Abstract
Yeasts associated with lepidopteran pests have been shown to play a role in their survival, development, and oviposition preference. It has been demonstrated that combining these yeasts with existing biological control agents can enhance their efficacy. The tortricid Thaumatotibia leucotreta is a phytosanitary pest in the South African citrus industry, with the baculovirus Cryptophlebia leucotreta granulovirus (CrleGV) being one of the components that can control this pest. Several yeast species were shown to be associated with T. leucotreta larvae, which affected their behaviour and development. A series of detached fruit bioassays were performed to determine whether the combination of yeast with CrleGV enhances its efficacy. These assays included determining the optimal yeast/virus ratio, testing all isolated yeast species in combination with CrleGV, and further improving yeast/virus formulation by adding an adjuvant. The optimal yeast concentration to use alongside CrleGV was determined to be 106 cells·mL−1. Pichia kluyveri, P. kudriavzevii, Kluyveromyces marxianus, and Saccharomyces cerevisiae in combination with CrleGV reduced larval survival compared to CrleGV alone. The addition of molasses and BREAK-THRU® S 240 to P. kudriavzevii and S. cerevisiae in combination with CrleGV did not notably improve their effectiveness; however, there was an observed decrease in larval survival. In future studies, field trials will be conducted with combinations of CrleGV and P. kudriavzevii or S. cerevisiae to investigate whether these laboratory findings can be replicated in orchard conditions.
1. Introduction
Thaumatotibia leucotreta (Meyrick) (Lepidoptera: Tortricidae) (false codling moth) is a significant pest of the South African citrus industry and other citrus-producing regions throughout sub-Saharan Africa [1]. The feeding of larvae on fruit causes damage, potentially reducing yields and resulting in financial losses [2]. Improved management practices in recent years have effectively suppressed T. leucotreta populations in South African orchards [3]. However, T. leucotreta is now primarily a phytosanitary risk rather than a destructive crop pest, with zero tolerance in the European Union and other export markets [4].
Several control options are available to manage T. leucotreta, including chemical, biological, cultural, and pheromone-based methods [3,4]. Treatments are strategically combined into an integrated pest management (IPM) programme to suppress T. leucotreta, beginning early in the season [5,6]. IPM programmes are considered the most effective approach to managing this pest and rely on the intelligent selection and implementation of pest monitoring and control options. Biological control agents are fast becoming an integral part of IPM programmes due to the stringent regulations around chemical insecticides, which limit their use and availability [7]. A number of biological agents are available for use against T. leucotreta in South Africa, most notably baculoviruses [3,8].
The baculovirus Cryptophlebia leucotreta granulovirus (CrleGV) has been extensively used for more than 15 years to control T. leucotreta throughout South Africa [3,9]. Despite the significant benefits of baculoviruses, they have a few drawbacks, including their sensitivity to ultraviolet (UV) degradation, slower speed of kill, short field persistence and narrow host range [8,9,10,11].
While CrleGV is an effective biological control agent against T. leucotreta [9], continued research and innovation are necessary to enhance its efficacy. Improving the effectiveness of baculoviruses is crucial for their long-term use as biopesticides. The principal biological limitation affecting the efficacy of baculoviruses is the possibility for them to be ingested by their intended host before penetrating the fruit [12]. Efforts to improve the performance of baculoviruses have mainly focused on increasing virus exposure time to larvae before they penetrate the fruit through the use of attractants and feeding stimulants [9,13]. Adding adjuvants such as molasses to virus formulations has been shown to significantly improve virus efficacy compared to applying the virus alone [9,14]. Recently, the incorporation of mutualistic microorganisms associated with the target pest into formulations has been proposed as a new larval attractant [15].
Previous attempts to enhance Cydia pomonella granulovirus (CpGV) performance through the combination of feeding stimulants and larval attractants have not yielded definitive improvements [13,16,17]. These efforts have predominantly centred around host plant volatiles that attract Cydia pomonella (Linnaeus) (Lepidoptera: Tortricidae) neonates, but they have primarily appeared to facilitate host location rather than stimulate feeding behaviour [18,19]. Microbes consumed and harboured within the gut of insects have the potential to profoundly influence both the survival and behaviour of their host [20]. The significance of microbial communities in insect–plant interactions is indispensable [21,22]. Cydia pomonella larvae have a close affiliation with yeasts from the genus Metschnikowia, which were isolated from their gut and feeding galleries. Larval feeding assays demonstrated that M. andauensis positively impacted C. pomonella larvae, accelerating their development and lowering mortality rates. Volatiles produced by M. andauensis also elicited upwind flight in adult C. pomonella females, which resulted in an increased number of eggs being oviposited on apples that had been inoculated with yeast [15]. The combination of yeasts, isolated from C. pomonella larvae, with CpGV occlusion bodies (OBs) noticeably improved the virus’s efficiency, both in controlled laboratory experiments and in practical field applications [23]. Yeasts from the genus Metschnikowia actively promoted larval feeding and facilitated the ingestion of CpGV [15,23]. Furthermore, considering the widespread availability of Saccharomyces cerevisiae in commercial use, its combination with CpGV was also evaluated. Larval mortality recorded in CpGV assays using S. cerevisiae closely resembled that of yeasts isolated from C. pomonella larvae [14].
A recent study aimed to identify yeast species present within the digestive tract of T. leucotreta larvae collected from citrus orchards across South Africa. This led to the identification of six yeast species: Meyerozyma guilliermondii, Pichia kluyveri, Pichia kudriavzevii, Hanseniaspora uvarum, Clavispora lusitaniae, and Kluyveromyces marxianus [24]. Larval development assays demonstrated that M. guilliermondii, H. uvarum, and P. kluyveri accelerated larval development and reduced mortality, while P. kluyveri, H. uvarum, P. kudriavzevii, and K. marxianus were shown to influence the feeding preference of neonate T. leucotreta in larval feeding assays. Additionally, the oviposition preference of adult T. leucotreta females was affected by M. guilliermondii, P. kudriavzevii, and H. uvarum, with an increased number of eggs being oviposited on Navel oranges inoculated with these yeasts.
Yeast strains hold promising potential as abundant sources of novel biological agents that can be harnessed for the augmentation and improvement of established control agents. In this study, we aimed to determine whether previously isolated yeast species associated with T. leucotreta larvae increase the efficacy of CrleGV when combined. Furthermore, the addition of an adjuvant (molasses) to improve the yeast/virus mixture efficacy was analysed.
2. Materials and Methods
2.1. Thaumatotibia leucotreta Culture
Thaumatotibia leucotreta eggs were obtained from the heterogeneous culture, known as “Mixed Colony”, held at Rhodes University’s Department of Zoology and Entomology, South Africa. Eggs were stored in Petri dishes sealed with parafilm in a 25 °C controlled environment (CE) room with a relative humidity of 30–60%. Once the eggs had turned dark brown, a piece of cotton wool moistened with double distilled water (ddH2O) was placed in the Petri dish to ensure that emerging T. leucotreta neonates did not dehydrate before being used.
2.2. Detached Fruit Bioassays
Three sets of detached fruit bioassays were conducted to (i) determine the optimal yeast concentration to use in combination with CrleGV, (ii) assess the effectiveness of combining each of the isolated yeasts with CrleGV, and (iii) evaluate the efficacy of the yeast/virus mixture through the addition of an adjuvant and surfactant. CrleGV was applied at an LC50 concentration of 9.31 × 107 OBs·mL−1 for all treatments [25]. The LC50 concentration was selected as this would result in 50% mortality of the T. leucotreta population.
Batches of Navel oranges were collected from orchards in the Sunday’s River Valley in the Eastern Cape Province of South Africa. No postharvest treatments had been applied to the oranges. The Navel oranges were stored in a 4 °C cold room to preserve the fruit until use. The oranges were checked weekly, with fruit showing any sign of disease or mould being discarded. Oranges were stored for a maximum period of 6–8 weeks. Navel oranges were removed from 4 °C cold storage one day prior to being used in a detached fruit bioassay. They were inspected for any sign of disease or mould before being thoroughly washed in a 0.5% bleach solution (v/v), rinsed twice in ddH2O, and allowed to air dry in a 25 °C CE room overnight.
Yeast cultures were grown in a Yeast extract Peptone Dextrose (YPD) medium containing 40 units·mL−1 of penicillin (Pen) and 40 µg·mL−1 of streptomycin (Strep) (Thermo Fisher Scientific, Waltham, MA, USA) for 20 h at 27 °C while shaking. Cell counts were adjusted appropriately with ddH2O.
A modified version of the detached fruit bioassay described by Moore et al. [25] was used to determine the effectiveness of combining CrleGV with yeast against T. leucotreta. The first set of bioassay treatments included a ddH2O control, CrleGV alone, P. kudriavzevii at 2 × 108 cells·mL−1 plus CrleGV, P. kudriavzevii at 2 × 106 cells·mL−1 plus CrleGV, and P. kudriavzevii at 2 × 104 cells·mL−1 plus CrleGV. A yeast concentration of 108 cells·mL−1 was selected as a starting point based on prior research on yeast/virus synergism in C. pomonella [23]. Pichia kudriavzevii was selected as the yeast isolate to use during these bioassays due to its attractiveness to T. leucotreta neonates and adult females [24]. The second set of detached fruit bioassays evaluated the efficacy of combining M. guilliermondii, P. kluyveri, H. uvarum, and K. marxianus with CrleGV. Saccharomyces cerevisiae was also included as a treatment, as it forms part of the artificial diet on which T. leucotreta larvae are reared [26]. Yeasts were applied at a concentration of 2 × 106 cells·mL−1 with CrleGV at 9.31 × 107 OBs·mL−1, as it was previously shown to be the optimal yeast/virus ratio. Finally, detached fruit bioassays were conducted to enhance the efficacy of the yeast/virus mixture by adding molasses and BREAK-THRU® S 240, as these have been shown to enhance the efficacy of the virus in field trials [9]. Pichia kudriavzevii was selected because it has been shown to influence T. leucotreta neonates and adult female behaviour [24]. Saccharomyces cerevisiae was included, as it is a commercially available yeast strain and has previously been shown to enhance the efficacy of CpGV [14]. The third set of bioassay treatments included a ddH2O control, CrleGV, P. kudriavzevii (at 2 × 106 cells·mL−1) plus CrleGV, and S. cerevisiae (at 2 × 106 cells·mL−1) plus CrleGV, each with an adjuvant (molasses) and surfactant (BREAK-THRU® S 240) (Evonik Industries AG, Essen, Germany) at 0.25% and 0.005%, respectively.
Navel oranges were placed onto a sterile metal rack and sprayed with a specific treatment until runoff using a handheld sprayer. The treated oranges were subsequently positioned on a platform with none of the fruit coming into contact with each other, transported to a 25 °C CE room and allowed to dry for 30–45 min. Five T. leucotreta neonates were placed on each fruit. Thirty Navel oranges were used per treatment, with each treatment replicated three times. Detached fruit bioassays were run for 14 days, after which the Navel oranges were dissected and inspected for the presence or absence of live T. leucotreta larvae.
2.3. Statistical Analysis
A generalized linear mixed model (GLMM) was utilised to determine whether the inclusion of yeast to CrleGV impacted T. leucotreta larval survival. The data from the three experiments were individually analysed, as each experiment was conducted sequentially. The GLMM was specified using a binomial error distribution and a logit link function. Significant differences in larval survival between treatments were assessed using likelihood ratio tests. Where significant differences were found, pairwise comparisons were performed using the “emmeans” R package and were adjusted for multiple comparisons using Tukey adjustment [27]. All statistical analyses were performed using R version 4.4.2 (R Core Team 2022) and all models were fitted using the “glmmTMB” R package [28]. Graphs were produced using GraphPad Prism version 10.0.3.
3. Results
3.1. Optimising the Yeast/Virus Ratio
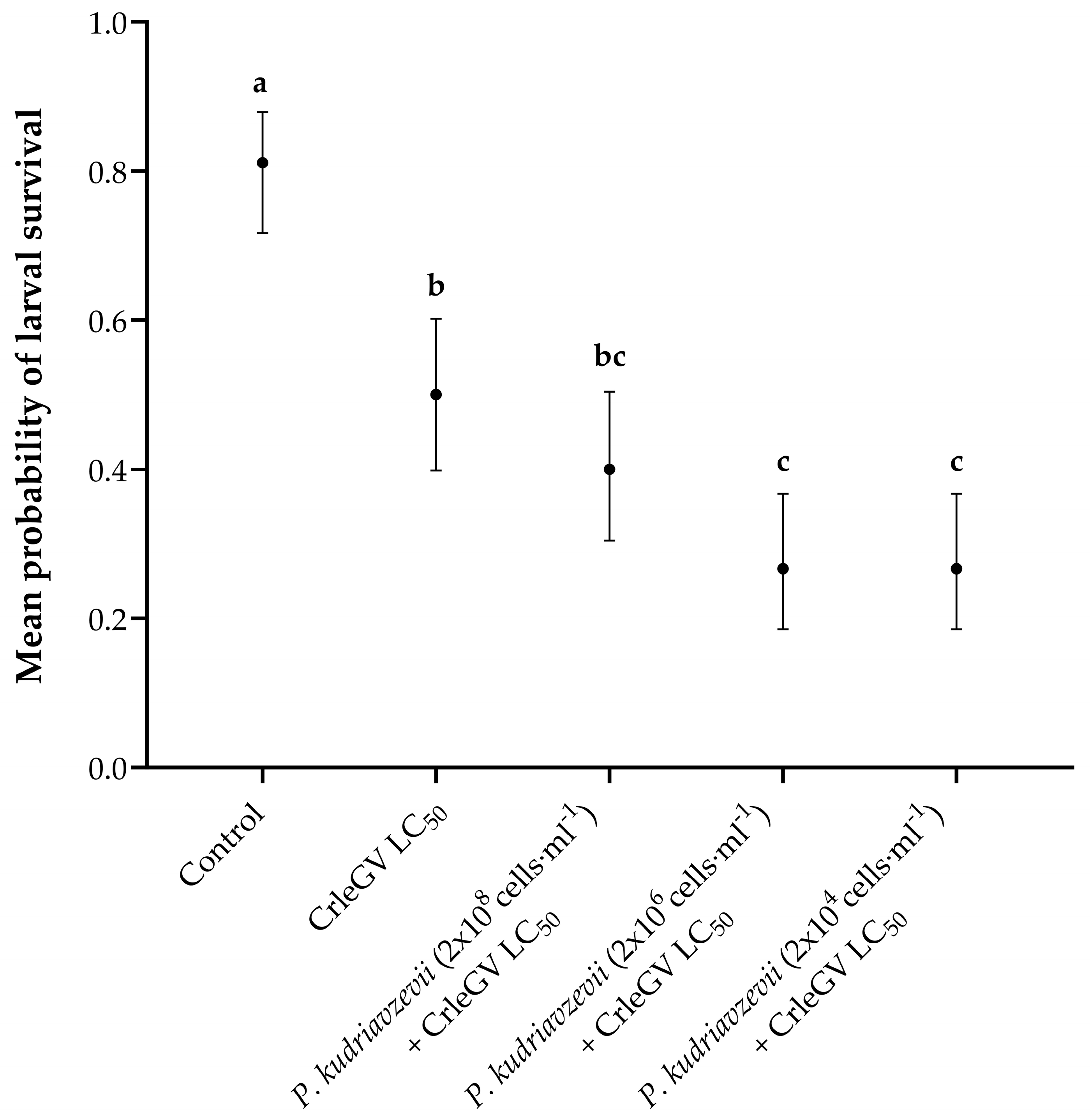
Detached fruit bioassays were conducted to determine the optimal yeast concentration to apply alongside CrleGV. There was evidence for a statistically significant difference in larval survival between treatments (X2 = 62.525, df = 4, p < 0.001). The application of P. kudriavzevii at a concentration of 2 × 108 cells·mL−1 (beta = 0.405, z-value = 1.346, p = 0.6623) in combination with CrleGV did not result in a significant reduction in larval survival when compared to the use of CrleGV alone. However, when P. kudriavzevii was applied at concentrations of 2 × 106 cells·mL−1 (beta = 1.012, z-value = 3.179, p = 0.0129) and 2 × 104 cells·mL−1 (beta = 1.012, z-value = 3.179, p = 0.0129), it led to a notable decrease in larval survival by 23.33% (Figure 1). All treatments were significantly different from the ddH2O control treatment.
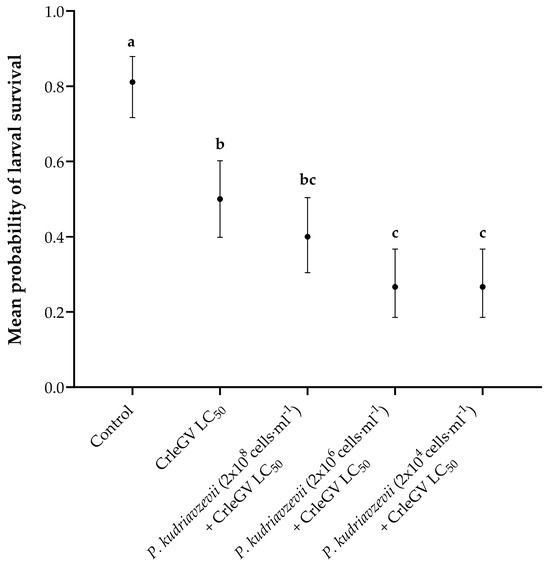
Figure 1.
Thaumatotibia leucotreta larval survival in 14-day detached fruit bioassays (n = 3). Navel oranges were treated with ddH2O, CrleGV at 9.31 × 107 OBs·ml−1, and P. kudriavzevii at varying concentrations ranging from 2 × 108 to 2 × 104 cells·mL−1 plus CrleGV. Different letters indicate statistically significant differences (p ≤ 0.05).
3.2. Combining CrleGV with Yeast
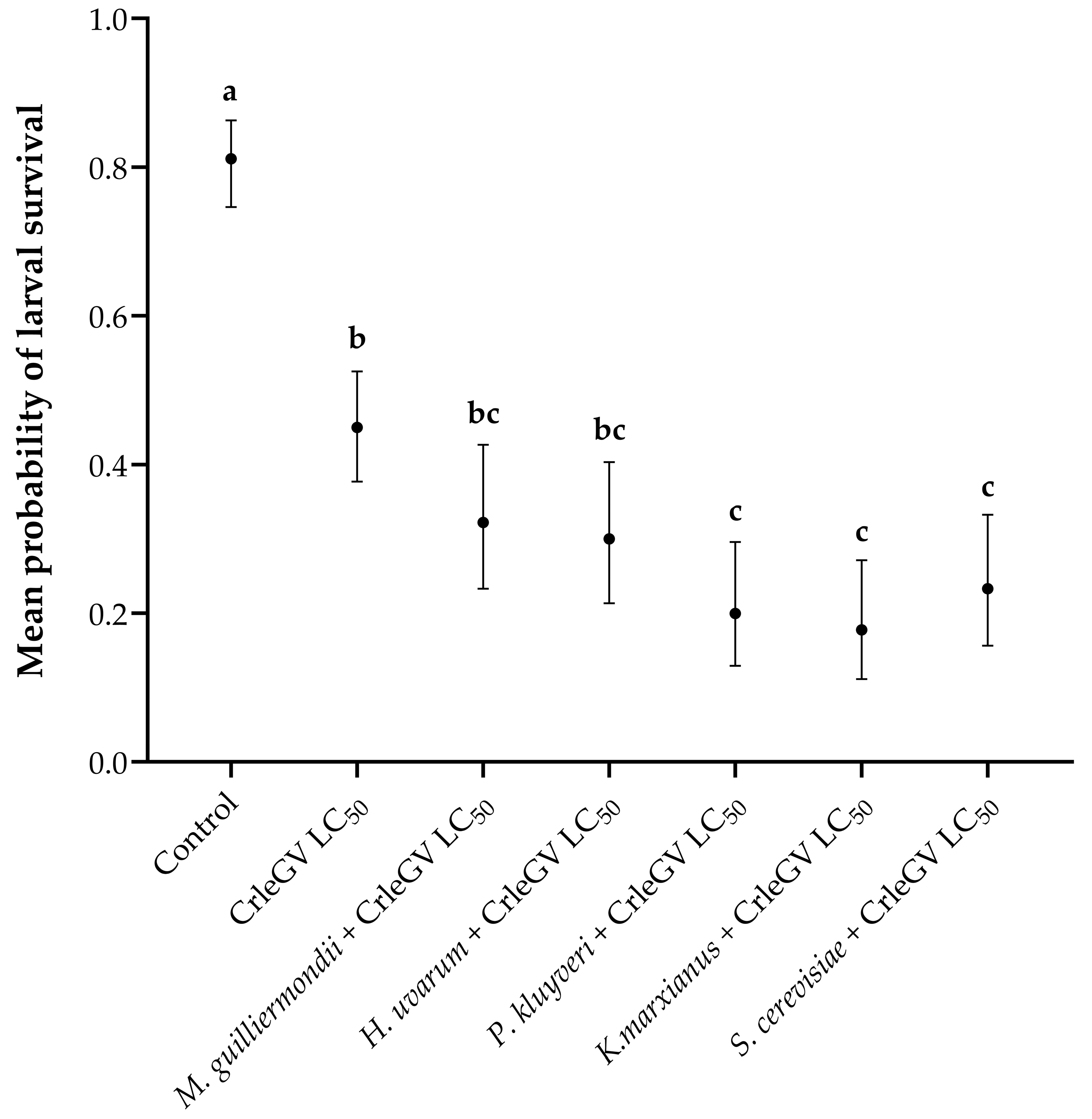
There was evidence for a statistically significant difference in larval survival between treatments (X2 = 143.82, df = 6, p < 0.001). In comparison to the use of CrleGV alone, the inclusion of P. kluyveri, K. marxianus, and S. cerevisiae alongside the virus resulted in reductions in larval survival by 25.01% (with beta = 1.187, z-value = 3.913, p = 0.0018), 27.24% (with beta = 1.332, z-value = 4.243, p = 0.0004), and 21.68% (with beta = 0.990, z-value = 3.402, p = 0.0119), respectively (Figure 2). Meyerozyma guilliermondii (beta = 0.543, z-value = 2.006, p = 0.4108) and H. uvarum (beta = 0.647, z-value = 2.357, p = 0.2174) did not significantly reduce larval survival when applied in combination with CrleGV compared to the virus alone. All treatments were significantly different from the ddH2O control treatment.
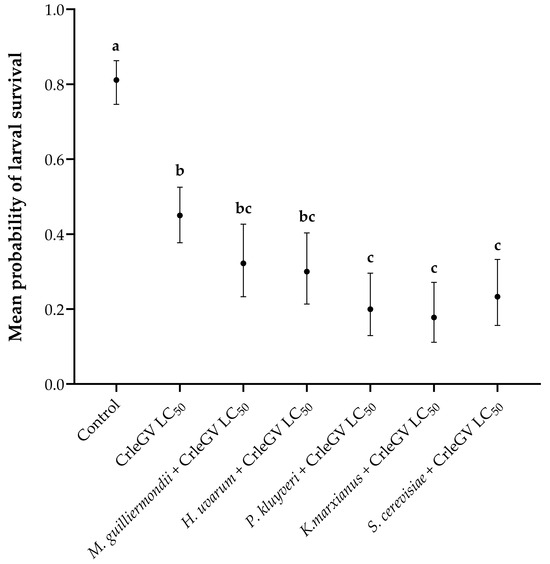
Figure 2.
Larval survival of T. leucotreta in 14-day detached fruit bioassays (n = 3). Navel oranges were treated with H. uvarum, K. marxianus, M. guilliermondii, P. kluyveri, and S. cerevisiae combined with CrleGV. Different letters indicate statistically significant differences (p ≤ 0.05).
3.3. Enhancing the Efficacy of Yeast/Virus Formulation
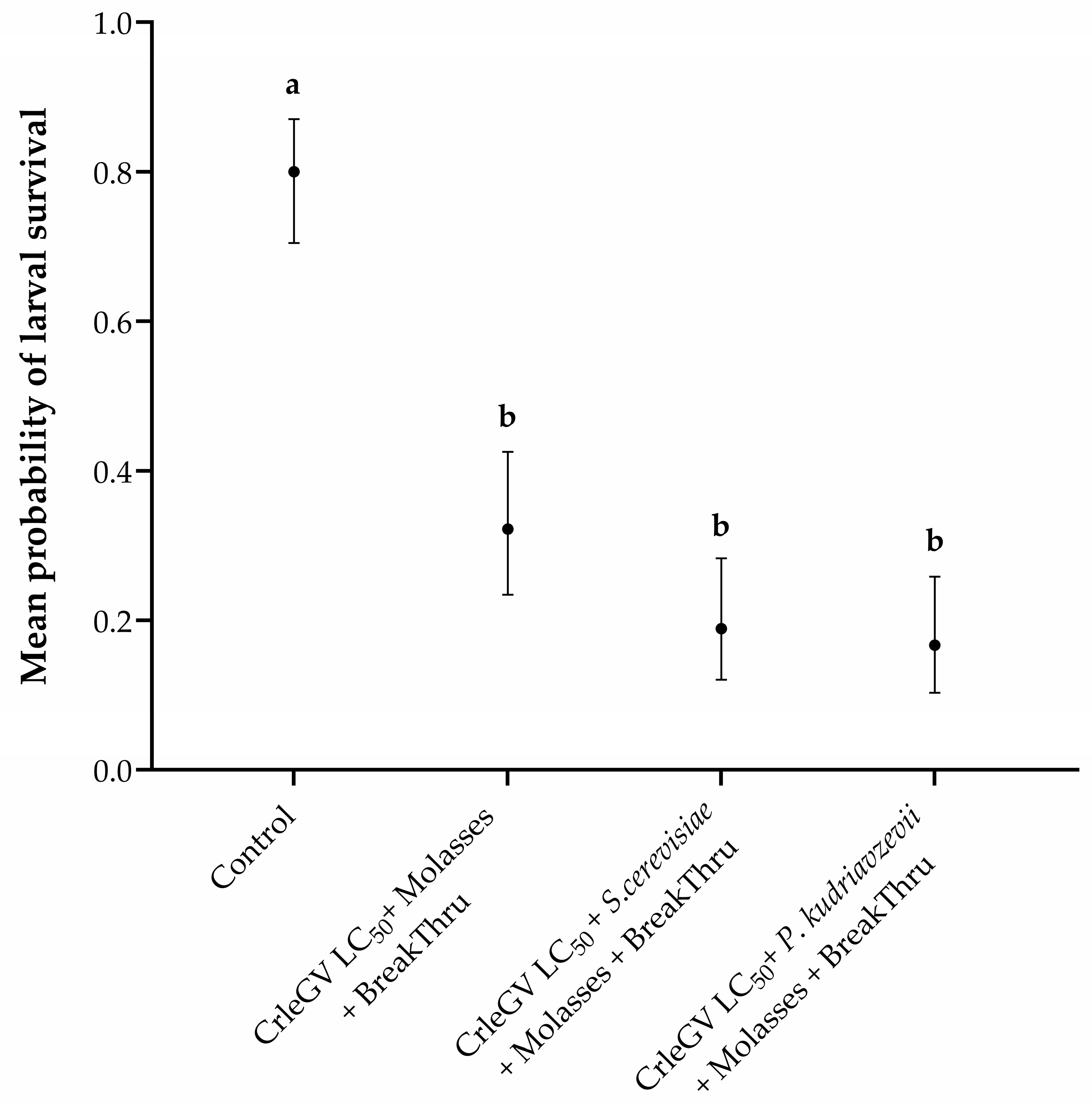
There was evidence for a statistically significant difference in larval survival between treatments (X2 = 80.059, df = 3, p < 0.001). The addition of molasses and BREAK-THRU® S 240 did not significantly enhance the efficacy of P. kudriavzevii (beta = 0.866, z–value = 2.393, p = 0.0783) or S. cerevisiae (beta = 0.714, z-value = 2.032, p = 0.1763) formulations compared to CrleGV alone (Figure 3). The inclusion of molasses and BREAK-THRU® S 240 resulted in a 15.56% and 13.34% decrease in larval survival, respectively, when P. kudriavzevii and S. cerevisiae were present, compared to treatments without these additives. Furthermore, no significant differences were recorded between the two yeast isolates (beta = −0.152, z-value = −0.390, p = 0.9799). All treatments were significantly different from the ddH2O control treatment.
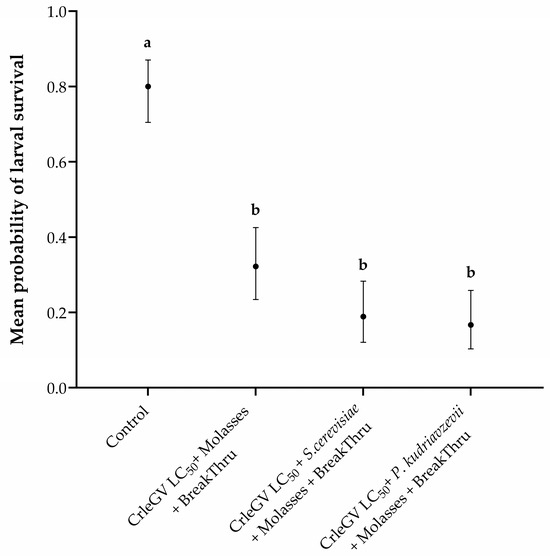
Figure 3.
Thaumatotibia leucotreta larval survival in 14-day detached fruit bioassays including molasses and BREAK-THRU® S 240 (n = 3). Navel oranges were treated with ddH2O, CrleGV alone, P. kudriavzevii plus CrleGV, and S. cerevisiae plus CrleGV, with yeasts applied at 2 × 106 cells·mL−1 and CrleGV at 9.31 × 107 OBs·mL−1. Except for the control, all treatments had the addition of molasses and BREAK-THRU® S 240 at 0.25% and 0.005%, respectively. Different letters indicate statistically significant differences (p ≤ 0.05).
4. Discussion
The influence of mutualistic yeast on insect behaviour and development has been studied recently [15,20,24,29,30]. Yeasts have demonstrated their vital role as a nutritional foundation for the growth of insect larvae and in their capacity to impact the feeding patterns and behaviour of newly hatched larvae [30,31,32,33]. Additionally, volatile compounds produced by yeasts evoke significant behavioural responses in insects [31,34,35]. Cydia pomonella larvae have a strong symbiotic relationship with yeast belonging to the genus Metschnikowia [15]. These yeasts have been demonstrated to play a critical role in promoting the growth of C. pomonella larvae by providing vital nutrients and safeguarding them against fungal infections, thereby reducing mortality [15]. The interactions between insect pests and their mutualistic microbes present an ideal target for manipulation and use in biological control. However, incorporating beneficial microbes to enhance larval feeding with existing biological control agents has been limited [14,23].
Previous research showed improved effectiveness of baculovirus treatments utilising mutualistic yeast at 108 cells·mL−1 [14,23]. However, no significant differences were recorded in this study when P. kudriavzevii was applied in mixtures with CrleGV at a similar concentration [36]. The concentration at which P. kudriavzevii was applied may have resulted in an unfavourable yeast/virus ratio and resulted in yeast cells not being thoroughly covered with CrleGV. Detached fruit bioassays using a 100-fold serial dilution of P. kudriavzevii, ranging from 2 × 108 to 2 × 104 cells·mL−1, were set up to determine the optimal yeast/virus ratio. Significant differences were recorded when using the lower yeast concentrations of 106 cells·mL−1 and 104 cells·mL−1 alongside CrleGV. The effectiveness of a biological control agent utilising microorganisms relies on how much of the agent used reaches the target pest [37]. Hence, the use of lower yeast/virus ratios may result in greater uptake of CrleGV OBs, assuming a consistent intake of contaminated yeast cells during larval feeding. An overabundance of yeast can also lead to high alcohol levels, which could negatively affect insect physiology and behaviour [38].
Previous studies have shown that neonate T. leucotreta exhibited altered behaviour when exposed to H. uvarum, P. kluyveri, P. kudriavzevii, and K. marxianus [24]. Specifically, they displayed an attraction to yeast broth that had been inoculated with these yeast species for feeding. It was also demonstrated that the mortality rate of T. leucotreta larvae significantly decreased when Navel oranges were treated with M. guilliermondii, P. kluyveri, H. uvarum, and S. cerevisiae [24]. These characteristics render these yeasts remarkably well-suited as potential feeding stimulants for T. leucotreta. The association of S. cerevisiae with several key agricultural pests has been extensively documented [39,40,41]. The addition of S. cerevisiae to CpGV resulted in a level of larval mortality comparable to that of the wild-type isolate (Metschnikowia pulcherrima) associated with C. pomonella [14]. Saccharomyces cerevisiae was included here, due to its commercial availability and inclusion in the artificial diet used to rear T. leucotreta [26]. A significant decrease in larval survival was recorded with most gut-associated yeasts and S. cerevisiae. However, M. guilliermondii and H. uvarum did not enhance the efficacy of CrleGV. The positive effects of their ingestion on the development and survival of T. leucotreta larvae may have reduced the effectiveness of CrleGV at the LC50 dose [24].
Once T. leucotreta larvae penetrate the fruit’s rind, they are unlikely to ingest any additional OBs [25]. Molasses has been used as a larval-feeding stimulant with some success in improving baculovirus efficacy [9,13]. Furthermore, owing to the adhesive properties of molasses, it may unintentionally lead to an increase in the number of OBs adhering to the fruit’s surface. BREAK-THRU® S240 was included as it decreases the surface tension of spray droplets, leading to enhanced retention and deposition of spray treatments [42]. Additionally, it exhibits a “super-spreading” effect that significantly enhances the coverage of surfaces by spray treatments, resulting in improved dispersion of spray residues [42]. Adding molasses and BREAK-THRU® S240 to P. kudriavzevii and S. cerevisiae plus CrleGV treatments did decrease larval survival but not significantly, compared to that of the virus alone. The efficacy of CrleGV could be further enhanced by adding molasses to yeast/virus formulations, as it has previously been demonstrated to be an effective adjuvant [9,13,14]. It might be necessary to fine-tune the ratio of molasses and BREAK-THRU® S240 utilized alongside P. kudriavzevii and S. cerevisiae to achieve the ideal working conditions. An LC50 concentration of CrleGV was selected, as this would result in 50% mortality of the T. leucotreta population. Thus, an increase or decrease in mortality could be observed when combining CrleGV with a specific treatment. The mortality rates of T. leucotreta observed in detached fruit bioassays using the LC50 concentration of CrleGV were comparable to previously reported rates [25]. As previously found with C. pomonella [14,23], the addition of mutualistic yeast to a virus treatment proved effective in increasing larval mortality.
The inclusion of yeast isolated from T. leucotreta to CrleGV formulations proved effective in increasing virus efficacy. Additionally, the inclusion of molasses and BREAK-THRU® S240 further increased the formulation’s effectiveness, compared to CrleGV being applied alone. Taken together, the results of this study indicate that P. kudriavzevii and S. cerevisiae hold potential for use in biocontrol, especially when combined with other well-established control techniques for use against T. leucotreta. These yeasts could potentially serve as a supplement to enhance larval feeding and thus virus ingestion, and as a distinctive approach for pest monitoring and attraction. Future work will entail conducting field trials with P. kudriavzevii and S. cerevisiae to determine whether their ability to increase the effectiveness of CrleGV in laboratory assays can be replicated in citrus orchards.
Author Contributions
Conceptualisation, S.D.M., M.P.H. and C.K.; methodology, S.D.M., M.v.d.M. and M.D.J.; formal analysis, M.v.d.M. and M.D.J.; investigation, M.v.d.M.; resources, S.D.M., M.P.H., C.K. and M.D.J.; data curation, M.v.d.M.; writing—original draft preparation, M.v.d.M.; writing—review and editing, M.v.d.M., M.D.J., C.K., M.P.H. and S.D.M.; visualisation, M.v.d.M.; supervision, C.K., M.P.H. and S.D.M.; project administration, M.v.d.M., M.P.H., S.D.M. and C.K.; funding acquisition, M.P.H. and S.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank Citrus Research International (CRI), Research for Citrus Exports (RCE) Programme of the Department of Science and Innovation (DSI), and the Research Council of Rhodes University for financial support. The South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation (NRF) of South Africa (grant code 84643) provided additional funding for this work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Special thanks must be given to Candice Coombes for maintaining the T. leucotreta colony held at Rhodes University, which was used for the duration of this research. The authors would also like to thank Guy Sutton for his assistance with the statistical analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mkiga, A.M.; Mohamed, S.A.; du Plessis, H.; Khamis, F.M.; Ekesi, S. Field and Laboratory Performance of False Codling Moth, Thaumatotibia leucotreta (Lepidoptera: Troticidae) on Orange and Selected Vegetables. Insects 2019, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Boardman, L.; Grout, T.G.; Terblanche, J.S. False Codling Moth Thaumatotibia leucotreta (Lepidoptera, Tortricidae) Larvae Are Chill-Susceptible. Insect Sci. 2012, 19, 315–328. [Google Scholar] [CrossRef]
- Moore, S.D. Biological Control of a Phytosanitary Pest (Thaumatotibia leucotreta): A Case Study. Int. J. Environ. Res. Public Health 2021, 18, 1198. [Google Scholar] [CrossRef]
- Hattingh, V.; Moore, S.; Kirkman, W.; Goddard, M.; Thackeray, S.; Peyper, M.; Sharp, G.; Cronjé, P.; Pringle, K. An Improved Systems Approach as a Phytosanitary Measure for Thaumatotibia leucotreta (Lepidoptera: Tortricidae) in Export Citrus Fruit From South Africa. J. Econ. Entomol. 2020, 113, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Malan, A.; von Diest, J.; Moore, S.D.; Addison, P. Control Options for False Codling Moth, Thaumatotibia leucotreta (Lepidoptera: Tortricidae), in South Africa, With Emphasis on the Potential Use of Entomopathogenic Nematodes and Fungi. Afr. Entomol. 2018, 26, 14–29. [Google Scholar] [CrossRef]
- Rodriguez, V.A.; Belaich, M.N.; Ghiringhelli, P.D.; Rodriguez, V.A.; Belaich, M.N.; Ghiringhelli, P.D. Baculoviruses: Members of Integrated Pest Management Strategies; IntechOpen: La Plata, Argentinia, 2012; ISBN 978-953-51-0050-8. [Google Scholar]
- Hatting, J.L.; Moore, S.D.; Malan, A.P. Microbial Control of Phytophagous Invertebrate Pests in South Africa: Current Status and Future Prospects. J. Invertebr. Pathol. 2019, 165, 54–66. [Google Scholar] [CrossRef]
- Moore, S.; Jukes, M. Advances in Microbial Control in IPM: Entomopathogenic Viruses. In Integrated Management of Insect Pests; Kogan, M., Heinrichs, E., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 593–648. ISBN 978-1-78676-260-3. [Google Scholar]
- Moore, S.D.; Kirkman, W.; Richards, G.I.; Stephen, P.R. The Cryptophlebia Leucotreta Granulovirus—10 Years of Commercial Field Use. Viruses 2015, 7, 1284–1312. [Google Scholar] [CrossRef]
- Lacey, L.A.; Frutos, R.; Kaya, H.K.; Vail, P. Insect Pathogens as Biological Control Agents: Do They Have a Future? Biol. Control 2001, 21, 230–248. [Google Scholar] [CrossRef]
- Wilson, K.; Grzywacz, D.; Curcic, I.; Scoates, F.; Harper, K.; Rice, A.; Paul, N.; Dillon, A. A Novel Formulation Technology for Baculoviruses Protects Biopesticide from Degradation by Ultraviolet Radiation. Sci. Rep. 2020, 10, 13301. [Google Scholar] [CrossRef]
- Archana, H.R.; Darshan, K.; Amrutha Lakshmi, M.; Ghoshal, T.; Bashayal, B.M.; Aggarwal, R. 22—Biopesticides: A Key Player in Agro-Environmental Sustainability. In Trends of Applied Microbiology for Sustainable Economy; Soni, R., Suyal, D.C., Yadav, A.N., Goel, R., Eds.; Developments in Applied Microbiology and Biotechnology; Academic Press: Cambridge, MA, USA, 2022; pp. 613–653. ISBN 978-0-323-91595-3. [Google Scholar]
- Ballard, J.; Ellis, D.J.; Payne, C.C. The Role of Formulation Additives in Increasing the Potency of Cydia Pomonella Granulovirus for Codling Moth Larvae, in Laboratory and Field Experiments. Biocontrol Sci. Technol. 2000, 10, 627–640. [Google Scholar] [CrossRef]
- Knight, A.L.; Basoalto, E.; Witzgall, P. Improving the Performance of the Granulosis Virus of Codling Moth (Lepidoptera: Tortricidae) by Adding the Yeast Saccharomyces cerevisiae with Sugar. Environ. Entomol. 2015, 44, 252–259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Witzgall, P.; Proffit, M.; Rozpedowska, E.; Becher, P.G.; Andreadis, S.; Coracini, M.; Lindblom, T.U.T.; Ream, L.J.; Hagman, A.; Bengtsson, M.; et al. “This Is Not an Apple”–Yeast Mutualism in Codling Moth. J. Chem. Ecol. 2012, 38, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Arthurs, S.P.; Hilton, R.; Knight, A.L.; Lacey, L.A. Evaluation of the Pear Ester Kairomone as a Formulation Additive for the Granulovirus of Codling Moth (Lepidoptera: Tortricidae) in Pome Fruit. J. Econ. Entomol. 2007, 100, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Ballard, J.; Ellis, D.; Payne, C. Uptake of Granulovirus from the Surface of Apples and Leaves by First Instar Larvae of the Codling Moth Cydia pomonella L. (Lepidoptera: Olethreutidae). Biocontrol Sci. Technol. 2000, 10, 617–625. [Google Scholar] [CrossRef]
- Light, D.M.; Beck, J.J. Characterization of Microencapsulated Pear Ester, (2E,4Z)-Ethyl-2,4-Decadienoate, a Kairomonal Spray Adjuvant against Neonate Codling Moth Larvae. J. Agric. Food Chem. 2010, 58, 7838–7845. [Google Scholar] [CrossRef]
- Light, D.M.; Beck, J.J. Behavior of Codling Moth (Lepidoptera: Tortricidae) Neonate Larvae on Surfaces Treated with Microencapsulated Pear Ester. Environ. Entomol. 2012, 41, 603–611. [Google Scholar] [CrossRef]
- Stefanini, I. Yeast-Insect Associations: It Takes Guts. Yeast 2018, 35, 315–330. [Google Scholar] [CrossRef]
- Douglas, A.E. Microbial Brokers of Insect-Plant Interactions Revisited. J. Chem. Ecol. 2013, 39, 952–961. [Google Scholar] [CrossRef]
- Giron, D.; Dubreuil, G.; Bennett, A.; Dedeine, F.; Dicke, M.; Dyer, L.A.; Erb, M.; Harris, M.O.; Huguet, E.; Kaloshian, I.; et al. Promises and Challenges in Insect–Plant Interactions. Entomol. Exp. Appl. 2018, 166, 319–343. [Google Scholar] [CrossRef]
- Knight, A.L.; Witzgall, P. Combining Mutualistic Yeast and Pathogenic Virus—A Novel Method for Codling Moth Control. J. Chem. Ecol. 2013, 39, 1019–1026. [Google Scholar] [CrossRef]
- van der Merwe, M.; Jukes, M.D.; Knox, C.; Moore, S.D.; Hill, M.P. Mutualism between Gut-Borne Yeasts and Their Host, Thaumatotibia leucotreta, and Potential Usefulness in Pest Management. Insects 2022, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.D.; Hendry, D.A.; Richards, G.I. Virulence of a South African Isolate of the Cryptophlebia Leucotreta Granulovirus to Thaumatotibia leucotreta Neonate Larvae. BioControl 2011, 56, 341–352. [Google Scholar] [CrossRef]
- Moore, S.D.; Richards, G.I.; Chambers, C.; Hendry, D. An Improved Larval Diet for Commercial Mass Rearing of the False Codling Moth, Thaumatotibia leucotreta (Meyrick) (Lepidoptera: Tortricidae). Afr. Entomol. 2014, 22, 216–219. [Google Scholar] [CrossRef]
- Lenth, R.V. Estimated Marginal Means, Aka Least-Squares Means [R Package Emmeans Version 1.6. 0]. Comprehensive R Archive Network (CRAN). 2021. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 8 October 2023).
- Brooks, M.; Kristensen, K.; van Benthem, K.; Magnusson, A.; Berg, C.; Nielsen, A.; Skaug, H.; Mächler, M.; Bolker, B. GlmmTMB Balances Speed and Flexibility among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Becher, P.G.; Flick, G.; Rozpędowska, E.; Schmidt, A.; Hagman, A.; Lebreton, S.; Larsson, M.C.; Hansson, B.S.; Piškur, J.; Witzgall, P.; et al. Yeast, Not Fruit Volatiles Mediate Drosophila melanogaster Attraction, Oviposition and Development. Funct. Ecol. 2012, 26, 822–828. [Google Scholar] [CrossRef]
- Bellutti, N.; Gallmetzer, A.; Innerebner, G.; Schmidt, S.; Zelger, R.; Koschier, E.H. Dietary Yeast Affects Preference and Performance in Drosophila suzukii. J. Pest Sci. 2018, 91, 651–660. [Google Scholar] [CrossRef]
- Ljunggren, J.; Borrero-Echeverry, F.; Chakraborty, A.; Lindblom, T.U.T.; Hedenström, E.; Karlsson, M.; Witzgall, P.; Bengtsson, M. Yeast Volatomes Differentially Affect Larval Feeding in an Insect Herbivore. Appl. Environ. Microbiol. 2019, 85, e01761-19. [Google Scholar] [CrossRef]
- Lewis, M.T.; Hamby, K.A. Differential Impacts of Yeasts on Feeding Behavior and Development in Larval Drosophila suzukii (Diptera:Drosophilidae). Sci. Rep. 2019, 9, 13370. [Google Scholar] [CrossRef]
- Malassigné, S.; Minard, G.; Vallon, L.; Martin, E.; Valiente Moro, C.; Luis, P. Diversity and Functions of Yeast Communities Associated with Insects. Microorganisms 2021, 9, 1552. [Google Scholar] [CrossRef]
- Davis, T.S.; Crippen, T.L.; Hofstetter, R.W.; Tomberlin, J.K. Microbial Volatile Emissions as Insect Semiochemicals. J. Chem. Ecol. 2013, 39, 840–859. [Google Scholar] [CrossRef]
- Spitaler, U.; Bianchi, F.; Eisenstecken, D.; Castellan, I.; Angeli, S.; Dordevic, N.; Robatscher, P.; Vogel, R.F.; Koschier, E.H.; Schmidt, S. Yeast Species Affects Feeding and Fitness of Drosophila suzukii Adults. J. Pest Sci. 2020, 93, 1295–1309. [Google Scholar] [CrossRef]
- van der Merwe, M. Yeast-Baculovirus Synergism: Investigating Mixed Infections for Improved Management of the False Codling Moth, Thaumatotibia leucotreta. Ph.D. Thesis, Rhodes University, Grahamstown, South Africa, 2018. Available online: http://hdl.handle.net/10962/62963 (accessed on 22 August 2023).
- Gonzalez, F.; Tkaczuk, C.; Dinu, M.M.; Fiedler, Ż.; Vidal, S.; Zchori-Fein, E.; Messelink, G.J. New Opportunities for the Integration of Microorganisms into Biological Pest Control Systems in Greenhouse Crops. J. Pest Sci. 2016, 89, 295–311. [Google Scholar] [CrossRef]
- Madden, A.A.; Epps, M.J.; Fukami, T.; Irwin, R.E.; Sheppard, J.; Sorger, D.M.; Dunn, R.R. The Ecology of Insect-Yeast Relationships and Its Relevance to Human Industry. Proc. Biol. Sci. 2018, 285, 20172733. [Google Scholar] [CrossRef]
- Günther, C.S.; Goddard, M.R. Do Yeasts and Drosophila Interact Just by Chance? Fungal Ecol. 2019, 38, 37–43. [Google Scholar] [CrossRef]
- Knight, A.L.; Basoalto, E.; Yee, W.; Hilton, R.; Kurtzman, C.P. Adding Yeasts with Sugar to Increase the Number of Effective Insecticide Classes to Manage Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in Cherry. Pest Manag. Sci. 2016, 72, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Meriggi, N.; Di Paola, M.; Cavalieri, D.; Stefanini, I. Saccharomyces cerevisiae—Insects Association: Impacts, Biogeography, and Extent. Front. Microbiol. 2020, 11, 1629. [Google Scholar] [CrossRef] [PubMed]
- Sieverding, E.; Dirkse, F.; Moneta, P.; Fleute-Schlachter, I. Optimization of agricultural sprays with the adjuvant break-thru S240® with reference to grape diseases. In Giornate Fitopatologiche 2008, Cervia (RA), 12–14 March 2008, Volume 2; Università di Bologna: Bologna, Italy, 2008; pp. 399–406. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).