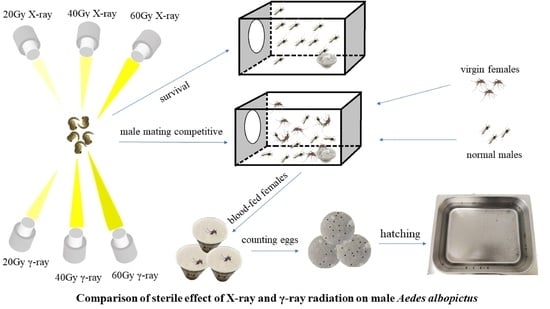
Sterility of Aedes albopictus by X-ray Irradiation as an Alternative to γ-ray Irradiation for the Sterile Insect Technique
Abstract
1. Introduction
2. Results
2.1. Effects of Male Pupae Radiation on the Emergence Rate, Egg Number, and Egg Hatch Rate
2.2. Effects of Radiation on the Survival of Male Mosquitoes
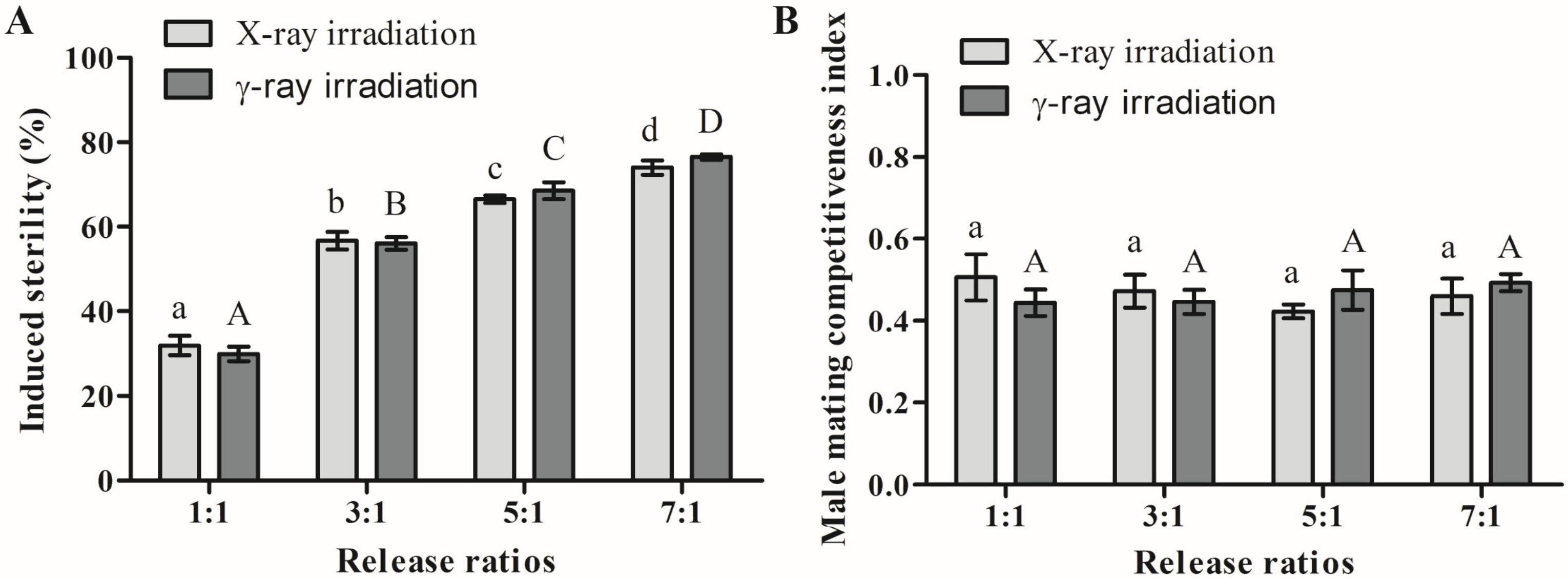
2.3. Effects of Radiation on Male Mating Competitiveness
3. Discussion
4. Materials and Methods
4.1. Mosquito
4.2. Radiation Effects on Emergence Rate, Egg Number, Hatch Rate, and Survival
4.3. Radiation Effects on Male Mating Competitiveness
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leta, S.; Beyene, T.J.; De Clercq, E.M.; Amenu, K.; Kraemer, M.U.G.; Revie, C.W. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int. J. Infect. Dis. 2018, 67, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Wilke, A.B.; Beier, J.C. Aedes albopictus (Asian Tiger Mosquito). Trends Parasitol. 2020, 36, 942–943. [Google Scholar] [CrossRef] [PubMed]
- Ricardo Machi, A.; Rodrigues Mayne, R.; Adriani Gava, M.; Bergamin Arthur, P.; Arthur, V. Gamma Radiation Sterilization Dose of Adult Males in Asian Tiger Mosquito Pupae. Insects 2019, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Reiner, R.C., Jr.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.-Y.; He, M.-Z.; Zhou, L.-Y.; Wu, X.-Y.; Wang, L.-M.; Li, N.; Deng, S.-Q. Mosquito Repellents: Efficacy Tests of Commercial Skin-Applied Products in China. Molecules 2022, 27, 5534. [Google Scholar] [CrossRef]
- Namias, A.; Jobe, N.B.; Paaijmans, K.P.; Huijben, S. The need for practical insecticide-resistance guidelines to effectively inform mosquito-borne disease control programs. Elife 2021, 10, e65655. [Google Scholar] [CrossRef]
- Deng, S.-Q.; Yang, X.; Wei, Y.; Chen, J.-T.; Wang, X.-J.; Peng, H.-J. A Review on Dengue Vaccine Development. Vaccines 2020, 8, 63. [Google Scholar] [CrossRef]
- Moyes, C.L.; Vontas, J.; Martins, A.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.-P.; et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef]
- Deng, S.-Q.; Zou, W.-H.; Li, D.-L.; Chen, J.-T.; Huang, Q.; Zhou, L.-J.; Tian, X.-X.; Chen, Y.-J.; Peng, H.-J. Expression of Bacillus thuringiensis toxin Cyt2Ba in the entomopathogenic fungus Beauveria bassiana increases its virulence towards Aedes mosquitoes. PLoS Negl. Trop. Dis. 2019, 13, e0007590. [Google Scholar] [CrossRef]
- Oliva, C.F.; Benedict, M.Q.; Collins, C.M.; Baldet, T.; Bellini, R.; Bossin, H.; Bouyer, J.; Corbel, V.; Facchinelli, L.; Fouque, F.; et al. Sterile Insect Technique (SIT) against Aedes Species Mosquitoes: A Roadmap and Good Practice Framework for Designing, Implementing and Evaluating Pilot Field Trials. Insects 2021, 12, 191. [Google Scholar] [CrossRef]
- Pérez-Staples, D.; Díaz-Fleischer, F.; Montoya, P. The Sterile Insect Technique: Success and Perspectives in the Neotropics. Neotrop. Èntomol. 2021, 50, 172–185. [Google Scholar] [CrossRef]
- Gato, R.; Menéndez, Z.; Prieto, E.; Argilés, R.; Rodríguez, M.; Baldoquín, W.; Hernández, Y.; Pérez, D.; Anaya, J.; Fuentes, I.; et al. Sterile Insect Technique: Successful Suppression of an Aedes aegypti Field Population in Cuba. Insects 2021, 12, 469. [Google Scholar] [CrossRef]
- Bouyer, J.; Yamada, H.; Pereira, R.; Bourtzis, K.; Vreysen, M.J. B Phased Conditional Approach for Mosquito Management Using Sterile Insect Technique. Trends Parasitol. 2020, 36, 325–336. [Google Scholar] [CrossRef]
- Dame, D.A.; Curtis, C.F.; Benedict, M.Q.; Robinson, A.S.; Knols, B.G. Historical applications of induced sterilisation in field populations of mosquitoes. Malar. J. 2009, 8, S2. [Google Scholar] [CrossRef]
- Bellini, R. Safety, regulatory and environmental issues with sterile insect technique-based mosquito vector control in European countries. Rev. Sci. Technol. 2022, 41, 170–177. [Google Scholar] [CrossRef]
- Bellini, R.; Medici, A.; Puggioli, A.; Balestrino, F.; Carrieri, M. Pilot Field Trials with Aedes albopictus Irradiated Sterile Males in Italian Urban Areas. J. Med. Èntomol. 2013, 50, 317–325. [Google Scholar] [CrossRef]
- Becker, N.; Langentepe-Kong, S.M.; Tokatlian Rodriguez, A.; Oo, T.T.; Reichle, D.; Lühken, R.; Schmidt-Chanasit, J.; Lüthy, P.; Puggioli, A.; Bellini, R. Integrated control of Aedes albopictus in Southwest Germany supported by the Sterile Insect Technique. Parasite Vector 2022, 15, 9. [Google Scholar] [CrossRef]
- Gouagna, L.C.; Damiens, D.; Oliva, C.F.; Boyer, S.; Le Goff, G.; Brengues, C.; Dehecq, J.S.; Raude, J.; Simard, F.; Fontenille, D. Strategic Approach, Advances, and Challenges in the Development and Application of the SIT for Area-Wide Control of Aedes albopictus Mosquitoes in Reunion Island. Insects 2020, 11, 770. [Google Scholar] [CrossRef]
- Du, W.; Hu, C.; Yu, C.; Tong, J.; Qiu, J.; Zhang, S.; Liu, Y. Comparison between pupal and adult X-ray radiation, designed for the sterile insect technique for Aedes albopictus control. Acta Trop. 2019, 199, 105110. [Google Scholar] [CrossRef]
- Yamada, H.; Parker, A.G.; Oliva, C.F.; Balestrino, F.; Gilles, J. X-Ray-Induced Sterility in Aedes albopictus (Diptera: Culicidae) and Male Longevity Following Irradiation. J. Med. Èntomol. 2014, 51, 811–816. [Google Scholar] [CrossRef]
- Mastrangelo, T.; Parker, A.G.; Jessup, A.; Pereira, R.; Orozco-Dávila, D.; Islam, A.; Dammalage, T.; Walder, J.M.M. A New Generation of X Ray Irradiators for Insect Sterilization. J. Econ. Èntomol. 2010, 103, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.; Parker, A. Characterization and dosimetry of a practical X-ray alternative to self-shielded gamma irradiators. Radiat. Phys. Chem. 2011, 80, 107–113. [Google Scholar] [CrossRef]
- Gómez-Simuta, Y.; Parker, A.; Cáceres, C.; Vreysen, M.J.; Yamada, H. Characterization and dose-mapping of an X-ray blood irradiator to assess application potential for the sterile insect technique (SIT). Appl. Radiat. Isot. 2021, 176, 109859. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Vreysen, M.J.B.; Gilles, J.R.L.; Munhenga, G.; Damiens, D.D. The effects of genetic manipulation, dieldrin treatment and irradiation on the mating competitiveness of male Anopheles arabiensis in field cages. Malar. J. 2014, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, V.; Agostini, V.; Moroni, E.; Colombo, G.; Lombardo, G.; Migliore, N.R.; Gabrieli, P.; Garofalo, M.; Gagliardi, S.; Gomulski, L.M.; et al. The worldwide spread of Aedes albopictus: New insights from mitogenomes. Front. Genet. 2022, 13, 931163. [Google Scholar] [CrossRef]
- Shuran, Y.; Qiyong, L. Trend in global distribution and spread of Aedes albopictus. Chin. J. Vector. Biol. Control. 2013, 24, 4. [Google Scholar]
- Martínez-García, E.N.; Díaz-González, E.E.; Marina, C.F.; Bond, J.G.; Rodríguez-Rojas, J.J.; Ponce-García, G.; Sánchez-Casas, R.M.; Fernández-Salas, I. Temporal Viability of Aedes aegypti and Aedes albopictus Eggs Using Two Hygroscopic Substances as Preservatives under a Sterile Insect Technique (SIT) Program in Southern Mexico. Insects 2021, 13, 15. [Google Scholar] [CrossRef]
- Mastronikolos, G.D.; Kapranas, A.; Balatsos, G.K.; Ioannou, C.; Papachristos, D.P.; Milonas, P.G.; Puggioli, A.; Pajović, I.; Petrić, D.; Bellini, R.; et al. Quality Control Methods for Aedes albopictus Sterile Male Transportation. Insects 2022, 13, 179. [Google Scholar] [CrossRef]
- Helinski, M.E.; Parker, A.G.; Knols, B.G. Radiation biology of mosquitoes. Malar. J. 2009, 8, S6. [Google Scholar] [CrossRef]
- Bond, J.G.; Osorio, A.R.; Avila, N.; Gómez-Simuta, Y.; Marina, C.F.; Fernández-Salas, I.; Liedo, P.; Dor, A.; Carvalho, D.O.; Bourtzis, K.; et al. Optimization of irradiation dose to Aedes aegypti and Ae. albopictus in a sterile insect technique program. PLoS ONE 2019, 14, e0212520. [Google Scholar] [CrossRef]
- Balestrino, F.; Medici, A.; Candini, G.; Carrieri, M.; Maccagnani, B.; Calvitti, M.; Maini, S.; Bellini, R. Gamma ray dosimetry and mating capacity studies in the laboratory on Aedes albopictus males. J. Med. Entomol. 2010, 47, 581–591. [Google Scholar] [CrossRef]
- Bond, J.G.; Aguirre-Ibáñez, S.; Osorio, A.R.; Marina, C.F.; Gómez-Simuta, Y.; Tamayo-Escobar, R.; Dor, A.; Liedo, P.; Carvalho, D.O.; Williams, T. Sexual Competitiveness and Induced Egg Sterility by Aedes aegypti and Aedes albopictus Gamma-Irradiated Males: A Laboratory and Field Study in Mexico. Insects 2021, 12, 145. [Google Scholar] [CrossRef]
- Madakacherry, O.; Lees, R.S.; Gilles, J.R.L. Aedes albopictus (Skuse) males in laboratory and semi-field cages: Release ratios and mating competitiveness. Acta Trop. 2014, 132, S124–S129. [Google Scholar] [CrossRef]
- Bellini, R.; Balestrino, F.; Medici, A.; Gentile, G.; Veronesi, R.; Carrieri, M. Mating Competitiveness of Aedes albopictus Radio-Sterilized Males in Large Enclosures Exposed to Natural Conditions. J. Med. Èntomol. 2013, 50, 94–102. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, D.; Li, Y.; Yang, C.; Wu, Y.; Liang, X.; Liang, Y.; Pan, X.; Hu, L.; Sun, Q.; et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 2019, 572, 56–61. [Google Scholar] [CrossRef]
- Rodriguez, S.D.; Brar, R.K.; Drake, L.L.; Drumm, H.E.; Price, D.P.; Hammond, J.I.; Urquidi, J.; Hansen, I.A. The effect of the radio-protective agents ethanol, trimethylglycine, and beer on survival of X-ray-sterilized male Aedes aegypti. Parasite Vector 2013, 6, 211–218. [Google Scholar] [CrossRef]
- Kittayapong, P.; Kaeothaisong, N.-O.; Ninphanomchai, S.; Limohpasmanee, W. Combined sterile insect technique and incompatible insect technique: Sex separation and quality of sterile Aedes aegypti male mosquitoes released in a pilot population suppression trial in Thailand. Parasite Vector 2018, 11, 657. [Google Scholar] [CrossRef]
- Poncio, L.D.C.; dos Anjos, F.A.; de Oliveira, D.A.; Rebechi, D.; de Oliveira, R.N.; Chitolina, R.F.; Fermino, M.L.; Bernardes, L.G.; Guimarães, D.; Lemos, P.A.; et al. Novel Sterile Insect Technology Program Results in Suppression of a Field Mosquito Population and Subsequently to Reduced Incidence of Dengue. J. Infect. Dis. 2021, 224, 1005–1014. [Google Scholar] [CrossRef]
- Yamada, H.; Maiga, H.; Juarez, J.; Carvalho, D.D.O.; Mamai, W.; Ali, A.; Bimbile-Somda, N.S.; Parker, A.G.; Zhang, D.; Bouyer, J. Identification of critical factors that significantly affect the dose-response in mosquitoes irradiated as pupae. Parasite Vector 2019, 12, 435. [Google Scholar] [CrossRef]
- Damiens, D.; Lebon, C.; Wilkinson, D.A.; Dijoux-Millet, D.; Le Goff, G.; Bheecarry, A.; Gouagna, L.C. Cross-Mating Compatibility and Competitiveness among Aedes albopictus Strains from Distinct Geographic Origins—Implications for Future Application of SIT Programs in the South West Indian Ocean Islands. PLoS ONE 2016, 11, e0163788. [Google Scholar] [CrossRef]
- Fried, M. Determination of Sterile-Insect Competitiveness. J. Econ. Èntomol. 1971, 64, 869–872. [Google Scholar] [CrossRef]
- Huang, Y.-J.S.; Higgs, S.; Vanlandingham, D.L. Emergence and re-emergence of mosquito-borne arboviruses. Curr. Opin. Virol. 2019, 34, 104–109. [Google Scholar] [CrossRef] [PubMed]


| Irradiation | Emergence Rate (%) | Egg Number | Hatch Rate (%) |
|---|---|---|---|
| Control | 94.1 ± 1.4 a | 61.7 ± 1.8 a | 86.1 ± 4.4 a |
| X-ray of 10 Gy | 93.7 ± 2.5 a | 62.4 ± 2.3 a | 32.4 ± 3.7 b |
| X-ray of 20 Gy | 92.6 ± 2.1 a | 67.0 ± 2.3 a | 21.7 ± 2.1 c |
| X-ray of 30 Gy | 91.9 ± 2.8 a | 63.6 ± 2.2 a | 13.0 ± 1.6 d |
| X-ray of 40 Gy | 91.5 ± 4.2 a | 62.1 ± 2.2 a | 5.1 ± 1.8 e |
| X-ray of 50 Gy | 91.9 ± 3.1 a | 62.9 ± 2.0 a | 3.2 ± 1.0 e |
| X-ray of 60 Gy | 89.6 ± 4.3 a | 64.6 ± 2.1 a | 0.8 ± 0.6 f |
| γ-ray of 10 Gy | 91.9 ± 2.8 a | 65.1 ± 2.1 a | 19.3 ± 2.2 c |
| γ-ray of 20 Gy | 91.1 ± 2.2 a | 59.3 ± 2.1 a | 6.2 ± 1.2 e |
| γ-ray of 30 Gy | 87.4 ± 2.1 a,b | 61.0 ± 2.2 a | 3.2 ± 0.9 e |
| γ-ray of 40 Gy | 86.7 ± 2.7 a,b,c | 64.7 ± 2.3 a | 0.7 ± 0.7 f |
| γ-ray of 50 Gy | 78.9 ± 3.1 b,c | 61.6 ± 2.2 a | 0.6 ± 0.5 f |
| γ-ray of 60 Gy | 75.6 ± 3.1 c | 62.8 ± 2.0 a | 0.2 ± 0.4 f |
| Radiation Dose (Gy) | Average Survival Time (X-rays, Days) | Average Survival Time (γ-rays, Days) |
|---|---|---|
| Control | 27.7 ± 1.0 a | |
| 10 | 26.4 ± 1.1 a,b | 23.5 ± 0.9 c,d |
| 20 | 24.9 ± 1.0 b,c | 21.1 ± 0.9 f |
| 30 | 22.1 ± 0.9 d,e,f | 18.6 ± 0.7 g |
| 40 | 20.1 ± 0.8 e,f | 17.1 ± 0.6 h,i |
| 50 | 17.7 ± 0.7 g,h | 13.3 ± 0.6 j |
| 60 | 15.9 ± 0.6 i | 11.8 ± 0.4 k |
| Release Ratio (S/F) | Hatch Rate (γ-rays, %) | Hatch Rate (X-rays, %) |
|---|---|---|
| 0:1 | 89.2 ± 3.8 a | 87.4 ± 2.4 a |
| 1:1 | 62.1 ± 4.1 b | 59.0 ± 6.6 b |
| 3:1 | 38.8 ± 3.3 c | 37.2 ± 4.9 c |
| 5:1 | 27.6 ± 4.0 d | 28.7 ± 1.9 d |
| 7:1 | 20.6 ± 1.6 e | 22.1 ± 4.3 e |
| 1:0 | 0.4 ± 0.5 f | 0.7 ± 0.7 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-M.; Li, N.; Ren, C.-P.; Peng, Z.-Y.; Lu, H.-Z.; Li, D.; Wu, X.-Y.; Zhou, Z.-X.; Deng, J.-Y.; Zheng, Z.-H.; et al. Sterility of Aedes albopictus by X-ray Irradiation as an Alternative to γ-ray Irradiation for the Sterile Insect Technique. Pathogens 2023, 12, 102. https://doi.org/10.3390/pathogens12010102
Wang L-M, Li N, Ren C-P, Peng Z-Y, Lu H-Z, Li D, Wu X-Y, Zhou Z-X, Deng J-Y, Zheng Z-H, et al. Sterility of Aedes albopictus by X-ray Irradiation as an Alternative to γ-ray Irradiation for the Sterile Insect Technique. Pathogens. 2023; 12(1):102. https://doi.org/10.3390/pathogens12010102
Chicago/Turabian StyleWang, Lin-Min, Ni Li, Cui-Ping Ren, Zhe-Yu Peng, Hong-Zheng Lu, Dong Li, Xin-Yu Wu, Zi-Xin Zhou, Jian-Yi Deng, Zi-Han Zheng, and et al. 2023. "Sterility of Aedes albopictus by X-ray Irradiation as an Alternative to γ-ray Irradiation for the Sterile Insect Technique" Pathogens 12, no. 1: 102. https://doi.org/10.3390/pathogens12010102
APA StyleWang, L.-M., Li, N., Ren, C.-P., Peng, Z.-Y., Lu, H.-Z., Li, D., Wu, X.-Y., Zhou, Z.-X., Deng, J.-Y., Zheng, Z.-H., Wang, R.-Q., Du, Y.-N., Wang, D.-Q., & Deng, S.-Q. (2023). Sterility of Aedes albopictus by X-ray Irradiation as an Alternative to γ-ray Irradiation for the Sterile Insect Technique. Pathogens, 12(1), 102. https://doi.org/10.3390/pathogens12010102