Third Case of Visceral Leishmaniasis in COVID-19: Mini Review Article
Abstract
1. Introduction
2. Methods of the Review
3. Findings from Bibliography Research
4. New Case Report
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garazzino, S.; Vecchio, A.L.; Pierantoni, L.; Carducci, F.I.C.; Marchetti, F.; Meini, A.; Castagnola, E.; Vergine, G.; Donà, D.; Bosis, S.; et al. Epidemiology, clinical features and prognostic factors of pediatric SARS-CoV-2 infection: Results from an Italian multicenter study. Front. Pediatr. 2021, 9, 649358. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.H. Diagnosing severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) related multisystem inflammatory syndrome in children (MIS-C): Focus on the gastrointestinal tract and the myocardium. Clin. Infect. Dis. 2021, 72, e402–e403. [Google Scholar] [CrossRef] [PubMed]
- Fink, E.L.; Robertson, C.L.; Wainwright, M.S.; Roa, J.D.; Lovett, M.E.; Stulce, C.; Yacoub, M.; Potera, R.M.; Zivick, E.; Holloway, A.; et al. Prevalence and risk factors of neurologic manifestations in hospitalized children diagnosed with acute SARS-CoV-2 or MIS-C. Pediatr. Neurol. 2022, 128, 33–44. [Google Scholar] [CrossRef]
- García-Salido, A.; Vicente, J.C.D.C.; Hofheinz, S.B.; Ramírez, J.B.; Barrio, M.S.; Gordillo, I.L.; Yuste, A.H.; Pardellans, C.G.; Tejedor, M.C.-M.; Labarga, B.H.; et al. Severe manifestations of SARS-CoV-2 in children and adolescents: From COVID-19 pneumonia to multisystem inflammatory syndrome: A multicentre study in pediatric intensive care units in Spain. Crit. Care 2020, 24, 666. [Google Scholar] [CrossRef] [PubMed]
- Kishore, R.; Dhakad, S.; Arif, N.; Dar, L.; Mirdha, B.R.; Aggarwal, R.; Kabra, S.K. COVID-19: Possible cause of Induction of relapse of Plasmodium vivax infection. Indian J. Pediatr. 2020, 87, 751–752. [Google Scholar] [CrossRef] [PubMed]
- Cascio, A.; Colomba, C.; Antinori, S.; Orobello, M.; Paterson, D.; Titone, L. Pediatric visceral leishmaniasis in Western Sicily, Italy: A retrospective analysis of 111 cases. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Miotti, A.M.; Patacca, A.; Grosso, C.; Cristini, F. COVID-19 in a Patient with visceral leishmaniasis. Infect. Dis. Ther. 2020, 8, 4–5. [Google Scholar]
- Pikoulas, A.; Piperaki, E.-T.; Spanakos, G.; Kallianos, A.; Mparmparousi, D.; Rentziou, G.; Trakada, G. Visceral leishmaniasis and COVID-19 coinfection—A case report. IDCases 2022, 27, e01358. [Google Scholar] [CrossRef] [PubMed]
- Venturini, E.; Montagnani, C.; Garazzino, S.; Donà, D.; Pierantoni, L.; Vecchio, A.L.; Krzysztofiak, A.; Nicolini, G.; Bianchini, S.; Galli, L.; et al. Treatment of children with COVID-19: Update of the Italian Society of Pediatric Infectious Diseases position paper. Ital. J. Pediatr. 2021, 47, 199. [Google Scholar] [CrossRef] [PubMed]
- Sebastião, C.S.; Gaston, C.; Paixão, J.P.; Sacomboio, E.N.M.; Neto, Z.; de Vasconcelos, J.N.; Morais, J. Coinfection between SARS-CoV-2 and vector-borne diseases in Luanda, Angola. J. Med. Virol. 2022, 94, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, D.; Sarkar, M.; Roy, A.; Roy, D.; Datta, K.; Sengupta, T.; Hazra, A.; Mondal, R. COVID-19 and co-infection in children: The Indian perspectives. J. Trop. Pediatr. 2021, 67, fmab073. [Google Scholar] [CrossRef] [PubMed]
- Jochum, J.; Kreuels, B.; Tannich, E.; Huber, S.; Wiesch, J.S.Z.; Schmiedel, S.; Ramharter, M.; Addo, M. Malaria in the time of COVID-19: Do not miss the real cause of illness. Trop. Med. Infect. Dis. 2021, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Colomba, C.; Saporito, L.; Vitale, F.; Reale, S.; Vitale, G.; Casuccio, A.; Tolomeo, M.; Maranto, D.; Rubino, R.; Di Carlo, P.; et al. Cryptic Leishmania infantum infection in Italian HIV infected patients. BMC Infect. Dis. 2009, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Cascio, A.; Colomba, C. Childhood Mediterranean visceral leishmaniasis. Infez. Med. 2003, 11, 5–10. [Google Scholar]
- Ahmed, M.; Advani, S.; Moreira, A.; Zoretic, S.; Martinez, J.; Chorath, K.; Acosta, S.; Naqvi, R.; Burmeister-Morton, F.; Burmeister, F.; et al. Multisystem inflammatory syndrome in children: A systematic review. EClinicalMedicine 2020, 26, 100527. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.F.G.; Vieira, T.M.; Moura, A.P.V.; Andrade, M.C. Should an intersection between visceral leishmaniasis endemicity and the COVID-19 pandemic be considered? Med. Hypotheses 2020, 144, 110289. [Google Scholar] [CrossRef]
- Tolomeo, M.; Bonura, S.; Abbott, M.; Anastasia, A.; Colomba, C.; Cascio, A. Good’s syndrome and recurrent leishmaniasis: A case report and review of literature. Heliyon 2020, 6, e05061. [Google Scholar] [CrossRef] [PubMed]
- Colomba, C.; Saporito, L.; Bonura, S.; Campisi, G.; Di Carlo, P.; Panzarella, V.; Caputo, V.; Cascio, A. Leishmania infection in psoriasis. J. Infect. 2020, 80, 578–606. [Google Scholar] [CrossRef]
- Colomba, C.; Adamoli, L.; Trizzino, M.; Siracusa, L.; Bonura, S.; Tolomeo, M.; Cajozzo, M.; Giammanco, G.M. A case of visceral leishmaniasis and pulmonary tuberculosis in a post-partum woman. Int. J. Infect. Dis. 2015, 33, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Saporito, L.; Giammanco, G.M.; De Grazia, S.; Colomba, C. Visceral leishmaniasis: Host-parasite interactions and clinical presentation in the immunocompetent and in the immunocompromised host. Int. J. Infect. Dis. 2013, 17, e572–e576. [Google Scholar] [CrossRef] [PubMed]
- Le Balc’h, P.; Pinceaux, K.; Pronier, C.; Seguin, P.; Tadié, J.M.; Reizine, F. Herpes simplex virus and cytomegalovirus reactivations among severe COVID-19 patients. Crit. Care 2020, 24, 530. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.A.; Buti, M. COVID-19 and hepatitis B infection. Antivir. Ther. 2020, 25, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Lupia, T.; Corcione, S.; De Rosa, F.G. Giardiasis reactivation during severe SARS-CoV-2 infection. Parasitol. Int. 2021, 80, 102241. [Google Scholar] [CrossRef] [PubMed]
- Heaney, A.K.; Head, J.R.; Broen, K.; Click, K.; Taylor, J.; Balmes, J.R.; Zelner, J.; Remais, J.V. Coccidioidomycosis and COVID-19 co-infection, United States, 2020. Emerg. Infect. Dis. 2021, 27, 1266–1273. [Google Scholar] [CrossRef]
- Bamorovat, M.; Sharifi, I.; Aflatoonian, M.R.; Karamoozian, A.; Tahmouresi, A.; Jafarzadeh, A.; Heshmatkhah, A.; Sharifi, F.; Salarkia, E.; Khaleghi, T.; et al. Prophylactic effect of cutaneous leishmaniasis against COVID-19: A case-control field assessment. Int. J. Infect. Dis. 2021, 122, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Antinori, S.; Calattini, S.; Longhi, E.; Bestetti, G.; Piolini, R.; Magni, C.; Orlando, G.; Gramiccia, M.; Acquaviva, V.; Foschi, A.; et al. Clinical use of polymerase chain reaction performed on peripheral blood and bone marrow samples for the diagnosis and monitoring of visceral leishmaniasis in HIV-infected and HIV-uninfected patients: A single-center, 8-year experience in Italy and review of the literature. Clin. Infect. Dis. 2007, 44, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Cascio, A.; Calattini, S.; Colomba, C.; Scalamogna, C.; Galazzi, M.; Pizzuto, M.; Camilli, R.; Gramiccia, M.; Titone, L.; Corbellino, M.; et al. Polymerase chain reaction in the diagnosis and prognosis of Mediterranean visceral leishmaniasis in immunocompetent children. Pediatrics 2002, 109, e27. [Google Scholar] [CrossRef] [PubMed]
- Cascio, A.; Di Martino, L.; Occorsio, P.; Giacchino, R.; Catania, S.; Gigliotti, A.R.; Aiassa, C.; Iaria, C.; Giordano, S.; Colomba, C.; et al. A 6 day course of liposomal amphotericin B in the treatment of infantile visceral leishmaniasis: The Italian experience. J. Antimicrob. Chemother. 2004, 54, 217–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Masi, F.; Ursini, T.; Iannece, M.D.; Chianura, L.; Baldasso, F.; Foti, G.; Di Gregorio, P.; Casabianca, A.; Storaci, N.; Nigro, L.; et al. Five-year retrospective Italian multicenter study of visceral leishmaniasis treatment. Antimicrob. Agents Chemother. 2014, 58, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.; Brunet, L.R. Microbes, immunoregulation, and the gut. Gut 2005, 54, 317–320. [Google Scholar] [CrossRef] [PubMed]
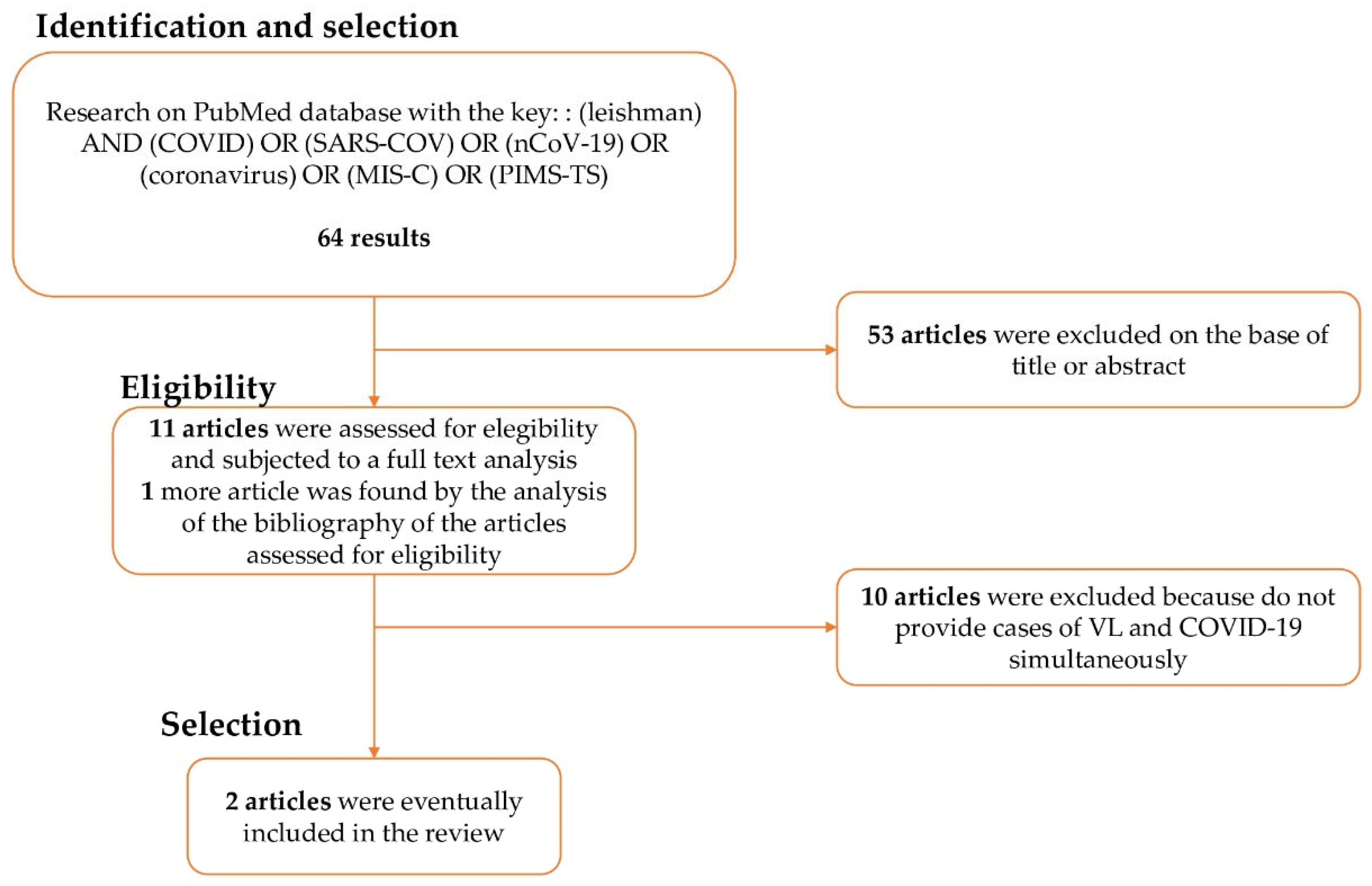

| Author/Year | Age | Sex | Immunocompetence | Fever and Pancytopenia | Sample for Diagnosis | Diagnosis | Therapy | Outcome |
|---|---|---|---|---|---|---|---|---|
| Miotti 2020 | 70 | M | No | Yes | Blood and bone marrow | Molecular diagnosis | Amphotericin B + dexamethason | Death |
| Pikoulas 2021 | 30 | F | Yes | Yes | Blood and bone marrow | Molecular diagnosis | Amphotericin B + dexamethason | Full recovery |
| Present case 2022 | 4 | F | Yes | Yes | Blood | Molecular diagnosis | Amphotericin B | Full recovery |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colomba, C.; Guccione, C.; Rubino, R.; Scalisi, M.; Condemi, A.; Bagarello, S.; Giordano, S.; Cascio, A. Third Case of Visceral Leishmaniasis in COVID-19: Mini Review Article. Pathogens 2022, 11, 913. https://doi.org/10.3390/pathogens11080913
Colomba C, Guccione C, Rubino R, Scalisi M, Condemi A, Bagarello S, Giordano S, Cascio A. Third Case of Visceral Leishmaniasis in COVID-19: Mini Review Article. Pathogens. 2022; 11(8):913. https://doi.org/10.3390/pathogens11080913
Chicago/Turabian StyleColomba, Claudia, Cristoforo Guccione, Raffaella Rubino, Michela Scalisi, Anna Condemi, Sara Bagarello, Salvatore Giordano, and Antonio Cascio. 2022. "Third Case of Visceral Leishmaniasis in COVID-19: Mini Review Article" Pathogens 11, no. 8: 913. https://doi.org/10.3390/pathogens11080913
APA StyleColomba, C., Guccione, C., Rubino, R., Scalisi, M., Condemi, A., Bagarello, S., Giordano, S., & Cascio, A. (2022). Third Case of Visceral Leishmaniasis in COVID-19: Mini Review Article. Pathogens, 11(8), 913. https://doi.org/10.3390/pathogens11080913








