Visible 405 nm Violet-Blue Light Successfully Inactivates HIV-1 in Human Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Plasma and HIV-1 Virus
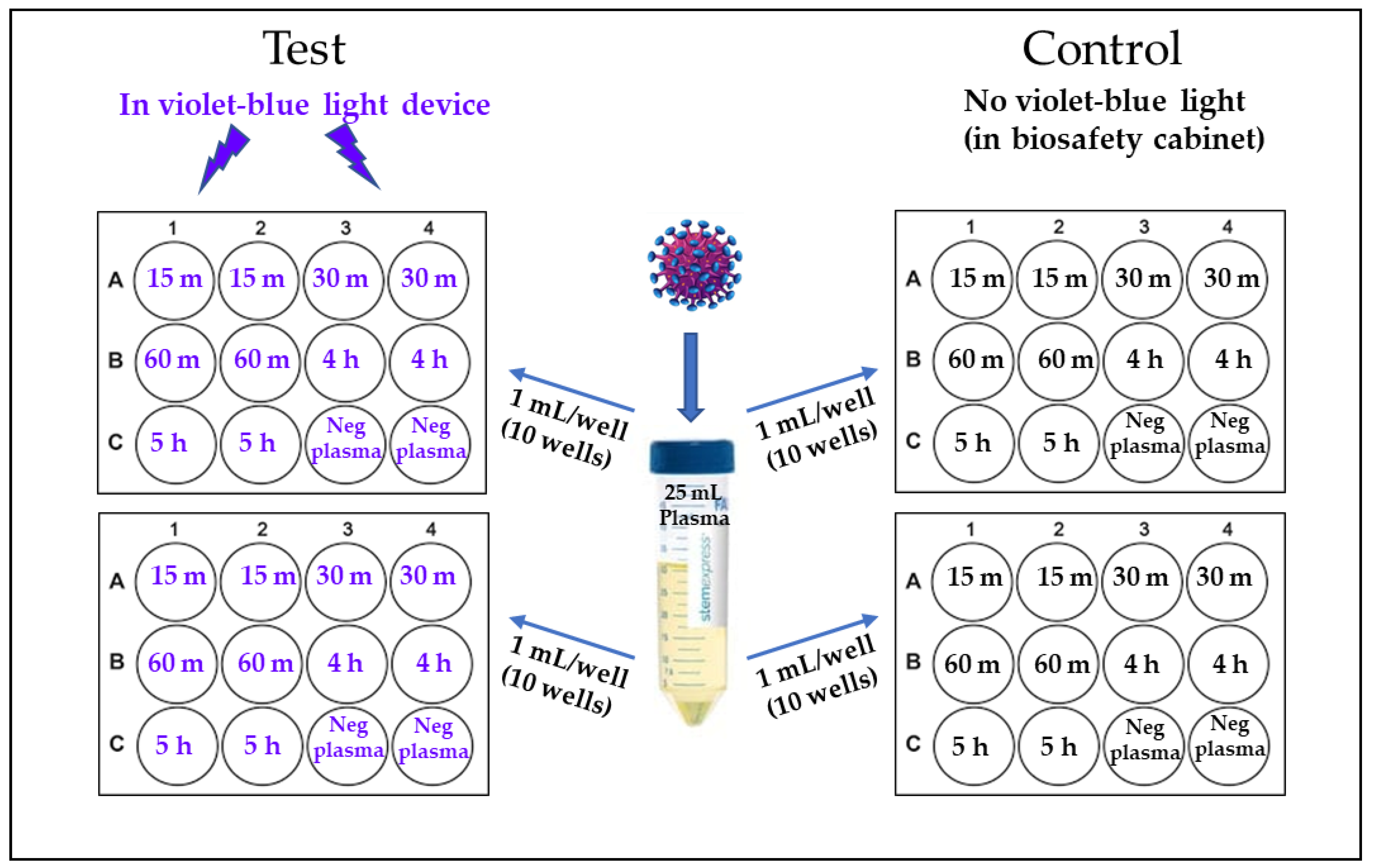
2.2. 405 nm Violet-Blue Light Treatment of the Virus
2.3. Estimation of HIV-1 Infectivity in Plasma Samples
2.4. Statistical Analysis
3. Results
405 nm Light Inactivates HIV-1 in Human Plasma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escolar, G.; Diaz-Ricart, M.; McCullough, J. Impact of different pathogen reduction technologies on the biochemistry, function, and clinical effectiveness of platelet concentrates: An updated view during a pandemic. Transfusion 2022, 62, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Fong, I.W. Blood Transfusion-Associated Infections in the Twenty-First Century: New Challenges. In Current Trends and Concerns in Infectious Diseases; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Busch, M.P.; Bloch, E.M.; Kleinman, S. Prevention of transfusion-transmitted infections. Blood 2019, 133, 1854–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubbard, T.; Backholer, L.; Wiltshire, M.; Cardigan, R.; Ariens, R.A. Effects of riboflavin and amotosalen photoactivation systems for pathogen inactivation of fresh-frozen plasma on fibrin clot structure. Transfusion 2016, 56, 41–48. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Kim, I.S.; Bae, J.E.; Kang, J.W.; Cho, Y.J.; Cho, N.S.; Lee, S.W. Pathogen inactivation efficacy of Mirasol PRT System and Intercept Blood System for non-leucoreduced platelet-rich plasma-derived platelets suspended in plasma. Vox Sang. 2014, 107, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Maclean, M.; Anderson, J.G.; MacGregor, S.J.; White, T.; Atreya, C.D. A New Proof of Concept in Bacterial Reduction: Antimicrobial Action of Violet-Blue Light (405 nm) in Ex Vivo Stored Plasma. J. Blood Transfus. 2016, 2016, 2920514. [Google Scholar] [CrossRef] [Green Version]
- Tomb, R.M.; Maclean, M.; Coia, J.E.; Graham, E.; McDonald, M.; Atreya, C.D.; MacGregor, S.J.; Anderson, J.G. New Proof-of-Concept in Viral Inactivation: Virucidal Efficacy of 405 nm Light Against Feline Calicivirus as a Model for Norovirus Decontamination. Food Environ. Virol. 2017, 9, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Maclean, M.; Gelderman, M.P.; Kulkarni, S.; Tomb, R.M.; Stewart, C.F.; Anderson, J.G.; MacGregor, S.J.; Atreya, C.D. Non-ionizing 405 nm Light as a Potential Bactericidal Technology for Platelet Safety: Evaluation of in vitro Bacterial Inactivation and in vivo Platelet Recovery in Severe Combined Immunodeficient Mice. Front. Med. 2020, 6, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankowska, K.I.; Nagarkatti, R.; Acharyya, N.; Dahiya, N.; Stewart, C.F.; Macpherson, R.W.; Wilson, M.P.; Anderson, J.G.; MacGregor, S.J.; Maclean, M.; et al. Complete Inactivation of Blood Borne Pathogen Trypanosoma cruzi in Stored Human Platelet Concentrates and Plasma Treated with 405 nm Violet-Blue Light. Front. Med. 2020, 7, 617373. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.F.; Tomb, R.M.; Ralston, H.J.; Armstrong, J.; Anderson, J.G.; MacGregor, S.J.; Atreya, C.D.; Maclean, M. Violet-blue 405-nm Light-based Photoinactivation for Pathogen Reduction of Human Plasma Provides Broad Antibacterial Efficacy without Visible Degradation of Plasma Proteins. Photochem. Photobiol. 2022, 98, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, L.; Rose, J.K. Blockade of human immunodeficiency virus type 1 production in CD4+ T cells by an intracellular CD4 expressed under control of the viral long terminal repeat. Proc. Natl. Acad. Sci. USA 1993, 90, 2695–2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, S.K.; Gravell, M.; Wu, Y.N.; Mikulski, S.M.; Shogen, K.; Ardelt, W.; Youle, R.J. Inhibition of HIV-1 production and selective degradation of viral RNA by an amphibian ribonuclease. J. Biol. Chem. 1996, 271, 20783–20788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathnasinghe, R.; Jangra, S.; Miorin, L.; Schotsaert, M.; Yahnke, C.; Garciotaa-Sastre, A. The virucidal effects of 405 nm visible light on SARS-CoV-2 and influenza A virus. Sci. Rep. 2021, 11, 19470. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.; Becher, D.; Hessling, M. High Intensity Violet Light (405 nm) Inactivates Coronaviruses in Phosphate Buffered Saline (PBS) and on Surfaces. Photonics 2021, 8, 414. [Google Scholar] [CrossRef]
- Stasko, N.; Kocher, J.F.; Annas, A.; Henson, I.; Seitz, T.S.; Miller, J.M.; Arwood, L.; Roberts, R.C.; Womble, T.M.; Keller, E.G.; et al. Visible blue light inhibits infection and replication of SARS-CoV-2 at doses that are well-tolerated by human respiratory tissue. Sci. Rep. 2021, 11, 20595. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Y.; Bi, X.X. Experimental studies on the inactivation of HBV in blood via riboflavin photochemical treatment. Exp. Ther. Med. 2017, 13, 222–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hessling, M.; Lau, B.; Vatter, P. Review of Virus Inactivation by Visible Light. Photonics 2022, 9, 113. [Google Scholar] [CrossRef]
- Cabral, J.; Ag, R. Blue Light Disinfection in Hospital Infection Control: Advantages, Drawbacks, and Pitfalls. Antibiotics 2019, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Hadi, J.; Wu, S.; Brightwell, G. Antimicrobial Blue Light versus Pathogenic Bacteria: Mechanism, Application in the Food Industry, Hurdle Technologies and Potential Resistance. Foods 2020, 9, 1895. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, P.; Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Grant, M.H. Cytotoxic responses to 405 nm light exposure in mammalian and bacterial cells: Involvement of reactive oxygen species. Toxicol. In Vitro 2016, 33, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanstrom, R.; Coffin, J. HIV-1 pathogenesis: The virus. Cold Spring Harb. Perspect. Med. 2012, 2, a007443. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragupathy, V.; Haleyurgirisetty, M.; Dahiya, N.; Stewart, C.; Anderson, J.; MacGregor, S.; Maclean, M.; Hewlett, I.; Atreya, C. Visible 405 nm Violet-Blue Light Successfully Inactivates HIV-1 in Human Plasma. Pathogens 2022, 11, 778. https://doi.org/10.3390/pathogens11070778
Ragupathy V, Haleyurgirisetty M, Dahiya N, Stewart C, Anderson J, MacGregor S, Maclean M, Hewlett I, Atreya C. Visible 405 nm Violet-Blue Light Successfully Inactivates HIV-1 in Human Plasma. Pathogens. 2022; 11(7):778. https://doi.org/10.3390/pathogens11070778
Chicago/Turabian StyleRagupathy, Viswanath, Mohan Haleyurgirisetty, Neetu Dahiya, Caitlin Stewart, John Anderson, Scott MacGregor, Michelle Maclean, Indira Hewlett, and Chintamani Atreya. 2022. "Visible 405 nm Violet-Blue Light Successfully Inactivates HIV-1 in Human Plasma" Pathogens 11, no. 7: 778. https://doi.org/10.3390/pathogens11070778
APA StyleRagupathy, V., Haleyurgirisetty, M., Dahiya, N., Stewart, C., Anderson, J., MacGregor, S., Maclean, M., Hewlett, I., & Atreya, C. (2022). Visible 405 nm Violet-Blue Light Successfully Inactivates HIV-1 in Human Plasma. Pathogens, 11(7), 778. https://doi.org/10.3390/pathogens11070778





