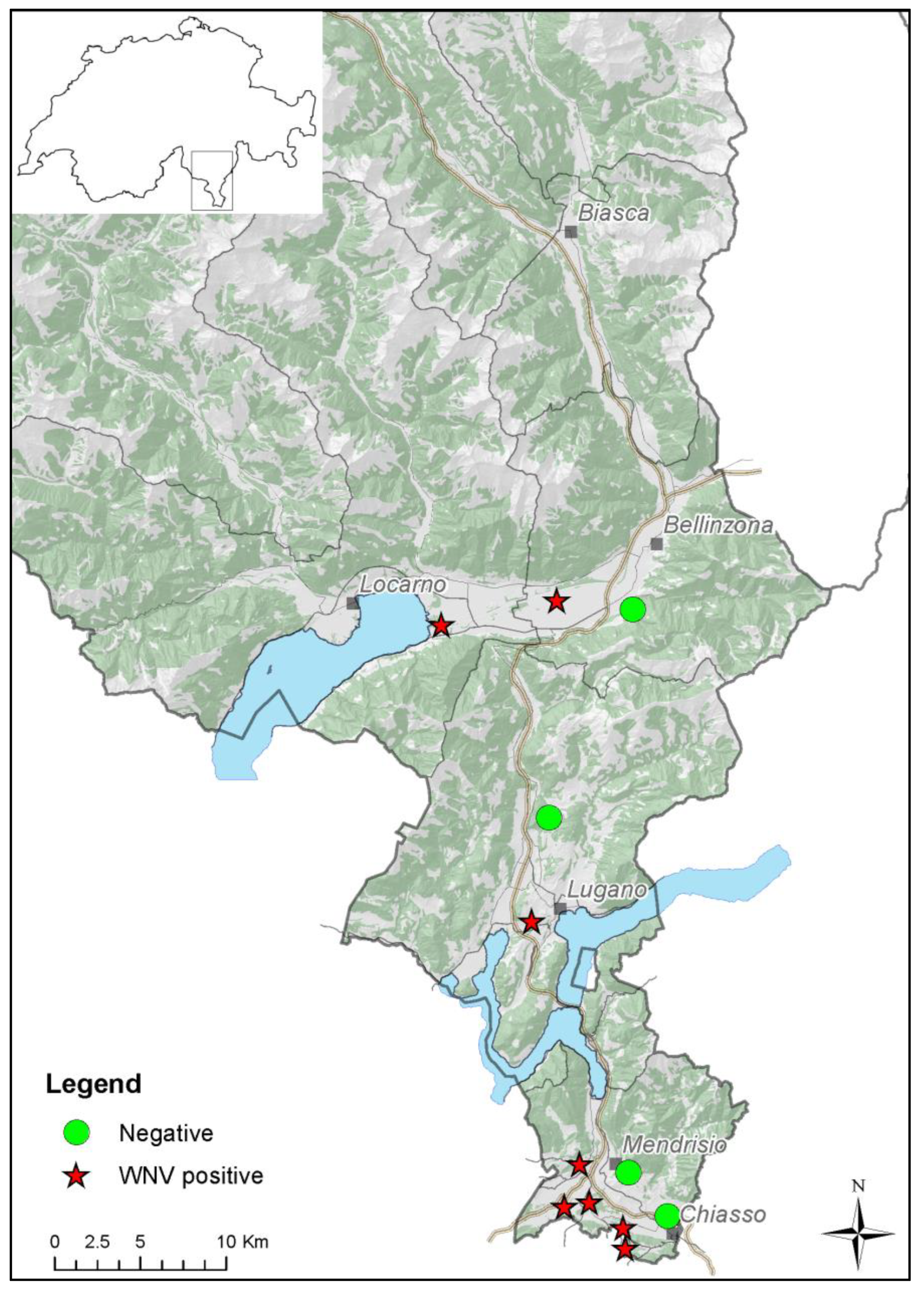
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calisher, C.H.; Karabatsos, N.; Dalrymple, J.M.; Shope, R.E.; Porterfield, J.S.; Westaway, E.G.; Brandt, W.E. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 1989, 70, 37–43. [Google Scholar] [CrossRef]
- Hubálek, Z.; Halouzka, J. West Nile fever a reemerging mosquito-borne viral disease in Europe. Emerg. Infect. Dis. 1999, 5, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, F.; Trachsel, D.S.; Stoeckle, S.D.; Sierra, J.B.; Lübke, S.; Groschup, M.H.; Gehlen, H.; Ziegler, U. Seroepidemiological Survey of West Nile Virus Infections in Horses from Berlin/Brandenburg and North Rhine-Westphalia, Germany. Viruses 2022, 14, 243. [Google Scholar] [CrossRef] [PubMed]
- Troupin, A.; Colpitts, T.M. Overview of West Nile Virus Transmission and Epidemiology. Methods Mol. Biol. 2016, 1435, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.W.; Shing, E.; Nelder, M.; Sander, B. Epidemiologic and clinical parameters of West Nile virus infections in humans: A scoping review. BMC Infect. Dis. 2017, 17, 609. [Google Scholar] [CrossRef] [PubMed]
- Bunning, M.L.; Bowen, R.A.; Cropp, B.C.; Sullivan, K.G.; Davis, B.S.; Komar, N.; Godsey, M.S.; Baker, D.; Hettler, D.L.; Holmes, D.A.; et al. Experimental Infection of Horses with West Nile virus. Emerg. Infect. Dis. 2002, 8, 380–386. [Google Scholar] [CrossRef]
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. Hyg. 1940, 20, 471–492. [Google Scholar] [CrossRef]
- Zeller, H.G.; Schuffenecker, I. West Nile virus: An overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 147–156. [Google Scholar] [CrossRef]
- Ziegler, U.; Lühken, R.; Keller, M.; Cadar, D.; van der Grinten, E.; Michel, F.; Albrecht, K.; Eiden, M.; Rinder, M.; Lachmann, L.; et al. West Nile virus epizootic in Germany, 2018. Antivir. Res. 2019, 162, 39–43. [Google Scholar] [CrossRef]
- Vlaskamp, D.R.M.; Thijsen, S.F.T.; Reimerink, J.; Hilkens, P.; Bouvy, W.H.; Bantjes, S.E.; Vlaminckx, B.J.M.; Zaaijer, H.; Van den Kerkhof, H.H.T.C.; Raven, S.F.H.; et al. First autochthonous human West Nile virus infection in the Netherlands, July to August 2020. Eurosurveillance 2020, 25, 2001904. [Google Scholar] [CrossRef]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile Virus: Review of the Literature. JAMA 2013, 310, 308–315. [Google Scholar] [CrossRef]
- Chiari, M.; Prosperi, A.; Faccin, F.; Avisani, D.; Cerioli, M.; Zanoni, M.; Bertoletti, M.; Moreno, A.M.; Bruno, R.; Monaco, F.; et al. West Nile Virus Surveillance in the Lombardy Region, Northern Italy. Transbound. Emerg. Dis. 2015, 62, 343–349. [Google Scholar] [CrossRef]
- Pautasso, A.; Radaelli, M.C.; Ballardini, M.; Francese, D.R.; Verna, F.; Modesto, P.; Grattarola, C.; Desiato, R.; Bertolini, S.; Vitale, N.; et al. Detection of West Nile and Usutu Viruses in Italian Free Areas: Entomological Surveillance in Piemonte and Liguria Regions, 2014. Vector Borne Zoonotic Dis. 2016, 16, 292–294. [Google Scholar] [CrossRef]
- Bundesamt für Lebensmittelsicherheit und Veterinärwesen; Bundesamt für Gesundheit. Bericht Zur Überwachung von Zoonosen und Lebensmittelbedingten Krankheitsausbrüchen—Daten 2021. 2022, pp. 1–50. Available online: https://www.blv.admin.ch/blv/de/home/tiere/publikationen/statistiken-berichte-tiere.html (accessed on 16 May 2023).
- Engler, O.; Savini, G.; Papa, A.; Figuerola, J.; Groschup, M.H.; Kampen, H.; Medlock, J.; Vaux, A.; Wilson, A.J.; Werner, D.; et al. European surveillance for West Nile virus in mosquito populations. Int. J. Environ. Res. Public Health 2013, 10, 4869–4895. [Google Scholar] [CrossRef]
- Wipf, N.C.; Guidi, V.; Tonolla, M.; Ruinelli, M.; Müller, P.; Engler, O. Evaluation of honey-baited FTA cards in combination with different mosquito traps in an area of low arbovirus prevalence. Parasites Vectors 2019, 12, 554. [Google Scholar] [CrossRef]
- Flacio, E.; Rossi-Pedruzzi, A.; Bernasconi-Casati, E.; Patocchi, N. Culicidae fauna from Canton Ticino and report of three new species for Switzerland. Mitteilungen Schweiz. Entomol. Ges. 2014, 87, 163–182. [Google Scholar]
- Scaramozzino, N.; Crance, J.M.; Jouan, A.; DeBriel, D.A.; Stoll, F.O.; Garin, D. Comparison of flavivirus universal primer pairs and development of a rapid, highly sensitive heminested reverse transcription-PCR assay for detection of flaviviruses targeted to a conserved region of the NS5 gene sequences. J. Clin. Microbiol. 2001, 39, 1922–1927. [Google Scholar] [CrossRef]
- Quick, J.; Grubaugh, N.; Pullan, S.T.; Claro, I.M.; Smith, A.D.; Gangavarapu, K.; Oliveira, G.; Robles-Sikisaka, R.; Rogers, T.F.; Beutler, N.A.; et al. Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nat. Protoc. 2017, 12, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijse, D.F.; van der Linden, A.; Kohl, R.H.; Sikkema, R.S.; Koopmans, M.P.; Munnink, B.B.O. Towards reliable whole genome sequencing for outbreak preparedness and response. BMC Genom. 2022, 23, 569. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequence. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Yu, G. Using ggtree to visualize data on tree-like structures. Curr. Protoc. Bioinform. 2020, 69, e96. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Reisen, K.R. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Riccardo, F.; Bella, A.; Monaco, F.; Ferraro, F.; Petrone, D.; Mateo-Urdiales, A.; Andrianou, X.D.; Del Manso, M.; Venturi, G.; Fortuna, C.; et al. Rapid increase in neuroinvasive West Nile virus infections in humans, Italy, July 2022. Eurosurveillance 2022, 27, 2200653. [Google Scholar] [CrossRef]
- Tsioka, K.; Gewehr, S.; Pappa, S.; Kalaitzopoulou, S.; Stoikou, K.; Mourelatos, S.; Papa, A. West Nile Virus in Culex Mosquitoes in Central Macedonia, Greece, 2022. Viruses 2023, 15, 224. [Google Scholar] [CrossRef]
- Barzon, L.; Montarsi, F.; Quaranta, E.; Monne, I.; Pacenti, M.; Michelutti, A.; Toniolo, F.; Danesi, P.; Marchetti, G.; Gobbo, F.; et al. Early start of seasonal transmission and co-circulation of West Nile virus lineage 2 and a newly introduced lineage 1 strain, northern Italy, June 2022. Eurosurveillance 2022, 27, 2200548. [Google Scholar] [CrossRef]
- ECDC. Communicable Disease Threats Report, Week 47, 20–26 November 2022. 2022, pp. 1–16. Available online: https://www.ecdc.europa.eu/en/publications-data/communicable-disease-threats-report-20-26-november-2022-week-47 (accessed on 26 October 2023).
- MeteoSvizzera. Bollettino del Clima Dell’anno 2022. 2022, pp. 1–17. Available online: https://www.meteosvizzera.admin.ch/servizi-e-pubblicazioni/pubblicazioni/rapporti-e-bollettini/2022/bollettino-del-clima-dell-anno-2022.html (accessed on 26 October 2023).
- Paz, S. Climate change impacts on West Nile virus transmission in a global context. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20130561. [Google Scholar] [CrossRef]
- Shaman, J.; Day, J.F.; Stieglitz, M. Drought-induced amplification and epidemic transmission of West Nile virus in southern Florida. J. Med. Entomol. 2005, 42, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Petrovic, T.; Savic, V.; Barbic, L.; Tabain, I.; Stevanovic, V.; Klobucar, A.; Mrzljak, A.; Ilic, M.; Bogdanic, M.; et al. Epidemiology of Usutu Virus: The European Scenario. Pathogens 2020, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Flacio, E.; Engeler, L.; Tonolla, M.; Lüthy, P.; Patocchi, N. Strategies of a thirteen years surveillance programme on Aedes albopictus (Stegomyia albopicta) in southern Switzerland. Parasites Vectors 2015, 8, 208. [Google Scholar] [CrossRef]
- Ravasi, D.; Parrondo Monton, D.; Tanadini, M.; Flacio, E. Effectiveness of integrated Aedes albopictus management in southern Switzerland. Parasites Vectors 2021, 14, 405. [Google Scholar] [CrossRef]
- Angelini, A.; Tamba, M.; Finarelli, A.C.; Bellini, R.; Albieri, A.; Bonilauri, P.; Cavrini, F.; Dottori, M.; Gaibani, P.; Martini, E.; et al. West Nile virus circulation in Emilia-Romagna, Italy: The integrated surveillance system 2009. Eurosurveillance 2010, 15, 19547. [Google Scholar] [CrossRef]
- Calzolari, M.; Monaco, F.; Montarsi, F.; Bonilauri, P.; Ravagnan, S.; Bellini, R.; Cattoli, G.; Cordioli, P.; Cazzin, S.; Pinoni, C.; et al. New incursions of West Nile virus lineage 2 in Italy in 2013: The value of the entomological surveillance as early warning system. Vet. Ital. 2013, 49, 315–319. [Google Scholar] [CrossRef]
- Lustig, Y.; Sofer, D.; Bucris, E.D.; Mendelson, E. Surveillance and Diagnosis of West Nile Virus in the Face of Flavivirus Cross-Reactivity. Front. Microbiol. 2018, 9, 2421. [Google Scholar] [CrossRef]

| Primer | Genome Position 1 | Sequence (5′–3′) 2 | Product Size (bp) |
|---|---|---|---|
| LSWNV3’F | 10,459–10,478 | CGCCACCGGAAGTTGAGTAG | 111 |
| LSWNV3’R | 10,569–10,550 | CTCCTTCCGAGACGGTTCTG | |
| LSWNV3’P | 10,483–10,503 | TGCTGCCTGCGDCTCAACCCC | |
| LSWNV5’F | 7–27 | TCGCCTGTGTGAGCTGACAAA | 112 |
| LSWNV5’R | 118–96 | GCCCTCCTGGTTTCTTAGACATC | |
| LSWNV5’P | 89–66 | TTCGTGCYAAGAAACAGCTCGCAC |
| Sample ID | Sampling Period | Matrix | Ct Value 1 | Accession No. | % Genome Covered |
|---|---|---|---|---|---|
| TI_SUPSI_C28 | 2–9 August | FTA card | 28.6 | OR091151 | 92.69 |
| TI_SUPSI_C37 | 9–17 August | FTA card | 24.2 | OR091152 | 97.58 |
| TI_SUPSI_C65 | 28 August–6 September | FTA card | 27.8 | OR091153 | 95.96 |
| TI_SUPSI_C70 | 6–12 September | FTA card | 28.0 | OR091154 | 98.26 |
| TI_SUPSI_M63 | 19 July–2 August | Culex pipiens | 31.5 | OR091155 | 95.83 |
| TI_SUPSI_M158 | 9–17 August | Culex pipiens | 29.3 | OR091156 | 96.76 |
| TI_SUPSI_M224 | 28 August–6 September | Culex pipiens | 27.9 | OR091158 | 98.23 |
| TI_SUPSI_M202 | 27 September–5 October | Culex pipiens | 14 | OR091157 | 99.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzin, S.; Liechti, N.; Jandrasits, D.; Flacio, E.; Beuret, C.; Engler, O.; Guidi, V. First Detection of West Nile Virus Lineage 2 in Mosquitoes in Switzerland, 2022. Pathogens 2023, 12, 1424. https://doi.org/10.3390/pathogens12121424
Cazzin S, Liechti N, Jandrasits D, Flacio E, Beuret C, Engler O, Guidi V. First Detection of West Nile Virus Lineage 2 in Mosquitoes in Switzerland, 2022. Pathogens. 2023; 12(12):1424. https://doi.org/10.3390/pathogens12121424
Chicago/Turabian StyleCazzin, Stefania, Nicole Liechti, Damian Jandrasits, Eleonora Flacio, Christian Beuret, Olivier Engler, and Valeria Guidi. 2023. "First Detection of West Nile Virus Lineage 2 in Mosquitoes in Switzerland, 2022" Pathogens 12, no. 12: 1424. https://doi.org/10.3390/pathogens12121424
APA StyleCazzin, S., Liechti, N., Jandrasits, D., Flacio, E., Beuret, C., Engler, O., & Guidi, V. (2023). First Detection of West Nile Virus Lineage 2 in Mosquitoes in Switzerland, 2022. Pathogens, 12(12), 1424. https://doi.org/10.3390/pathogens12121424






