Seroepidemiological Survey of Chronic Chagas Disease in a Rural Community in Southern Bahia, Brazil, Using Recombinant Chimeric Antigens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Design and Population
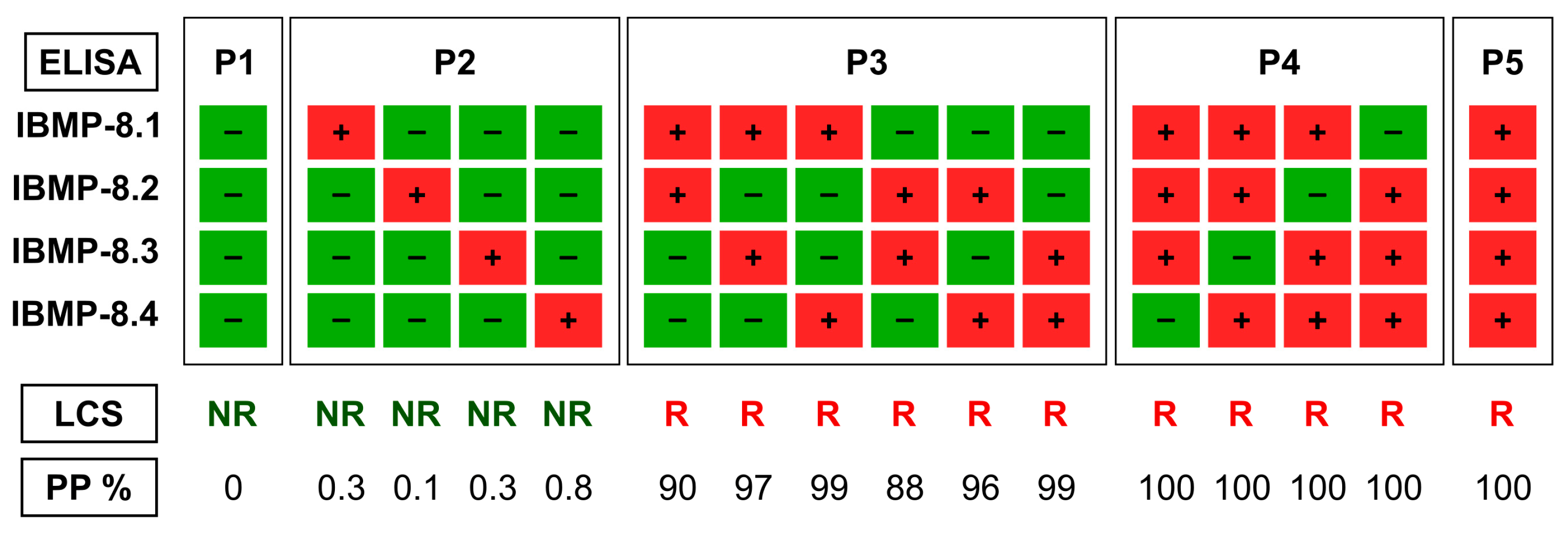
2.3. Serology Testing
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chagas, C. Nova Tripanomíase Humana. Estudos Sobre Morfologia e o Ciclo Evolutivo Do Schizotrypanum Cruzi, n. Gen., n. Sp., Agente Etiológico Da Nova Entidade Mórbida Do Homem. Mem. Inst. Oswaldo Cruz 1909, 1, 159–218. [Google Scholar] [CrossRef]
- Freitas, N.E.M.; Habib, F.L.; Santos, E.F.; Silva, Â.A.O.; Fontes, N.D.; Leony, L.M.; Sampaio, D.D.; Almeida, M.C.; Dantas-Torres, F.; Santos, F.L.N. Technological Advances in the Serological Diagnosis of Chagas Disease in Dogs and Cats: A Systematic Review. Parasites Vectors 2022, 15, 343. [Google Scholar] [CrossRef]
- Kribs-Zaleta, C.M. Alternative Transmission Modes for Trypanosoma Cruzi. Math. Biosci. Eng. 2010, 7, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.F.; Silva, Â.A.O.; Leony, L.M.; Freitas, N.E.M.; Daltro, R.T.; Regis-Silva, C.G.; Del-Rei, R.P.; Souza, W.V.; Ostermayer, A.L.; Costa, V.M.; et al. Acute Chagas Disease in Brazil from 2001 to 2018: A Nationwide Spatiotemporal Analysis. PLoS Negl. Trop. Dis. 2020, 14, e0008445. [Google Scholar] [CrossRef]
- Rassi, A.J.; Rassi, A.J.; Marcondes de Rezende, J. American Trypanosomiasis (Chagas Disease). Infect. Dis. Clin. N. Am. 2012, 26, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Useche, Y.; Pérez, A.R.; de Meis, J.; Bonomo, A.; Savino, W. Central Nervous System Commitment in Chagas Disease. Front. Immunol. 2022, 13, 975106. [Google Scholar] [CrossRef] [PubMed]
- Hasslocher-Moreno, A.M.; Xavier, S.S.; Saraiva, R.M.; Sousa, A.S. Indeterminate form of Chagas Disease: Historical, Conceptual, Clinical, and Prognostic Aspects. Rev. Soc. Bras. Med. Trop. 2021, 54, e02542021. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.P.; Beaton, A.; Acquatella, H.; Bern, C.; Bolger, A.F.; Echeverría, L.E.; Dutra, W.O.; Gascon, J.; Morillo, C.A.; Oliveira-Filho, J.; et al. Chagas Cardiomyopathy: An Update of Current Clinical Knowledge and Management: A Scientific Statement from the American Heart Association. Circulation 2018, 138, e169–e209. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization Chagas Disease in Latin America: An Epidemiological Update Based on 2010 Estimates. Wkly. Epidemiol. Rec. 2015, 90, 33–43. [Google Scholar]
- World Health Organization. Chagas Disease (American Trypanosomiasis)—Fact Sheet (Revised in August 2012). Wkly. Epidemiol. Rec. 2012, 87, 519–522. [Google Scholar]
- Coura, J.R.; Viñas, P.A. Chagas Disease: A New Worldwide Challenge. Nature 2010, 465, S6–S7. [Google Scholar] [CrossRef] [PubMed]
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas Disease: From Discovery to a Worldwide Health Problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef]
- IBGE Camamu (BA). Available online: https://www.ibge.gov.br/cidades-e-estados/ba/camamu.html? (accessed on 4 July 2023).
- Whether Spark Clima e Condições Meteorológicas Médias Em Camamu No Ano Todo. Available online: https://pt.weatherspark.com/y/30990/Clima-característico-em-Camamu-Brasil-durante-o-ano (accessed on 4 July 2023).
- Santos, F.L.N.; Celedon, P.A.F.; Zanchin, N.I.T.; Brasil, T.A.C.; Foti, L.; Souza, W.V.; Silva, E.D.; Gomes, Y.M.; Krieger, M.A. Performance Assessment of Four Chimeric Trypanosoma Cruzi Antigens Based on Antigen-Antibody Detection for Diagnosis of Chronic Chagas Disease. PLoS ONE 2016, 11, e0161100. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.L.N.; Celedon, P.A.; Zanchin, N.I.; Souza, W.V.; Silva, E.D.; Foti, L.; Krieger, M.A.; Gomes, Y.M. Accuracy of Chimeric Proteins in the Serological Diagnosis of Chronic Chagas Disease—A Phase II Study. PLoS Negl. Trop. Dis. 2017, 11, e0005433. [Google Scholar] [CrossRef] [PubMed]
- Del-Rei, R.P.; Leony, L.M.; Celedon, P.A.F.; Zanchin, N.I.T.; Reis, M.G.; Gomes, Y.M.; Schijman, A.G.; Longhi, S.A.; Santos, F.L.N. Detection of Anti-Trypanosoma Cruzi Antibodies by Chimeric Antigens in Chronic Chagas Disease-Individuals from Endemic South American Countries. PLoS ONE 2019, 14, e0215623. [Google Scholar] [CrossRef]
- Dopico, E.; Del-Rei, R.P.; Espinoza, B.; Ubillos, I.; Zanchin, N.I.T.; Sulleiro, E.; Moure, Z.; Celedon, P.A.F.; Souza, W.V.; Silva, E.D.; et al. Immune Reactivity to Trypanosoma Cruzi Chimeric Proteins for Chagas Disease Diagnosis in Immigrants Living in a Non-Endemic Setting. BMC Infect. Dis. 2019, 19, 251. [Google Scholar] [CrossRef]
- Santos, E.F.; Silva, Â.A.O.; Freitas, N.E.M.; Almeida, M.C.C.; Araújo, F.L.V.; Celedon, P.A.F.; Krieger, M.A.; Zanchin, N.T.I.; Reis, M.G.; Santos, F.L.N. Performance of Chimeric Trypanosoma Cruzi Antigens in Serological Screening for Chagas Disease in Blood Banks. Front. Med. 2022, 9, 852864. [Google Scholar] [CrossRef]
- World Health Organization. Second WHO Consultation on the Development of a WHO Reference Panel for the Control of Chagas Diagnostic Tests; World Health Organization: Geneva, Switzerland, 2007.
- Brazil. Protocolo Clínico e Diretrizes Terapêuticas Doença de Chagas; Ministry of Health of Brazil: Brasília, Brazil, 2018. [Google Scholar]
- Santos, F.L.N.; Campos, A.C.P.; Amorim, L.D.A.F.; Silva, E.D.; Zanchin, N.I.T.; Celedon, P.A.F.; Del-Rei, R.P.; Krieger, M.A.; Gomes, Y.M. Highly Accurate Chimeric Proteins for the Serological Diagnosis of Chronic Chagas Disease: A Latent Class Analysis. Am. J. Trop. Med. Hyg. 2018, 99, 1174–1179. [Google Scholar] [CrossRef]
- Santos, E.F.; Leony, L.M.; Silva, Â.A.O.; Daltro, R.T.; Freitas, N.E.M.; Vasconcelos, L.C.M.; Araújo, F.L.V.; Celedon, P.A.F.; Krieger, M.A.; Zanchin, N.I.T.; et al. Assessment of Liaison XL Murex Chagas Diagnostic Performance in Blood Screening for Chagas Disease Using a Reference Array of Chimeric Antigens. Transfusion 2021, 61, 2701–2709. [Google Scholar] [CrossRef]
- Martins-Melo, F.R.; Ramos, A.N.; Alencar, C.H.; Heukelbach, J. Prevalence of Chagas Disease in Brazil: A Systematic Review and Meta-Analysis. Acta Trop. 2014, 130, 167–174. [Google Scholar] [CrossRef]
- Escolano, P.; Liporaci, N.; Manzan, C.; Barbosa, A.; Alves, V.; Teixeira, R.; Afonso, P.; Buffulin, L.; Bentlin, M.R.; Morais, C. Prevalence of Chagas Infection in Catolândia-Bahia. Rev. Soc. Bras. Med. Trop. 1989, 22, 159–160. [Google Scholar] [CrossRef]
- Aras, R.; Gomes, I.; Veiga, M.; Melo, A. Transmissão Vetorial Da Doença de Chagas Em Mulungu Do Morro, Nordeste Do Brasil. Rev. Soc. Bras. Med. Trop. 2003, 36, 359–363. [Google Scholar] [CrossRef]
- Niborski, L.L.; Grippo, V.; Lafón, S.O.; Levitus, G.; García-Bournissen, F.; Ramirez, J.C.; Burgos, J.M.; Bisio, M.; Juiz, N.A.; Ayala, V.; et al. Serological Based Monitoring of a Cohort of Patients with Chronic Chagas Disease Treated with Benznidazole in a Highly Endemic Area of Northern Argentina. Mem. Inst. Oswaldo Cruz 2016, 111, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, M.G.; Hernández, Y.; Bertocchi, G.; Fernández, M.; Lococo, B.; Ramírez, J.C.; Cura, C.; Albizu, C.L.; Schijman, A.; Abril, M.; et al. New Scheme of Intermittent Benznidazole Administration in Patients Chronically Infected with Trypanosoma Cruzi: A Pilot Short-Term Follow-Up Study with Adult Patients. Antimicrob. Agents Chemother. 2016, 60, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Murcia, L.; Carrilero, B.; Saura, D.; Iborra, M.A.; Segovia, M. Diagnosis and Treatment of Chagas Disease. Enferm. Infecc. Microbiol. Clin. 2013, 31 (Suppl. S1), 26–34. [Google Scholar] [CrossRef] [PubMed]
- Torrico, F.; Gascón, J.; Barreira, F.; Blum, B.; Almeida, I.C.; Alonso-Vega, C.; Barboza, T.; Bilbe, G.; Correia, E.; Garcia, W.; et al. New Regimens of Benznidazole Monotherapy and in Combination with Fosravuconazole for Treatment of Chagas Disease (BENDITA): A Phase 2, Double-Blind, Randomised Trial. Lancet Infect. Dis. 2021, 21, 1129–1140. [Google Scholar] [CrossRef]
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; Sosa-Estani, S.; Rassi, A.; Rosas, F.; Villena, E.; Quiroz, R.; Bonilla, R.; Britto, C.; et al. Randomized Trial of Benznidazole for Chronic Chagas’ Cardiomyopathy. N. Engl. J. Med. 2015, 373, 1295–1306. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, N.M.; Barreto, N.M.P.V.; Farias, M.M.B.; de Lima Oliveira, C.; Araújo, W.A.C.; de Souza, J.N.; Teixeira, M.C.A.; Gonçalves, N.L.S.; Sampaio, D.D.; Pavan, T.B.S.; et al. Seroepidemiological Survey of Chronic Chagas Disease in a Rural Community in Southern Bahia, Brazil, Using Recombinant Chimeric Antigens. Pathogens 2023, 12, 1222. https://doi.org/10.3390/pathogens12101222
Soares NM, Barreto NMPV, Farias MMB, de Lima Oliveira C, Araújo WAC, de Souza JN, Teixeira MCA, Gonçalves NLS, Sampaio DD, Pavan TBS, et al. Seroepidemiological Survey of Chronic Chagas Disease in a Rural Community in Southern Bahia, Brazil, Using Recombinant Chimeric Antigens. Pathogens. 2023; 12(10):1222. https://doi.org/10.3390/pathogens12101222
Chicago/Turabian StyleSoares, Neci Matos, Nilo Manoel Pereira Vieira Barreto, Marina Morena Brito Farias, Cíntia de Lima Oliveira, Weslei Almeida Costa Araújo, Joelma Nascimento de Souza, Márcia Cristina Aquino Teixeira, Noilson Lázaro Sousa Gonçalves, Daniel Dias Sampaio, Tycha Bianca Sabaini Pavan, and et al. 2023. "Seroepidemiological Survey of Chronic Chagas Disease in a Rural Community in Southern Bahia, Brazil, Using Recombinant Chimeric Antigens" Pathogens 12, no. 10: 1222. https://doi.org/10.3390/pathogens12101222
APA StyleSoares, N. M., Barreto, N. M. P. V., Farias, M. M. B., de Lima Oliveira, C., Araújo, W. A. C., de Souza, J. N., Teixeira, M. C. A., Gonçalves, N. L. S., Sampaio, D. D., Pavan, T. B. S., Celedon, P. A. F., Zanchin, N. I. T., & Santos, F. L. N. (2023). Seroepidemiological Survey of Chronic Chagas Disease in a Rural Community in Southern Bahia, Brazil, Using Recombinant Chimeric Antigens. Pathogens, 12(10), 1222. https://doi.org/10.3390/pathogens12101222





