A Simplified Multiplex PCR Assay for Simultaneous Detection of Six Viruses Infecting Diverse Chilli Species in India and Its Application in Field Diagnosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Virus Materials
2.2. Nucleic-acid Extraction and cDNA Synthesis
2.3. Primer Design
2.4. Singleplex PCR and Its Optimization
2.5. Development of Multiplex PCR and Its Optimization
2.6. Sensitivity Determination of Multiplex PCR
2.7. Evaluation of the Stability and Validation of the Multiplex PCR Using Field Samples
3. Results
3.1. Singleplex PCR and Its Optimization
3.2. Development of Multiplex PCR and Its Optimization
3.3. Sensitivity Determination of the Multiplex PCR
3.4. Determination of Stability of Multiplex PCR
3.5. Detection of Multiple Viruses in Chilli Collected from the Fields Using Multiplex PCR
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeWitt, D.; Bosland, P.W. Peppers of the World; Ten Speed Press: Berkeley, CA, USA, 1996; ISBN 9780898158403. [Google Scholar]
- Pickersgill, B. Genetic resources and breeding of Capsicum spp. Euphytica 1997, 96, 129–133. [Google Scholar] [CrossRef]
- FAO. Statistical Yearbook—World Food and Agriculture; FAO: Rome, Italy, 2021; ISBN 978-92-5-134332-6. [Google Scholar]
- NHB. Horticulture crops for 2019–20 (Second Advance Estimates), Area, and Production of Horticulture Crops: All India. New Delhi: Ministry of Agriculture and Farmers Welfare, Govt. of India, 3.; National Horticulture Board, 2020. Available online: https://pib.gov.in/Pressreleaseshare.aspx?PRID=1628695 (accessed on 10 November 2022).
- Sanatombi, K.; Sen-Mandi, S.; Sharma, G.J. DNA profiling of Capsicum landraces of Manipur. Sci. Hortic. 2010, 124, 405–408. [Google Scholar] [CrossRef]
- Kothari, S.L.; Joshi, A.; Kachhwaha, S.; Ochoa-Alejo, N. Chilli Peppers—A review on tissue culture and transgenesis. Biotechnol. Adv. 2010, 28, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Sanabam, R.; Chanu, N.T.; Sharma, S.K.; Roy, S.S.; Ansari, M.A.; Prakash, N. Genetic Diversity of Chilli veinal mottle virus infecting different chilli landraces in North East India indicates the possibility of transboundary movement of virus. 3 Biotech 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Tripathi, S.; Verma, R. Begomoviruses in India. In Begomoviruses: Occurrence and Management in Asia and Africa; Springer: Singapore, 2017; pp. 171–186. [Google Scholar] [CrossRef]
- Reddy, M.K.; Srivastava, A.; Kumar, S.; Kumar, R.; Chawda, N.; Ebert, A.W.; Vishwakarma, M. Chilli (Capsicum annuum L.) Breeding in India: An Overview. SABRAO J. Breed Genet 2014, 46, 160–173. [Google Scholar]
- Nigam, K.; Suhail, S.; Verma, Y.; Singh, V.; Gupta, S. Molecular characterization of begomovirus associated with leaf curl disease in chilli. World J. Pharm. Res. 2013, 4, 1579–1592. [Google Scholar]
- Thakur, H.; Jindal, S.K.; Sharma, A.; Dhaliwal, M.S. Chilli leaf curl virus disease: A serious threat for chilli cultivation. J. Plant Dis. Prot. 2018, 125, 239–249. [Google Scholar] [CrossRef]
- Zehra, S.B. Chilli leaf curl virus an emerging threat to chilli in India. Int. J. Pure Appl. Biosci. 2017, 5, 404–414. [Google Scholar] [CrossRef]
- Green, S.K.; Kim, J.S. Characteristics and Control of Viruses Infecting Peppers: A Literature Review; Asian Vegetable Research and Development Center: Taipei, Taiwan, 1991; Volume 18, ISBN 9789290580454.
- Banerjee, A.; Dutta, R.; Roy, S.; Ngachan, S.V. First report of Chilli veinal mottle virus in Naga chilli (Capsicum chinense) in Meghalaya, India. VirusDisease 2013, 25, 142–143. [Google Scholar] [CrossRef][Green Version]
- Krishnareddy, M.; Rani, R.U.; Kumar, K.S.A.; Reddy, K.M.; Pappu, H.R. Capsicum chlorosis virus (Genus Tospovirus) infecting chili pepper (Capsicum annuum) in India. Plant Dis. 2008, 92, 1469. [Google Scholar] [CrossRef]
- Haokip, B.; Alice, D.; Malathi, V.; Nagendran, K.; Renukadevi, P.; Karthikeyan, G. Detection of Capsicum chlorosis virus (CaCV), an emerging virus infecting chilli in Tamil Nadu, India. Vegetos-Int. J. Plant Res. 2016, 29, 130. [Google Scholar] [CrossRef]
- Rialch, N.; Sharma, V.; Sharma, A.; Sharma, P.N. Characterization and complete nucleotide sequencing of Pepper mild mottle virus infecting bell pepper in India. Phytoparasitica 2015, 43, 327–337. [Google Scholar] [CrossRef]
- Meetei, N.T.; Singh, A.K.; Singh, B.K.; Mandal, N. Disease incidence and molecular indexing of viruses infecting King Chilli (Capsicum Chinense Jacq) in North East India. Indian Phytopathol. 2019, 73, 117–124. [Google Scholar] [CrossRef]
- Vinodhini, J.; Rajendran, L.; Abirami, R.; Karthikeyan, G. Co-existence of chlorosis inducing strain of Cucumber mosaic virus with tospoviruses on hot pepper (Capsicum annuum) in India. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef]
- Senanayake, D.M.J.B.; Mandal, B.; Lodha, S.; Varma, A. First report of Chilli leaf curl virus affecting chilli in India. Plant Pathol. 2007, 56, 343. [Google Scholar] [CrossRef]
- Sharma, S.K.; Chanu, N.T.; Anand, Y.R.; Singh, Y.H.; Singh, S.; Raj, C.; Baranwal, V.K.; Rai, R.; Sanabam, R.; Roy, S.S.; et al. First report of large cardamom chirke virus, a macluravirus naturally infecting chili crop in India. Plant Dis. 2019, 103, 777. [Google Scholar] [CrossRef]
- Kunkalikar, S.; Poojari, S.; Rajagopalan, P.; Zehr, U.B.; Naidu, R.A.; Kankanallu, R.S. First report of Capsicum chlorosis virus in tomato in India. Plant Health Prog. 2007, 8. [Google Scholar] [CrossRef]
- German, T.L.; Ullman, D.E.; Moyer, J.W. Tospoviruses: Diagnosis, molecular biology, phylogeny, and vector relationships. Annu. Rev. Phytopathol. 1992, 30, 315–348. [Google Scholar] [CrossRef]
- Sharman, M.; Thomas, J.E.; Tree, D.; Persley, D.M. Natural host range and thrips transmission of capsicum chlorosis virus in Australia. Australas. Plant Pathol. 2019, 49, 45–51. [Google Scholar] [CrossRef]
- Colson, P.; Richet, H.; Desnues, C.; Balique, F.; Moal, V.; Grob, J.-J.; Berbis, P.; Lecoq, H.; Harlé, J.-R.; Berland, Y.; et al. Pepper mild mottle virus, a plant virus associated with specific immune responses, fever, abdominal pains, and pruritus in humans. PLoS ONE 2010, 5, e10041. [Google Scholar] [CrossRef]
- Ahmad, A.; Tiberini, A.; Ashfaq, M.; Tomassoli, L. First report of Pepper mild mottle virus infecting chilli pepper in Pakistan. New Dis. Rep. 2015, 32, 31. [Google Scholar] [CrossRef]
- Secrist, K.; Ali, A. First complete genome sequence of Pepper mild mottle virus from chili pepper in the United States. Genome Announc. 2018, 6, e00331-18. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dong, J.H.; Zhang, T.J.; Wang, R.; Luo, Z.P.; Luo, H.Y.; Zhang, Z.K. A new isolate of Chilli veinal mottle virus that infects tobacco in China. J. Plant Pathol. 2013, 95, 187–190. [Google Scholar] [CrossRef]
- Rao, S.; Chen, X.; Qiu, S.; Peng, J.; Zheng, H.; Lu, Y.; Wu, G.; Chen, J.; Jiang, W.; Zhang, Y.; et al. Identification of two new isolates of Chilli veinal mottle virus from different regions in China: Molecular diversity, phylogenetic and recombination analysis. Front. Microbiol. 2020, 11, 616171. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Xu, C.; Li, J.; Gu, Y.; Xia, C.; Xie, Q.; Xie, Y.; An, M.; Xia, Z.; Wu, Y. Characterization and a RT-RPA assay for rapid detection of Chilli veinal mottle virus (ChiVMV) in Tobacco. Virol. J. 2020, 17, 1–9. [Google Scholar] [CrossRef]
- Arogundade, O.; Ajose, T.; Osijo, I.; Onyeanusi, H.; Matthew, J.; Aliyu, T.H. Management of viruses and viral diseases of pepper (Capsicum Spp.) in Africa. Capsicum 2020, 73–86. [Google Scholar] [CrossRef]
- Moury, B.; Palloix, A.; Caranta, C.; Gognalons, P.; Souche, S.; Selassie, K.G.; Marchoux, G. Serological, molecular, and pathotype diversity of Pepper veinal mottle virus and Chili veinal mottle virus. Phytopathology 2005, 95, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.S.; Huang, Y.C.; Zhang, D.Y.; Reddy, K.; Hidayat, S.H.; Srithongchai, W.; Green, S.K.; Jan, F.J. Molecular characterization of the CP gene and 3′UTR of Chilli veinal mottle virus from South and Southeast Asia. Plant Pathol. 2008, 57, 408–416. [Google Scholar] [CrossRef]
- Palukaitis, P.; Roossinck, M.J.; Dietzgen, R.G.; Francki, R.I.B. Cucumber mosaic virus. Adv. Virus Res. 1992, 41, 281–348. [Google Scholar] [CrossRef]
- Peden, K.W.C.; Symons, R.H. Cucumber mosaic virus contains a functionally divided genome. Virology 1973, 53, 487–492. [Google Scholar] [CrossRef]
- Zitter, T.A.; Murphy, J.F. Cucumber mosaic virus. Plant Health Instr. 2009. [Google Scholar] [CrossRef]
- Kenyon, L.; Kumar, S.; Tsai, W.-S.; Hughes, J. d’A. Virus diseases of peppers (Capsicum Spp.) and their control. Control. Plant Virus Dis.-Seed-Propagated Crops 2014, 90, 297–354. [Google Scholar] [CrossRef]
- Rahman, M.S.; Akanda, A.M.; Mian, I.H.; Bhuiyan, M.K.A.; Hossain, M.M. New sources of resistance to Cucumber mosaic virus in Capsicum annuum. J. Crop Sci. Biotechnol. 2016, 19, 249–258. [Google Scholar] [CrossRef]
- Sastry, K.S. Impact of virus and viroid diseases on crop yields. In Plant Virus and Viroid Diseases in the Tropics; Springer: Dordrecht, The Netherlands, 2013; pp. 99–159. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Proudlove, W. Further studies on Cucumber mosaic virus infection of narrow-leafed lupin (Lupinus angustifolius): Seed-borne infection, aphid transmission, spread and effects on grain yield. Ann. Appl. Biol. 1991, 118, 319–329. [Google Scholar] [CrossRef]
- Sidharthan, V.K.; Sharma, S.K.; Baranwal, V.K. The first near-complete genome sequence of Large cardamom chirke virus mined from the transcriptome dataset of large cardamom. Plant Gene 2021, 28, 100324. [Google Scholar] [CrossRef]
- Mandal, B.; Vijayanandraj, S.; Shilpi, S.; Pun, K.B.; Singh, V.; Pant, R.P.; Jain, R.K.; Varadarasan, S.; Varma, A. disease distribution and characterisation of a new macluravirus associated with chirke disease of large cardamom. Ann. Appl. Biol. 2012, 160, 225–236. [Google Scholar] [CrossRef]
- Vijayanandraj, S.; Yogita, M.; Das, A.; Ghosh, A.; Mandal, B. highly efficient immunodiagnosis of Large cardamom chirke virus using the polyclonal antiserum against Escherichia coli expressed recombinant coat protein. Indian J. Virol 2013, 24, 227–234. [Google Scholar] [CrossRef][Green Version]
- Chattopadhyay, B.; Singh, A.K.; Yadav, T.; Fauquet, C.M.; Sarin, N.B.; Chakraborty, S. Infectivity of the cloned components of a begomovirus: DNA beta complex causing chilli leaf curl disease in India. Arch. Virol. 2008, 153, 533–539. [Google Scholar] [CrossRef]
- Varma, A.; Mandal, B.; Singh, M.K. Global emergence and spread of whitefly (Bemisia tabaci) transmitted geminiviruses. In The Whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) Interaction with Geminivirus-Infected Host Plants; Thompson, W., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 205–292. [Google Scholar] [CrossRef]
- Bhatt, B.S.; Chahwala, F.D.; Rathod, S.; Singh, A.K. Identification and molecular characterization of a new recombinant begomovirus and associated betasatellite DNA infecting Capsicum annuum in India. Arch. Virol. 2016, 161, 1389–1394. [Google Scholar] [CrossRef]
- Khan, Z.A.; Khan, J.A. Characterization of a new begomovirus and betasatellite associated with chilli leaf curl disease in India. Arch. Virol. 2016, 162, 561–565. [Google Scholar] [CrossRef]
- Ertunc, F. Emerging Plant Viruses. Emerg. Reemerging Viral Pathog. 2020, 1041–1062. [Google Scholar] [CrossRef]
- Moreno, A.B.; López-Moya, J.J. When viruses play team sports: Mixed infections in plants. Phytopathology 2019, 110, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kushwaha, N.; Chakraborty, S. synergistic interaction among begomoviruses leads to the suppression of host defense-related gene expression and breakdown of resistance in chilli. Appl. Microbiol. Biotechnol. 2016, 100, 4035–4049. [Google Scholar] [CrossRef]
- Chakraborty, S.; Vanitharani, R.; Chattopadhyay, B.; Fauquet, C.M. Supervirulent pseudorecombination and asymmetric synergism between genomic components of two distinct species of begomovirus associated with severe tomato leaf curl disease in India. J. Gen. Virol. 2008, 89, 818–828. [Google Scholar] [CrossRef]
- Padidam, M.; Sawyer, S.; Fauquet, C.M. Possible emergence of new geminiviruses by frequent recombination. Virology 1999, 265, 218–225. [Google Scholar] [CrossRef]
- Davino, S.; Miozzi, L.; Panno, S.; Rubio, L.; Davino, M.; Accotto, G.P. Recombination profiles between Tomato yellow leaf curl virus and Tomato yellow leaf curl sardinia virus in laboratory and field conditions: Evolutionary and taxonomic implications. J. Gen. Virol. 2012, 93, 2712–2717. [Google Scholar] [CrossRef]
- Murphy, J.F.; Bowen, K.L. Synergistic disease in pepper caused by the mixed infection of Cucumber mosaic virus and Pepper mottle virus. Phytopathology 2006, 96, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Galipienso, L.; Ferriol, I. Detection of plant viruses and disease management: Relevance of genetic diversity and evolution. Front. Plant Sci. 2020, 11, 1092. [Google Scholar] [CrossRef]
- Pallás, V.; Sánchez-Navarro, J.A.; James, D. Recent advances on the multiplex molecular detection of plant viruses and viroids. Front. Microbiol. 2018, 9, 2087. [Google Scholar] [CrossRef]
- Dincer, C.; Bruch, R.; Kling, A.; Dittrich, P.S.; Urban, G.A. Multiplexed point-of-care testing - XPOCT. Trends Biotechnol. 2017, 35, 728–742. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Song, C.; Zhao, M.; Li, Y. Several concerns about the primer design in the universal molecular beacon real-time PCR assay and its application in HBV DNA Detection. Anal. Bioanal. Chem. 2007, 388, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, K.; Wu, W.; Mi, W.; Hao, X.; Wu, Y. A multiplex reverse transcription PCR assay for simultaneous detection of six main RNA viruses in tomato plants. J. Virol. Methods 2019, 265, 53–58. [Google Scholar] [CrossRef]
- Nemes, K.; Salánki, K. A multiplex RT-PCR assay for the simultaneous detection of prevalent viruses infecting pepper (Capsicum annuum L.). J. Virol. Methods 2020, 278, 113838. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Peng, H.; Chen, W.; Cheng, J.; Wu, Y. Development of multiplex real-time PCR for simultaneous detection of three Potyviruses in tobacco plants. J. Appl. Microbiol. 2012, 114, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Elnifro, E.M.; Ashshi, A.M.; Cooper, R.J.; Klapper, P.E. Multiplex PCR: Optimization and application in diagnostic virology. Clin. Microbiol. Rev. 2000, 13, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Michailides, T.J. Approaches for eliminating PCR inhibitors and designing PCR primers for the detection of phytopathogenic fungi. Crop Prot. 2007, 26, 145–161. [Google Scholar] [CrossRef]
- Chou, Q.; Russell, M.; Birch, D.E.; Raymond, J.; Bloch, W. Prevention of pre-PCR mis-priming and primer dimerization improves low-copy-number amplifications. Nucleic Acids Res. Spec. Publ. 1992, 20, 1717–1723. [Google Scholar] [CrossRef]
- Brownie, J. The elimination of primer-dimer accumulation in PCR. Nucleic Acids Res. Spec. Publ. 1997, 25, 3235–3241. [Google Scholar] [CrossRef]
- Wei, T.; Pearson, M.N.; Blohm, D.; Nölte, M.; Armstrong, K. Development of a short oligonucleotide microarray for the detection and identification of multiple Potyviruses. J. Virol. Methods 2009, 162, 109–118. [Google Scholar] [CrossRef]
- Henegariu, O.; Heerema, N.A.; Dlouhy, S.R.; Vance, G.H.; Vogt, P.H. Multiplex PCR: Critical parameters and step-by-step protocol. Biotechniques 1997, 23, 504–511. [Google Scholar] [CrossRef]
- Thompson, J.R.; Wetzel, S.; Klerks, M.M.; Vašková, D.; Schoen, C.D.; Špak, J.; Jelkmann, W. Multiplex RT-PCR detection of four aphid-borne strawberry viruses in Fragaria Spp. in combination with a plant mRNA specific internal control. J. Virol. Methods 2003, 111, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Navarro, J.A.; Aparicio, F.; Herranz, M.C.; Minafra, A.; Myrta, A.; Pallás, V. Simultaneous detection and identification of eight stone fruit viruses by one-step RT-PCR. Eur. J. Plant Pathol. 2005, 111, 77–84. [Google Scholar] [CrossRef]

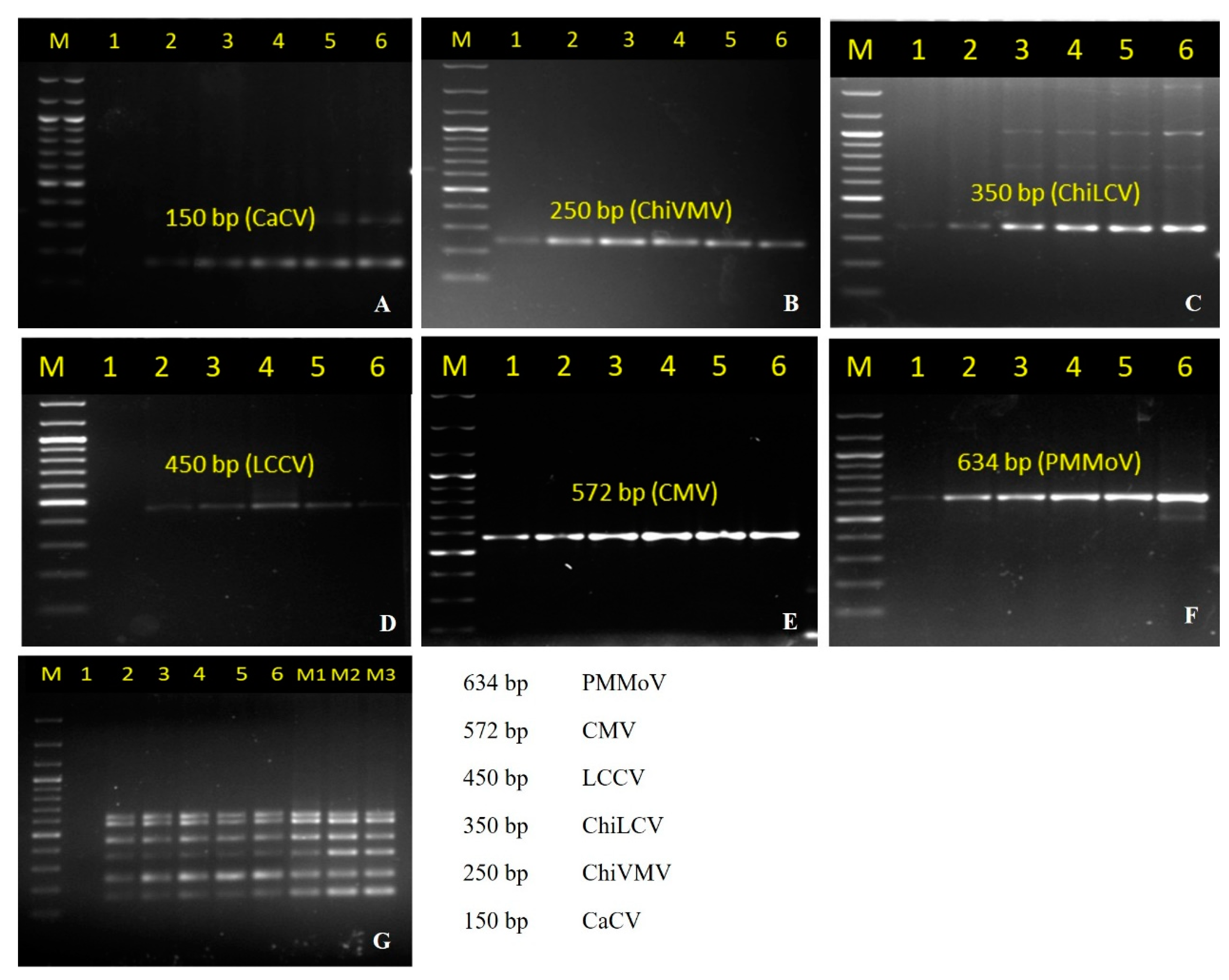
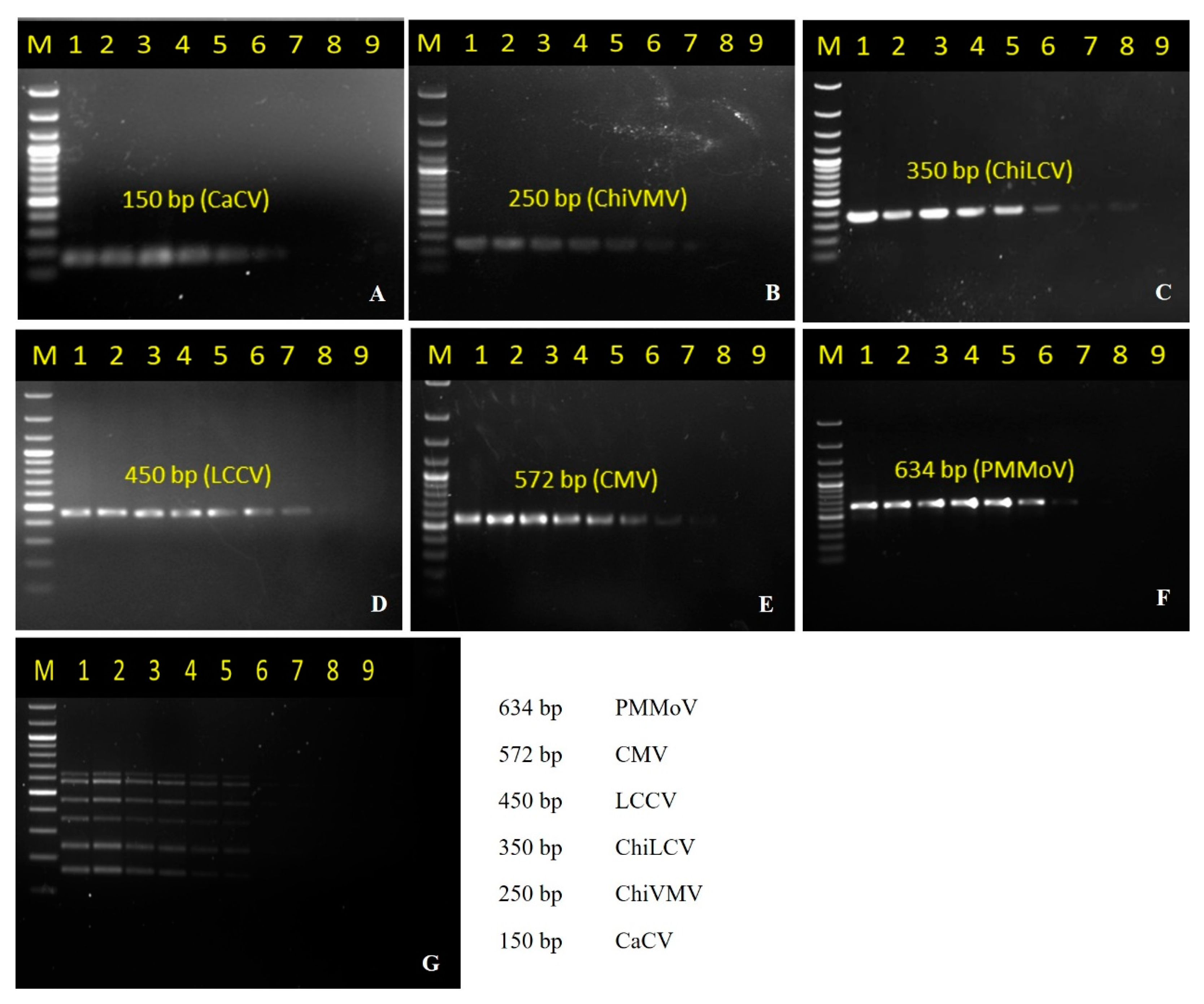
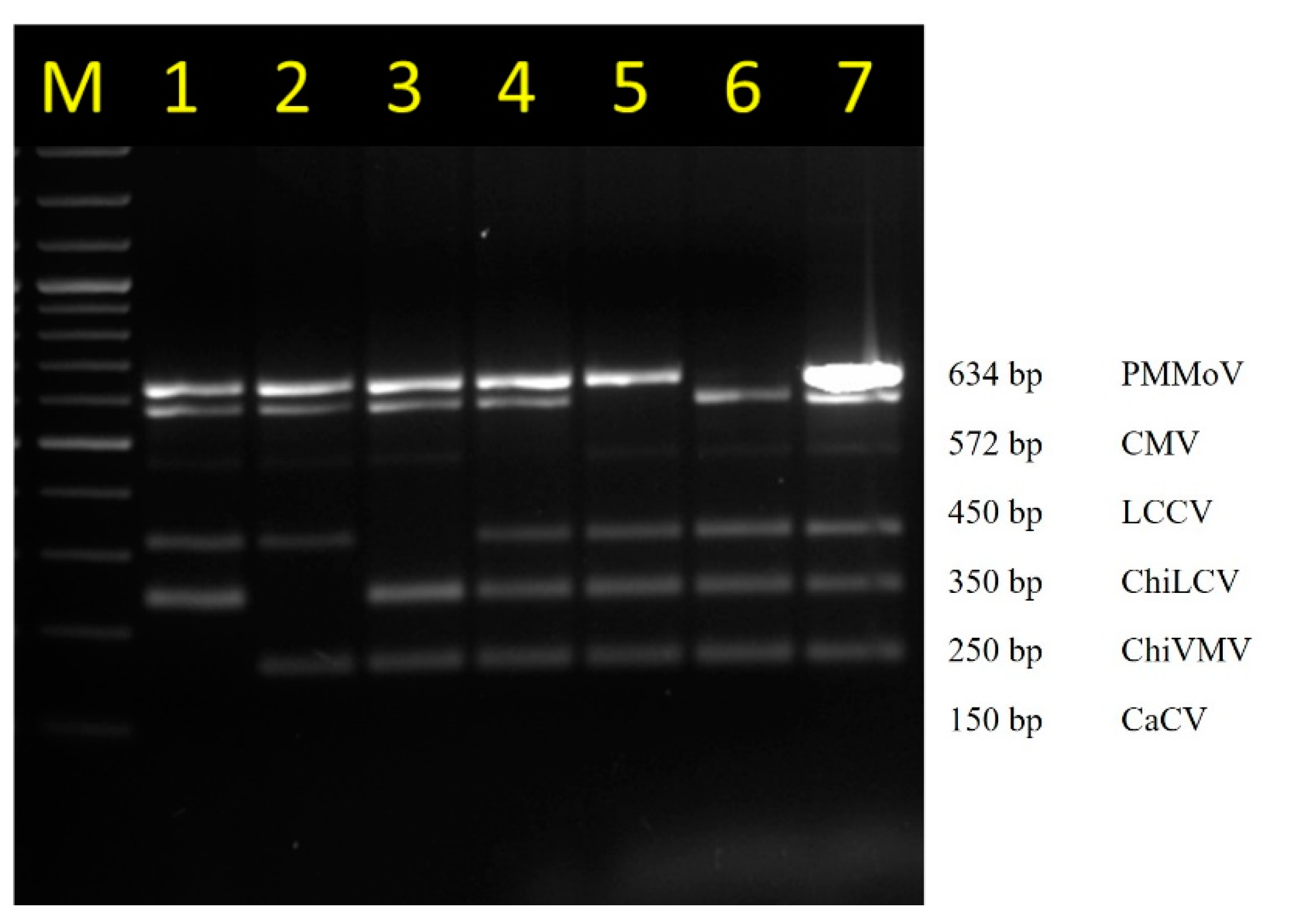

| Primer | Sequence (5′–3′) | Target Virus | Length (bp) | Tm (°C) | Amplicon Size (bp) | Primer Binding Site |
|---|---|---|---|---|---|---|
| CaCVmCF | GGGAAAATAATTGGTGCAAGG | CaCV | 21 | 57.5 | 150 | 317–337 |
| CaCVmCR | AGATGTATGCAAAGGTAATGGAATT | 25 | 59.2 | 473–449 | ||
| ChiVMVmF | AGCGCAACTCTGAGAAGCC | ChiVMV | 19 | 59.5 | 250 | 9141–9159 |
| ChiVMVmR | TTCTATTCACATCCTCTGCTG | 21 | 57.5 | 9392–9372 | ||
| LCVmF | CCCATAGAGTAGGTAAGCG | ChiLCV | 19 | 57.5 | 350 | 610–628 |
| LCVmR | CATATTTACCAGCTTCCTGC | 20 | 56.4 | 964–945 | ||
| Chirke-mF | GTGGAGAATACCTCAAATACC | LCCV | 21 | 57.5 | 450 | 117–137 |
| Chirke-mR | CTGAGTATACCCTCTTTTTGTG | 22 | 58.4 | 573–552 | ||
| CMVmF | ATGGACAAATCTGAATCAACC | CMV | 21 | 55.4 | 572 | 1–21 |
| CMVmR | GTCTTTTGAATACACGAGTAC | 21 | 55.4 | 573–553 | ||
| PMMoVmCF | AAAGGAAGTAATAAGTATGTAGGTAAGAG | PMMoV | 29 | 63.3 | 634 | 5551–5579 |
| PMMoVmCR | GTTCGTCCAACTTATTTATGCC | 23 | 58.4 | 6184–6163 |
| Sample No. | State | Location | Species of Chilli | Symptoms | Virus Detection | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CaCV | ChiVMV | ChiLCV | LCCV | CMV | PMMoV | |||||
| 1 | Manipur | Chandrakhong | King chilli (C. chinense) | Mos, Nec, Wst | + | − | − | + | + | − |
| 2 | Manipur | Khoijuman | King chilli (C. chinense) | Chl, Nec, Mos, Stn | + | + | + | + | + | + |
| 3 | Tripura | Khwai | Chilli (C. annuum) | Mos, Ss | − | − | − | − | + | − |
| 4 | Mizoram | Kolasib | King chilli (C. chinense) | Dis | − | − | − | − | − | − |
| 5 | Manipur | Mongsang, Tamenglong | Bird’s eye chilli (C. frutescens) | Mos, Nec, Chl | + | − | − | − | − | − |
| 6 | Manipur | Tamenglong | Chilli (C. annuum) | Mos, Nec, Chl | + | − | − | + | + | − |
| 7 | Manipur | Gelnal, Kangpokpi | King chilli (C. chinense) | Ss, Mot, Dis | − | + | − | − | − | − |
| 8 | Manipur | Gelnal, Kangpokpi | Chilli (C. annuum) | Ss, Mot, Dis | − | + | − | − | − | − |
| 9 | Manipur | Maklode, Senapati | Chilli (C. annuum) | Dis, Mot, Mos, Chl, Nec, Stn | + | − | + | + | + | + |
| 10 | Manipur | Tupul, Tamenglong | Bird’s eye chilli (C. frutescens) | Mos, Ss, Cur | + | + | − | + | + | − |
| 11 | Manipur | Mongsang, Tamenglong | Chilli (C. annuum) | Mos, Ss, Cur | + | + | − | + | + | − |
| 12 | Manipur | Sirarakhong, Ukhrul | King chilli (C. chinense) | Mos, Stn, Wst | + | + | + | + | + | + |
| 13 | Manipur | Purul, Senapati | King chilli (C. chinense) | Dis, Ss, Chl, Nec, Stn | + | + | − | + | + | − |
| 14 | Manipur | Sawombung | King chilli (C. chinense) | Dis, Mos, Nec | + | − | − | − | − | − |
| 15 | Manipur | Kachai, Ukhrul | King chilli (C. chinense) | Mos, Chl, Nec, Stn | + | − | - | − | − | − |
| 16 | Manipur | Hiyangthang | King chilli (C. chinense) | Chl, Mos, Stn, Ss | + | + | + | + | + | + |
| 17 | Sikkim | Lingi Payong | Dale chilli (C. annuum) | Mot, Nec, Wst | + | + | − | + | + | − |
| 18 | Sikkim | Rishi | King chilli, C. chinense | Dis, Stn, Wst | + | - | - | + | + | - |
| 19 | Arunachal Pradesh | Pasighat | Chilli (C. annuum) | Chl, Stn | − | − | − | + | − | − |
| 20 | Manipur | Tera, Imphal | Black chilli (C. annuum) | Wst | − | − | − | + | − | − |
| 21 | Nagaland | Wokha | King chilli (C. chinense) | Dis, Mos, Cur | − | + | + | − | − | + |
| 22 | Manipur | Nambol kakyai | King chilli (C. chinense) | Dis, Mot, Mos, Ss | − | + | − | − | − | − |
| 23 | Manipur | Tuisen, Ukhrul | King chilli (C. chinense) | Mos, Chl, Nec, Stn | + | + | − | - | + | + |
| 24 | Mizoram | Thenzawl | King chilli (C. chinense) | Mot, Mos, Ss, Cur, Chl, Nec, Stn, | + | + | + | − | + | + |
| 25 | Meghalaya | Barapani | King chilli (C. chinense) | Dis, Mot, Mos, Stn, | − | − | − | − | + | − |
| 26 | Manipur | Sekta | King chilli (C. chinense) | Dis | − | − | − | − | − | − |
| 27 | Manipur | Pungdongbam | King chilli (C. chinense) | Chl, Nec | + | − | − | − | − | − |
| 28 | Manipur | Sirarakhong | King chilli (C. chinense) | Mot, Ss, Stn | − | + | − | − | − | − |
| 29 | Manipur | Muolvaiphei | King chilli (C. chinense) | Dis, Mot, Mos, Ss, Chl, Stn, | + | − | + | − | + | + |
| 30 | Manipur | Wangoi | King chilli (C. chinense) | Mos, Cur, Chl, Stn | + | + | − | − | + | − |
| 31 | Manipur | Nambol | King chilli (C. chinense) | Mos, Ss, Cur, Chl, Nec, Stn | + | + | + | − | + | + |
| 32 | Manipur | Sinam Village | King chilli (C. chinense) | Dis, Mot, Mos, Ss, Cur, Chl, Nec | + | + | − | − | + | − |
| 33 | Manipur | Wari | King chilli (C. chinense) | Mot, Stn, Wst | + | + | + | − | + | + |
| 34 | Manipur | Paorabi | King chilli (C. chinense) | Mot, Mos | − | − | − | − | + | − |
| 35 | Manipur | Sagolmang | King chilli (C. chinense) | As | − | − | − | − | − | − |
| 36 | Manipur | Sagolmang | Bird’s eye chilli (C. fructescens) | As | − | − | − | − | − | − |
| 37 | Manipur | Buhsau | King chilli (C. chinense) | Chl, Nec | + | − | − | − | − | − |
| 38 | Manipur | Zeezaw | King chilli (C. chinense) | Mot, Ss | − | + | − | − | − | − |
| 39 | Manipur | P. Sejol | King chilli (C. chinensis) | Dis, Mos, Cur, Chl | + | − | + | + | + | + |
| 40 | Manipur | P. Sejol | Bird’s eye chilli (C. fructescens) | Dis, Mos, Cur, Chl | + | − | + | + | + | + |
| 41 | Manipur | Saiton Khunou | King chilli (C. chinense) | Mot, Mos, Cur, Chl, Ne | + | + | − | + | + | − |
| 42 | Manipur | Khurai Angom Leikai | King chilli (C. chinense) | Mot, Nec, Stn, Wst | + | + | + | + | + | + |
| 43 | Manipur | Uchiwa | King chilli (C. chinense) | Dis, Mot, Mos | + | + | − | + | + | − |
| 44 | Manipur | Mayang Imphal | Chilli (C. annuum) | Chl, Stn, Wst | + | + | + | + | + | + |
| 45 | Manipur | Heibongpokpi | King chilli (C. chinense) | Dis, Mot, Mos | − | − | − | − | + | − |
| 46 | Manipur | Heibongpokpi | Chilli (C. annuum) | Dis, Mot, Mos | − | − | − | − | + | − |
| 47 | Manipur | Haorangsabal | King chilli (C. chinense) | Cur | − | − | − | − | − | − |
| 48 | Manipur | Lamsang | King chilli (C. chinense) | Chl, Stn | + | − | − | − | − | − |
| 49 | Manipur | Lambal | King chilli (C. chinense) | Dis, Mot, Ss | + | − | − | + | + | − |
| 50 | Manipur | Lambal | Bird’s eye chilli (C. fructescens) | Dis, Mot, Ss | − | + | − | − | − | − |
| 51 | Manipur | Songpijang | King chilli (C. chinense) | Dis, Mot, Mos, Chl | + | − | + | + | + | + |
| 52 | Manipur | Gelnel | King chilli (C. chinense) | Stn, Wst, Mot | + | + | − | + | + | − |
| 53 | Manipur | Chlava | King chilli (C. chinense) | Mot, Mos, Ss, Stn | + | + | + | + | + | + |
| 54 | Manipur | Rengpang | King chilli (C. chinense) | Dis, Mot, Mos, Ss, Chl | + | + | − | + | + | − |
| 55 | Manipur | Gopibung | King chilli (C. chinense) | Mot, Mos, Chl, Stn | + | + | + | + | + | + |
| 56 | Manipur | Khongshang | King chilli (C. chinense) | Dis, Mos, Stn | − | − | - | − | + | − |
| 57 | Nagaland | New chumoukedima | King chilli (C. chinense) | Dis | − | − | − | − | − | − |
| 58 | Nagaland | Medziphema | King chilli (C. chinense) | Dis, Chl, Stn | + | − | − | − | − | − |
| 59 | Nagaland | Old Tesen | King chilli (C. chinense) | Mot, Ss, Stn, | − | + | − | − | − | − |
| 60 | Nagaland | Duekwaram | King chilli (C. chinense) | Mos, Cur | + | − | + | − | + | + |
| 61 | Nagaland | Jharnapani | King chilli (C. chinense) | Dis, Mos, Chl, Nec | + | + | − | − | + | − |
| Total positive sample | 37 | 31 | 17 | 22 | 35 | 18 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devi, O.P.; Sharma, S.K.; Sanatombi, K.; Devi, K.S.; Pathaw, N.; Roy, S.S.; Chanu, N.T.; Sanabam, R.; Devi, H.C.; Singh, A.R.; et al. A Simplified Multiplex PCR Assay for Simultaneous Detection of Six Viruses Infecting Diverse Chilli Species in India and Its Application in Field Diagnosis. Pathogens 2023, 12, 6. https://doi.org/10.3390/pathogens12010006
Devi OP, Sharma SK, Sanatombi K, Devi KS, Pathaw N, Roy SS, Chanu NT, Sanabam R, Devi HC, Singh AR, et al. A Simplified Multiplex PCR Assay for Simultaneous Detection of Six Viruses Infecting Diverse Chilli Species in India and Its Application in Field Diagnosis. Pathogens. 2023; 12(1):6. https://doi.org/10.3390/pathogens12010006
Chicago/Turabian StyleDevi, Oinam Priyoda, Susheel Kumar Sharma, Keithellakpam Sanatombi, Konjengbam Sarda Devi, Neeta Pathaw, Subhra Saikat Roy, Ngathem Taibangnganbi Chanu, Rakesh Sanabam, Huirem Chandrajini Devi, Akoijam Ratankumar Singh, and et al. 2023. "A Simplified Multiplex PCR Assay for Simultaneous Detection of Six Viruses Infecting Diverse Chilli Species in India and Its Application in Field Diagnosis" Pathogens 12, no. 1: 6. https://doi.org/10.3390/pathogens12010006
APA StyleDevi, O. P., Sharma, S. K., Sanatombi, K., Devi, K. S., Pathaw, N., Roy, S. S., Chanu, N. T., Sanabam, R., Devi, H. C., Singh, A. R., & Baranwal, V. K. (2023). A Simplified Multiplex PCR Assay for Simultaneous Detection of Six Viruses Infecting Diverse Chilli Species in India and Its Application in Field Diagnosis. Pathogens, 12(1), 6. https://doi.org/10.3390/pathogens12010006








