Abstract
This is a single-centre observational study of adult patients with severe pneumonia requiring hospitalization conducted at the emergency department. During the observation period (94 weeks), 398 patients were diagnosed with severe pneumonia and required further treatment at the hospital. The median age of patients was 73 years. About 65% of patients had at least one chronic comorbidity. Almost 30% of patients had cardiovascular disorders, and 13% had diabetes mellitus. The average Emergency Department length of stay was 3.56 days. The average length of hospitalization was 15.8 days. Overall, 94% of patients treated for pneumonia received a beta-lactam antibiotic. The median time from ED admission to the administration of the first dose of antimicrobial agent was less than 6 h. Microbiology test samples were obtained from 48.7% patients. Gram-positive cocci were isolated most commonly (52.9%) from blood samples. Biological material from the lower respiratory tract was collected from 8.3% of patients, and from 47.2% of positive samples, fungi were cultured. The urine samples were obtained from 35.9% patients, and Gram-negative rods (76%) were isolated most commonly. Overall, 16.1% of patients died during the hospitalization. The mean age of patients who died was 79 years. This observational study is the first single-centre study conducted as part of the Polish Emergency Department Research Organization (PEDRO) project. It aims to provide up-to-date information about patients with pneumonia in order to improve medical care and develop local diagnostic and therapeutic recommendations.
1. Introduction
1.1. Background
The emergency department (ED) plays a pivotal role in providing the community access to acute health care. Because of the unscheduled and episodic nature of health emergencies, clinical decision making by ED staff is a real challenge and a complex process. Every patient who comes through the door is an unknown and may suddenly develop worsening symptoms. Clinicians at the ED are obligated to handle plenty of various issues simultaneously, most of which cannot be foreseen in advance and optimized to “exist at the edge of chaos”. In order to ensure proper care of patients, it is necessary to quickly answer some questions: what is the likely diagnosis, what is the correct diagnostic pathway, what medication should be administered, and which unit should the patient be transferred to [1].
The goal of EDs is to provide evidence-based, high-quality, intensive, short-term observation and optimal antibiotic regimens for selected ED patients and reduce unnecessary hospital admissions and/or premature discharges due to over- and underdiagnosis [2]. Pneumonia remains a challenge to the clinician at the emergency department. It is a common cause of ED visits, including visits of elderly patients, whose pneumonia can be difficult to diagnose due to the non-specificity of symptoms and other comorbidities [3]. The experience of researchers from other countries shows that pneumonia is a common infection and causes significant morbidity and mortality [4,5,6,7]. No complex, up-to-date studies among Polish patients with pneumonia have been conducted so far. We do not know the real scale of the problem in Poland, characteristics of patients, and aetiological factors of infection [8].
The national recommendations for the Management of a patient with suspected severe infection at the Hospital Emergency Department, published in 2014, state that there are no Polish data describing the management of a patient with symptoms of severe infection at the ED [9]. Despite the fact that almost 8 years have passed since the communication of the need to obtain and compile the data, we still do not have any national experience and recommendations regarding the management of a patient in severe condition, including pneumonia requiring hospitalization, at EDs. The authors of the study refer to experiences of other researchers that were published between 1990 and 2006. Since then, there have been many changes, including but not limited to demographic conditions (e.g., aging of the population, high percentage of immunosuppressed people in the population), aetiological factors of infections (new and re-emerging infections), microbial antibiotic susceptibility profiles, diagnostic methods, and cost policy in hospitals; therefore, there is a need to characterize patients diagnosed at the ED in order to improve patient care [9]. It should be noted that Poland compares very unfavourably in terms of the pneumonia death rate to Europe. According to the Eurostat estimate, 131,450 people died from pneumonia in the European Union countries (EU-28) in 2016. The standardised death rate (SDR; three-year average) from pneumonia stood at 27 deaths per 100,000 EU inhabitants in 2017 (Figure 1). The SDR for the EU population aged ≥ 65 years was nearly 5-fold higher (126.52/100,000). In Poland, very high SDRs have been reported for years, with over 55 deaths per 100,000 inhabitants in some voivodeships [10].
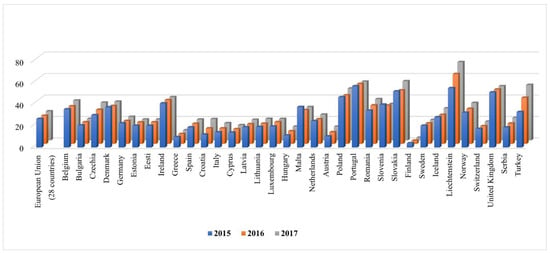
Figure 1.
Deaths from pneumonia in EU region.
1.2. Objective
The objective of this study is to evaluate demographic features, clinical patterns, and history of hospitalisation associated with patients who suffer from pneumonia requiring hospitalization and their impact on mortality.
The analysis of clinical records of adult patients admitted to the ED between 1 January 2019 and 15 October 2020 is a starting point for organized activities with the participation of other emergency departments in Poland to understand the characteristics and impact of this disease, improve the quality of care, and, above all, reduce mortality rates due to pneumonia in Poland.
2. Methods
This research was designed as a single-centre observational study of adult patients with severe pneumonia requiring hospitalization. The study was conducted at the Emergency Department of Clinical Hospital located in the centre of Warsaw, Poland. It is the first study conducted as part of the PEDRO (Polish Emergency Department Research Organization) project.
We reviewed and compared clinical records of patients who were admitted to the ED between 1 January 2019 and 15 October 2020; after 15 October 2020 only patients with confirmed COVID-19 were admitted to the hospital’s ED; therefore, the study was terminated. The data set for the analysis included only patients with first-listed diagnosis of pneumonia, meaning that pneumonia was the main reason for hospitalisation.
The key inclusion criteria were patients over 18 years of age with signs and symptoms of pneumonia who needed hospitalisation. The diagnosis of pneumonia was suspected based on the American Thoracic Society criteria, i.e., when:
- -
- The patient reported at least one of the following signs: cough, fever or chills, difficulty, breathing, chest pain, low energy and poor appetite, nausea, and diarrhoea;
- -
- Reduced or abnormal sounds were heard in the lungs during the physical examination;
- -
- Radiological (chest X-ray/computed tomography scan, CT) evidence of (an) infiltrate(s) consistent with pneumonia were observed [11].
The assessment of indications for hospitalization was conducted in accordance with the national recommendations stating that a decision whether to refer a patient to the hospital or not should take into consideration the patient safety and economic aspects. Therefore, indications for hospitalization result from the severity of the patient’s condition, presence of comorbidities that may contribute to the unfavourable course of infection, ability to take oral medications, and social conditions of the patient [12].
We considered patients with lung disease that was not related to pulmonary infection and COVID-19 patients (people infected with SARS-CoV-2 were being transferred to a dedicated COVID-19 hospital) to be exclusion criteria in this study.
For each patient, information about demographics (age and sex), mortality, ED and hospital length of stay (LOS), clinical data (based on triage, signs and symptoms, and medical history), microbiological test results, and radiological features was collected. All data were entered in the questionnaire and checked by two persons (double checked).
Microbiology testing was conducted at the hospital laboratory. A decision to perform microbiological testing was made on the basis of the national Recommendations for the management of community-acquired respiratory tract infections [12], in accordance with the recommendations:
- The performance of microbiology testing, in particular a sputum culture, should be considered when the presence of a risk factor for infection with multidrug-resistant microorganism is identified, or aetiology of infection may be different than the most common one;
- Sputum culture was performed in patients hospitalized for moderate or severe community-acquired pneumonia who expectorated purulent secretion; sputum cultures are recommended prior to initiating antibiotic therapy. Peripheral blood culture can also be performed in those patients;
- In the event of severe pneumonia, in particular when the history shows lack of response to therapy with beta-lactam antibiotics, the determination of Streptococcus pneumoniae and Legionella pneumophila antigens in the urine is recommended [12].
Microbiology test results were interpreted in accordance with the principles specified in the Indications for the performance of microbiological testing in hospitalized patients [13] and the ATS/IDSA Clinical Guideline on Community-Acquired Pneumonia [14].
Statistical Analysis
In descriptive statistics, means, medians, standard deviations, and extreme values were used for continuous variables, while counts and proportions for categorical variables. Goodness-of-fit χ2 tests were applied to check if categorical variables follow uniform distribution. To compare categorical variables between groups, Pearson’s χ2 tests for independence were used. For comparisons of continuous variables between two groups, Mann–Whitney tests were applied. Survival times were compared with log-rank tests. All statistical hypotheses were verified at 0.05 level of significance. Calculations were performed using IBM SPSS Statistics ver. 20.0.
3. Results
Between 1 January 2019 and 15 October 2020 (94 weeks, 21.5 months), 61,108 adults presented at the ED of the University Hospital located in the centre of Warsaw (according to the Central Statistical Office of Poland (Polish abbreviation: GUS), the number of inhabitants was 1790.6 thousand and 1794.2 thousand in 2020 and 2021, respectively) [15]. In total, 8022 (13,1%) people were admitted to the hospital for further treatment; i.e., the average weekly number of patients admitted to hospital units following the ED visit was 85.3. During the observation period, 398 patients were diagnosed with severe pneumonia and required further treatment at the hospital, which was 5% of all admissions to our hospital during the 94-week observation period.
3.1. Demographic Characteristics and in-Hospital History of Patients Visiting the Emergency Department Who Were Diagnosed with Pneumonia during the 94-Week Observation Period
The median age of patients diagnosed with severe pneumonia at the ED was 73 years. The ratio of admitted women to men was 1:1.7. (a statistically significant difference, p < 0.001). The analysis of incidence of pneumonia requiring hospitalization across age groups 18–65, 66–80, and >80 years did not reveal any statistically significant differences; the ratio of hospitalized patients was 1.07:1:1.06. In total, 278 patients (70.2%) were brought to the hospital by the emergency medical team (EMT).
Forty-five (11.3%) patients were referred for further treatment at the intensive care unit (IUT) from the ED due to a diagnosis of severe pneumonia. The majority of patients (340; 85.4%) continued their therapy at the internal medicine unit. Sixty-four (16.1%) patients died during the hospitalization. Demographic characteristics, history of patients visiting the ED who were diagnosed with pneumonia, and intra-hospital movement and in-hospital mortality rates are shown in Table 1.

Table 1.
Demographics and history of patients visiting the ED who were diagnosed with pneumonia, intra-hospital movement and in-hospital mortality rates.
Triage—the emergency medical segregation that enables to select patients who need immediate care—has been recommended in Poland since 2019. Triage is based on the assessment of vital signs and is a part of the Mode of Patient Care at the Emergency Department (Polish abbreviation: TOPSOR) [16].
Initial patient assessment includes measurements of body temperature (T), blood pressure (systolic; SP/diastolic; DP), heart rate (HR), oxygen saturation (OS), respiratory rate, glycaemia, body weight, assessment of consciousness level using the Glasgow Coma Scale (GCS) or Alert Verbal Painful Unresponsiveness (AVPU) scale, assessment of pain severity, and performance of an electrocardiogram (ECG). The assessment of the patient health condition at hospital admission (TRIAGE) was performed in the majority of patients (between 306 and 362, depending on the assessed parameter). The triage data (those recorded in medical records) are presented in Table 2. The patient stratification at the ED did not take in account the severity of community-acquired pneumonia assessed using CURB, CURB-65, Pneumonia Severity Index for (PSI) for community-acquired pneumonia (CAP), and parameters included in Severe Community-Acquired Pneumonia (SCAP).

Table 2.
Triage patient characteristics.
3.2. Main Presentation Symptoms, Clinical Diagnosis, and Co-Occurring/Coexisting Conditions
Symptoms reported most commonly by patients presenting at the ED included dyspnoea, malaise, and fever/feeling of fever. The majority of patients (58.0%) presented only one symptom. The presence of two symptoms was reported by 24.9% of patients. Around 65% of patients with pneumonia diagnosed at the ED had at least one chronic comorbidity. Almost 30% of patients had cardiovascular disorders, and 13% of patients suffered from diabetes mellitus. Aspiration pneumonia was diagnosed in 16 subjects (4%): patients with disorders of consciousness, in advanced age, with alcohol dependence, or as a complication following gastroscopy or gastric lavage after attempted suicide. Detailed characteristics are presented in Table 3.

Table 3.
Symptoms, clinical diagnosis, and comorbidities in patients visiting the ED who were diagnosed with pneumonia.
3.3. Length of Patient Treatment at the ED and Overall Hospitalization Time
The average ED length of stay of patients diagnosed with pneumonia was 3.56 days (median: 1.88 days). The average length of hospitalization was 15.8 days (median: 12 days). Detailed information is provided in Table 4.

Table 4.
The ED length of stay and hospitalization duration.
3.4. Antimicrobial Therapy, Imaging Examinations, and Microbiological Test Results
The majority of patients (347; 95%) received an antimicrobial agent during the ED stay. In 18 (5%) patients, the therapy was initiated after the transfer to the unit. Overall, 68% of patients received an antibiotic within ≤1 h of being admitted to the ED. The median time from ED admission to the administration of the first dose of antimicrobial agent was less than 6 h (Table 5).

Table 5.
Time from ED admission to antibiotic administration.
In the course of hospitalization, 94% of patients treated for pneumonia received a beta-lactam antibiotic. Ceftriaxone, belonging to third-generation cephalosporin, was the most commonly administered medication (77.5% of patients). The main indications for ceftriaxone administration include both community-acquired and hospital-acquired pneumonia as well as bacteraemia associated with those infections. The empirical treatment was in line with the national recommendations and the ATS/IDSA Clinical Guidelines on the Management of Community Acquired Pneumonia [12,14]. The combination therapy with two antibiotics was used at the ED in 108 (31%) patients (72; 42% in 2019 and 36; 14% in 2020). Two (0.6%) subjects received three antimicrobial agents. Meropenem was administered to colonised patients or those infected with multidrug resistant (MDR) bacteria. The antiviral agent (ganciclovir) was ordered to a patient with transplanted organ who was suspected to have CMV pneumonia. Oseltamivir was administered in the case of confirmed or suspected influenza, while pentamidine was administered to one OLTX and LTX patient after CMV and SARS-CoV-2 infections were excluded and with suspected Pneumocystis jirovecii infection. The patient was allergic to sulfamethoxazole + trimethoprim. In eight patients, infections with other fungi were suspected, and antifungal agents (fluconazole, caspofungin) were administered. Antimicrobial treatments of inpatients are shown in Table 6.

Table 6.
Antimicrobial treatment of inpatients with pneumonia.
3.4.1. Imaging Examinations
The imaging examination result was one of diagnostic criteria of pneumonia. Chest X-ray or chest CT scan was performed in patients, with chest X-ray done more often (93% of patients) during the first year of observation (2019) and chest X-ray and CT performed in the case of doubts (38%). In the subsequent year of observation (2020), chest CT was ordered much more often (74,8%) (statistically significant, p < 0.001). (Table 7). It was related to the epidemic situation and SARS-CoV-2 infections. Chest CT offers advantage over chest X-ray in detecting lesions in the lungs in COVID-19 patients.

Table 7.
The number of performed chest X-ray and chest CT tests.
3.4.2. Microbiological Examination
Microbiology test samples were obtained from 194 (48.7%) patients. No bacteria were cultured, and no fungal antigens were detected from 69.6% of samples.
3.4.3. Blood Microbiological Testing
Positive results were produced for 30.4% of blood samples, with bacteria grown THAT were considered contamination in 3.1% of cases (in accordance with the clinical and laboratory criteria recommended by the Centers for Disease Control and Prevention (CDC) and the third international consensus definitions of sepsis and septic shock) [17]. In total, 51 microbial isolates were cultured from samples considered to be true-positive (27.3%). Gram-positive cocci (52.9%) were isolated most commonly, of which coagulase-negative staphylococci (51.8%) made up the largest group. Candida spp. antigens were detected in 11.8% of blood sample tests. The species of detected microorganisms are presented in Table 8.

Table 8.
Microbial species cultured from blood, lower respiratory tract, and urine samples.
3.4.4. Microbiological Testing of Material from the Lower Respiratory Tract
Biological material from the lower respiratory tract was collected from 33 (8.3%) patients.
The tracheal aspirate (20/33; 60.6%) was collected most frequently, while the sputum (6/33; 18.2%), bronchoalveolar lavage fluid (BALF) (3/33; 9.1%), and pleural fluid (4/33; 12.1%) were obtained much less often. Bacteria and fungi were cultured from 25 (75.7%) respiratory tract samples. No significant numbers of microorganisms were found in 12.1% (4/33) of samples (negative result). Bacteria grown were considered specimen contamination in four (12.1%) cases. Fungi were cultured from 47.2% of samples. The species of detected microorganisms are presented in Table 8.
3.4.5. Urine Microbiological Testing
The urine for microbiological testing was obtained from 143 (35.9%) patients. No microorganisms were cultured from 62.2% of urine samples (negative result). The urine sample contamination requiring repeated test occurred in 9.1% of cases. Significant numbers of microorganisms indicating a positive test result were found in samples obtained from 41 (28.7%) patients. A total of 13 microbial species were cultured. Gram-negative rods (76%) were isolated most commonly. The species of detected microorganisms are presented in Table 8.
3.5. Characteristics of Mortality after ED Admission with Recognized Severe Pneumonia
Among patients with severe pneumonia who were admitted to the hospital, 64 people died. As many as 26 (40.6%) patients died during ≤7 days of hospitalization. One person died during the ED stay, and 23 people (23/64; 35.9%) died during therapy at the ICU, which was 51% of patients continuing their therapy at the ICU. Survival times were significantly shorter in patients transferred from the ED to the ICU as compared to those hospitalized at other units (p < 0.001). The mean age of those who died was 79 years with the median of 81 years, and it was not significantly different in subsequent years (mean age: 80.5 years; median: 81 years in 2019; mean age: 77.8 years; median: 81 years in 2020). Thirty-four men (mean age: 77.3 years; median: 74.5) and thirty women (mean age: 80.7; median: 84.5) died. The median survival of hospitalized patients was 9 and 12 days (range: 1 to 60 days; mean: 11.7 and 13.6) in 2019 and 2020, respectively. The following were regarded to be major causes of death by ICD-10 codes: cardiac arrest, cause unspecified (I46.9): 23, 35.9%; shock, unspecified (R57): 14, 21.9%; heart failure, unspecified (I50.9): 8, 12.5%; and respiratory arrest (R09.2): 7, 10.9%. The majority of those who died at the hospital were brought to the ED by the medical transport team (92.1%; p < 0.001).
It was noted that the most common symptoms at admission to the ED (dyspnoea, malaise, and fever/feeling of fever) were not reported by people who died or their families; therefore, they did not have any statistically significant effect on the prognosis as the incidence proportion of death. Similarly, the most common comorbidities (listed in Table 3) did not significantly affect the incidence proportion of death. The statistical analysis showed that only oncological history, solid organ transplantation, and mental disorders increased the risk of death as independent factors (Table 9).

Table 9.
The influence of symptoms and comorbidities on the mortality rate.
Patients received the antibiotic(s) in accordance with the applicable guidelines. Antimicrobial therapy was applied in 87.5% (56/64) of patients. It shows that the time of administration ≤ 3.20 h and ≤6 h from ED admission does not significantly affect the incidence proportion of death (p-values were 0.301 and 0.117, respectively). It has been demonstrated that the survival time was significantly longer when the antibiotic was administered no later than 12 h after patient admission (p = 0.043).
4. Discussion
The clinical presentation of pneumonia varies, ranging from mild pneumonia characterized by fever and productive cough to severe pneumonia that is characterized by respiratory distress and requires hospitalization [18,19]. There is a common view that clinical diagnosis of pneumonia does not present any problem if the symptoms are classic. In the presented study, nearly 66% of patients were aged more than 65 years. The symptoms of pneumonia in the elderly can be subtle, and fever is not a dominant symptom [20,21].
During the observation carried out, patients diagnosed with severe pneumonia most frequently reported the presence of dyspnoea (42.7%) and malaise (31.4%). Fever occurred in 20.6% of patients and cough in 7% of patients only. One symptom was reported by as many as 58% of patients, and two symptoms were present in 24.9% of subjects. In view of the above, subtle clinical symptoms of CAP should be proactively sought in very elderly people admitted to the ED due to unexplained falls, urinary incontinence, dementia, or sudden exacerbation of any comorbidity (e.g., diabetes mellitus, congestive heart failure, Parkinson’s disease) [20].
Pneumonia remains an important cause of death in the elderly population aged over 65 years. The older adults have higher rates of hospitalization and are more likely to die as a result of pneumonia [22,23]. The causes are complex and include the presence of multiple comorbidities, changes in basic lung physiology, changes in the immune system, and upper airway colonization with virulent organisms, which predisposes to the occurrence of silent aspiration/micro-aspiration of bacteria and fungi from the upper respiratory and digestive tracts [20,23,24].
According to the data published by the Central Statistical Office of Poland (GUS) with regard to the size and structure of the population in Poland between 1989 and 2019, the number of people aged over 65 years increased by 25.3% in the reported period [25].The median age of patients included in our study was 73 years, with people aged over 65 years accounting for over 65% of the cohort. This forces the adaptation of the national health care system to the changing Polish population, especially since pneumonia remains one of the most important causes of mortality in Poland [10].
Pneumonia is a leading cause of hospitalization. Approximately one million people hospitalized annually across the European Union require hospital treatment, and the need for hospitalization in adults increases with patient age [26].
During the 94-week observation at the ED, pneumonia was an indication for hospitalization in 5% of patients admitted to the hospital. Among adults aged ≥ 50 years, the rate of CAP-related hospitalizations in Poland in the period between 2007 and 2009 was 363.9/100,000 person-years. For comparison, in neighbouring countries of Poland, CAP-related hospitalization rates were 456.6/100,000 (Czech Republic) and 504.6/100,000 (Slovakia) in 2010 [27]. It has been demonstrated that the hospitalized rate among adults aged ≥ 65 years in the U.S. was 18.3% (between 847 and 3500 per 100,000) of community-acquired pneumonia cases and that adults aged over 90 years were more than five times more likely to be hospitalized than individuals aged 65–69 years in the pre-COVID-19 era [4,28].
In Poland (during the 7-year observation; 2009–2016), the number of community-acquired pneumonia cases varied between 5.5 and 6.6 occurrences per 100,000 population among patients aged ≥ 60 years [29].
In the study conducted by Kaplan and co-workers, the death rate among inpatients with confirmed community-acquired pneumonia was nearly 11%, and the mortality doubled with age: from 7.8% among patients aged 65–69 years to 15.4% among individuals aged 90 years and older [4]. It is not surprising that mortality rates in the elderly population with pneumonia are higher than in younger adults. In patients aged over 65 years, mortality rates ranged from 10% up to 30% in those managed at the ICU [4,5,6,22].
In our observation, the death rate among hospitalized patients was 16%; the mean age of those who died was 79 years (median: 81 years). The median ages of women and men who died were not statistically different; they were 84.5 and 74.5 years (p = 0.557); 36% of deaths occurred at the ICU. In the study conducted by Tichopad et al., the average fatality rate for all adults aged ≥ 50 years was 18.6% in Poland (2007–2010) and in the neighbouring countries, 21.7% (Czech Republic) and 20.9% (Slovakia) in 2010, and the incidence, fatality, and likelihood of hospitalization increased with advancing age [25].
The identification of individuals with severe infection requiring hospitalization or ICU admission is helpful in the stratification of patients. In many countries, the Pneumonia Severity Index (PSI), parameters included in the Severe Community-Acquired Pneumonia (SCAP), and CURB-65 are widely used and helpful [14,20]. The application of those criteria may have an impact on the ED waiting time of a patient and a decision regarding the place of further treatment. The avoidance of delays in consultation can affect patient flow and contribute to prolonged lengths of stay (LOS) at the ED, which in turn leads to unnecessary ED overcrowding. Moreover, a prolonged LOS at the ED increases the patient’s mortality [30,31,32].
In the conducted study, the median ED length of stay was 1 day and 21 h (the mean time was 4 days and 6 h). It is well-known that the ED LOS depends on the patient’s condition and specific sub-groups of the ED population but even more on the organization of care. An improvement in the consultation process can enhance the flow of patients and, as a result, shorten the ED LOS.
The most important factors that are associated with the hospitalization time of patients with pneumonia include predictors related to acute disease (abnormal blood results, clinical signs of severity, severity markers, development of complications) or comorbidities (alcohol consumption, dysphagia, chronic renal failure, neoplastic disease, urinary catheterization, secondary urinary tract infection) and other factors that focus on the social situation of the patient (assessment of the family caregiver’s involvement, active and early involvement of the family in the discharge process, effective communication with the family) [30].
According to Garau and co-workers, PSI high-risk classes (IV, V), positive blood culture results in microbiological testing, ICU admission, multilobar involvement, and alcohol consumption were associated independently with the prolonged LOS. In the study conducted by Garoua and co-workers (3233 patients with CAP, mean age: 66.6 ± 18.5 years; range: 18–100 years, admitted to Spanish university tertiary-care hospitals), the mean LOS was 11.5 days (range: 1–111 days), with the median LOS of 9 days (interquartile range: 7–14 days) [33].
The optimal hospital LOS is difficult to define and varies markedly among centres. In our study, the average hospitalization time was 15 days and 7 h (median: 12 days). In the multicentre study of 875 patients conducted by Suter-Widmer and co-workers, the mean LOS was 9.8 days. The authors revealed that older age, respiratory rate > 20 pm, nursing home residence, chronic pulmonary disease, diabetes mellitus, multilobar CAP, and pneumonia severity index class were independently associated with longer hospitalization [30].
In our observation, longer hospitalization time might be associated with the fact that we had nearly four times more patients with comorbidities. More than 65% of patients had at least one comorbidity versus 16% of patients with coexisting illnesses, as reported by Suter-Widmer et al., who analysed 875 patients with community-acquired pneumonia in Switzerland [30]. The LOS depended not only on the resolution of acute disease but also on organizational issues.
In the studied group of patients, the three most commonly co-occurring disorders included hypertension, renal and heart diseases, and diabetes mellitus. Almost 35% of the cohort had only one comorbid disease, while two comorbidities were present in 17% of patients with pneumonia. Sorino and co-workers found that chronic kidney disease and end-stage renal disease increase the risk of pneumonia [34]. Smoking, alcoholism, compromised immune status, chronic obstructive pulmonary disease (COPD), cardiovascular disease, cerebrovascular disease, chronic liver or renal disease, diabetes mellitus, and dementia have also been mentioned in research studies [7,35]. In the international multicentre observational point-prevalence study of adult patients hospitalized for CAP in 54 countries worldwide, which was conducted by Di Pasquale et al., up to 18% of hospitalized patients had at least one risk factor for immunosuppression. Risk factors for immunosuppression were independently associated neither with Pseudomonas aeruginosa nor with fungal infections and viral infections other than influenza [36].
Pneumonia can be caused by various microorganisms: bacteria, viruses, and fungi. Textbooks and elaborations still state that the most common bacterial aetiological factors include Streptococcus pneumoniae and type b Haemophilus influenzae (Hib) [8,14,29,37,38,39]. The aetiological factor of pneumonia is not always identified. The clinical picture and radiological examination do not allow pathogens to be identified. Microbiological testing is not always done. The IDSA/ATS recommendations for microbiological testing in adults with suspected community-acquired pneumonia without any innate and acquired immunity impairment are as follows:
- Routine microbiological testing of sputum is not recommended in outpatients;
- Lower respiratory tract samples for microbiological testing should be collected from inpatients:
- a.
- With severe pneumonia before antimicrobial therapy is started, who were treated for methicillin resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa infection,
- b.
- Previously treated for MRSA or P. aeruginosa infection, mostly due to respiratory tract infection, and
- c.
- Who were hospitalized or treated with parenteral antibiotics within the past 90 days [14,19].
According to the National Health Statistics Reports based on the follow-up at 94 hospitals in the U.S., microbiological testing was performed in 31.2% of patients at the ED and in 11.5% of CTs [40]. Biological material from the lower respiratory tract was obtained from 8.3% of patients, with blood (48.7%) and urine (35.9%) collected more often for microbiological testing. In the observation carried out, community-acquired pneumonia (CAP) could be suspected in the majority of patients; however, taking into account the characteristics of those patients (with transplant history, cancer and renal diseases, requiring haemodialysis, advanced age), it can be recognized that typical aetiological factors involving Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus infections are not necessarily dominant in this group. Bacteria and fungi were cultured from 25 (75.7%) respiratory tract samples. The presence of fungi (Candida spp. and Aspergillus spp.) was detected in 47.2% of samples. In the study conducted by Gajewska M. et al., who used national public statistical data from the National Institute of Public Health—National Institute of Hygiene to monitor 1,038,810 pneumonia hospitalisations in the period between 2009 and 2016 in Poland, the most commonly recorded cause of pneumonia according to the ICD-10 code was J18—pneumonia, organism unspecified (190.4–220.6/100,000). Bacterial pneumonia, not elsewhere classified (J15) and viral pneumonia, not elsewhere classified (J12) were recognised in 75.4–92.2/100,000 and 22.7–37.5/100,000 of the population, respectively [29]. In our observation, Hemophilus influenza was cultured from the respiratory tract of one patient only. Streptococcus pneumoniae was identified in blood samples obtained from two patients (negative result of the lower respiratory tract culture). We draw attention to the incidence of fungi cultured from the upper respiratory tract. Fungi (most commonly Candida spp., 44%) were considered to be the aetiological factor based on microbiological testing of material from the lower respiratory tract in almost 47% of patients. Invasive fungal infections most commonly affect immunosuppressed patients, who constituted a significant percentage of our cohort. Correct interpretation of the microbiological test result is difficult or even impossible sometimes, in particular if Candida spp. belonging to the microbiome of the upper respiratory and digestive tract mucosa are identified [41]. Pseudomonas aeruginosa (8.3%) and Klebsiella pneumoniae (8.3%) were relatively often identified. Those bacteria are the most common cause of hospital-acquired infections as well as aspiration pneumonia, which develops as a result of aspiration of contents from the upper respiratory or digestive tracts and occurs most frequently in the elderly with alcoholism or after endoscopic procedures, who were members of our study group. Because of the small number of microbiological tests conducted, the actual aetiological agents of pneumonia in our study are largely speculative. We believe that it is reasonable to have microbiological testing performed in order to thoroughly understand the profile of patients presenting to the ED of our hospital. Local microbiological epidemiology and drug-resistance profile are critical for successful empirical antibiotic therapy [42,43].
Lower respiratory tract infections are not only one of the most frequent reasons for consultation as part of the primary care and at the ED, but they are also a cause of high antimicrobial-prescription rate [37]. Empirical treatment depends on the aetiology of infection influenced by the following factors: comorbidity, baseline functional status, severity of pneumonia, previously received antimicrobial drugs, contact with the hospital system, or even place of residence [37]. Generally, therapy should be initiated as soon as possible.
In the conducted study, almost 92% of patients received an antimicrobial drug, with the vast majority receiving it during the ED stay. Antimicrobial drugs were prescribed in accordance with the national guidelines [12], which are in line with the ATS/IDSA Clinical Guideline on Community-Acquired Pneumonia [14]. In our study, patients received an antibiotic after an average of almost 42 h from ED admission (median time: almost 6 h). Nearly 70% of patients received an antimicrobial therapy within ≤ 1 h of being admitted to the ED. It can be assumed that the short time from reporting to the ED to the first dose of antibiotic is beneficial for patients with suspected community-acquired pneumonia and correlates with a favourable outcome. This correlation could not be clearly established even in studies involving large groups of patients. In a study of 13,771 patients (≥ 65 years of age), Houck at al. reported that administering an antibiotic within 4 h of ED arrival led to a 15% reduction in 30-day mortality [44]. Conversely, a study of 16,313 adults found that administering antibiotics within 4 h did not benefit short-term mortality [45]. Current treatment recommendations for patients with CAP do not assign a specific period for antibiotics administration [46].
It is important to collect samples for microbiological testing before administering anti-infective drugs.
This observational study is the first study conducted as part of the PEDRO project. It has been initiated so that emergency physicians can heuristically use the best available evidence in a local setting with pragmatic interventions to improve critical outcomes in patients with severe CAP.
Author Contributions
Conceptualization, D.K.; methodology, D.K. and A.M.; software, A.M. and D.K.; validation, D.K., J.C. and A.M.; formal analysis, D.K. and J.C.; investigation, D.K. and J.C.; resources, D.K. and J.C.; data curation, D.K. and J.C.; writing—original draft preparation, A.M.; writing—review and editing, D.K., A.M. and J.C.; visualization, A.M.; supervision, D.K.; project administration, D.K.; funding acquisition, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Warsaw.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Smith, M.; Feied, C. The Emergency Department as a Complex System; George Washington University School of Medicine and Health Sciences: Washington, DC, USA, 1999. [Google Scholar]
- Guidelines for Emergency Department Short Stay Units; State of Victoria, Department of Health and Human Services: Melbourne, Australia, 2017.
- Atamna, A.; Shiber, S.; Yassin, M.; Yassin, M.; Drescher, M.J.; Bishara, J. The accuracy of a diagnosis of pneumonia in the emergency department. Int. J. Infect. Dis. 2019, 89, 62–65. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, V.; Angus, D.; Clermont, D.C.; Clermont, G.; Watson, R.S.; Linde-Zwirble, W.T. Hospitalized community-acquired pneumonia in the elderly: Age-and sex-related patterns of care and outcome in the United States. Am. J. Respir. Crit. Care Med. 2002, 165, 766–772. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Yong, Y.; Tan, W.; Shen, L.; Ng, H.S.; Fong, K.Y. Prognostic factors for mortality due to pneumonia among adults from different age groups in Singapore and mortality predictions based on PSI and CURB-65. Singap. Med. J. 2018, 59, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Brito, V.; Niederman, M.S. Predicting mortality in the elderly with community-acquired pneumonia: Should we design a new car or set a new ‘speed limit’? Thorax 2010, 65, 944–945. [Google Scholar] [CrossRef] [Green Version]
- Hespanhol, V.; Bárbara, C. Pneumonia mortality, comorbidities matter? Pulmonology 2020, 26, 123–129. [Google Scholar] [CrossRef]
- Kawecki, D.; Majewska, A. Pneumonia in the Covid-19 era– emergency room physician’s perspective. Part I–etiology and epidemiology. Emerg. Med. Serv. 2021, 8, 32–38. [Google Scholar] [CrossRef]
- Hryniewicz, W.; Ładny, J.R.; Kubner, A.; Ozorowski, T. Postępowanie z Pacjentem z Podejrzeniem Ciężkiego Zakażenia w Szpitalnym Oddziale Ratunkowym (SOR); Narodowy Instytut Leków: Warszawa, Polska, 2014.
- Jabłońska, J.; Suchacz, M. Ostre zakażenia dolnych dróg oddechowych. In Choroby Zakaźne i Pasożytnicze; Boroń-Kaczmarska, A., Wiercińska-Drapało, A., Eds.; PZWL: Warszawa, Polska, 2017. [Google Scholar]
- Eurostat. Deaths from pneumonia in EU Regions. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191112-1 (accessed on 2 May 2022).
- American Thoracic Society. What is Pneumonia? Am. J. Respir. Crit. Care Med. 2016, 193, 1–2. [Google Scholar] [CrossRef]
- Hryniewicz, W.; Albrecht, P.; Radzikowski, A. Rekomendacje Postępowania w Pozaszpitalnych Zakażeniach Układu Oddechowego; Narodowy Instytut Leków: Warszawa, Polska, 2016.
- Hryniewicz, W.; Ozorowski, T.; Pawlik, K.; Stefaniuk, E. Wskazania do Wykonywania Badań Mikrobiologicznych u Pacjentów Hospitalizowanych; Narodowy Instytut Leków: Warszawa, Polska, 2015.
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and treatment of adults with Community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
- Central Statistical Office of Poland, 2022. Available online: https://stat.gov.pl/en/ (accessed on 2 May 2022).
- Rozporządzenie Ministra Zdrowia z dnia 30 czerwca 2021 r. w Sprawie Systemu Zarządzającego Trybami Obsługi Pacjenta w Szpitalnym Oddziale Ratunkowym. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20210001182 (accessed on 2 May 2022).
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Kierzkowska, M.; Markowska, K.; Majewska, A. Knowledge, attitude, and practice regarding Staphylococcus pettenkoferi. Infect. Dis. Rep. 2022, 14, 112–120. [Google Scholar] [CrossRef]
- Kawecki, D.; Majewska, A. Pneumonia in the COVID-19 era–emergency room physician’s perspective. Part II–diagnosis and therapy. Emerg. Med. Serv. 2021, 3, 179–189. [Google Scholar] [CrossRef]
- Elshamly, M.; Nour, M.O.; Omar, A.M.M. Clinical presentations and outcome of severe community-acquired pneumonia. Egypt J. Chest. Dis. Tuberc. 2016, 65, 831–839. [Google Scholar] [CrossRef] [Green Version]
- Janssens, J.P.; Krause, K.H. Pneumonia in the very old. Lancet Infect. Dis. 2004, 4, 112–124. [Google Scholar] [CrossRef]
- Cunha, B.A. Pneumonia in the elderly. Clin. Microbiol. Infect. 2001, 7, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Stupka, J.E.; Mortensen, E.M.; Anzueto, A.; Restrepo, M.I. Community-acquired pneumonia in elderly patients. Aging Health 2009, 5, 763–774. [Google Scholar] [CrossRef] [Green Version]
- Kothe, H.; Bauer, T.; Marre, R.; Suttorp, N.; Welte, T.; Dalhoff, K.; Competence Network for Community-Acquired Pneumonia Study Group. Outcome of community-acquired pneumonia: Influence of age, residence status and antimicrobial treatment. Eur. Respir J. 2008, 32, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Savvateeva, E.N.; Rubina, A.Y.; Gryadunov, D.A. Biomarkers of Community-Acquired Pneumonia: A Key to Disease Diagnosis and Management. BioMed Res. Int. 2019, 2019, 1701276. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.J.; Loddenkemper, R.; Sibille, Y.; Sibille, Y. Eurpoean Lung White Book. Acute Lower Respiratory Infections; European Respiratory Society: Sheffield, UK, 2013. [Google Scholar]
- Tichopad, A.; Roberts, C.; Gembula, I.; Hajek, P.; Skoczynska, A.; Hryniewicz, W.; Jahnz-Rozyk, K.; Prymula, R.; Solovič, I.; Kolek, V. Clinical and economic burden of community-acquired pneumonia among adults in the Czech Republic, Hungary, Poland and Slovakia. PLoS ONE 2013, 8, e71375. [Google Scholar] [CrossRef]
- McLaughlin, J.M.; Khan, F.L.; Thoburn, E.A.; Isturiz, R.E.; Swerdlow, D.L. Rates of hospitalization for community-acquired pneumonia among US adults: A systematic review. Vaccine 2020, 38, 741–751. [Google Scholar] [CrossRef]
- Gajewska, M.; Goryński, P.; Paradowska-Stankiewicz, I.; Lewtak, K.; Piotrowicz, M.; Urban, E.; Cianciara, D.; Wysocki, M.J.; Książek, A.; Izurieta, P. Monitoring of community-acquired pneumonia hospitalisations before the introduction of pneumococcal conjugate vaccine into Polish National Immunisation Programme (2009–2016): A nationwide retrospective database analysis. Vaccine 2020, 38, 194–201. [Google Scholar] [CrossRef]
- Suter-Widmer, I.; Christ-Crain, M.; Zimmerli, W.; Albrich, W.; Mueller, B.; Schuetz, P.; ProHOSP Study Group. Predictors for length of hospital stay in patients with community-acquired pneumonia: Results from a Swiss multicenter study. BMC Pulm. Med. 2012, 12, 21. [Google Scholar] [CrossRef] [Green Version]
- Han, S.B.; Kim, J.H.; Lee, Y.J.; Durey, A. Impact of changing the admission process of patients with pneumonia on the length of stay in the emergency department. Am. J. Emerg. Med. 2021, 41, 170–173. [Google Scholar] [CrossRef]
- Andersson, J.; Nordgren, L.; Cheng, I.; Nilsson, U.; Kurland, L. Long emergency department length of stay: A concept analysis. Int. Emerg. Nurs. 2020, 53, 100930. [Google Scholar] [CrossRef]
- Garau, J.; Baquero, F.; Pérez-Trallero, E.; Pérez, J.L.; Martín-Sánchez, A.M.; García-Rey, C.; Martín-Herrero, J.E.; Dal-Ré, R.; NACER Group. Factors impacting on length of stay and mortality of community-acquired pneumonia. Clin. Microbiol. Infect. 2008, 14, 322–329. [Google Scholar] [CrossRef] [Green Version]
- Sorino, C.; Scichilone, N.; Pedone, C.; Negri, S.; Visca, D.; Spanevello, A. When kidneys and lungs suffer together. J. Nephrol. 2019, 32, 699–707. [Google Scholar] [CrossRef]
- Torres, A.; Blasi, F.; Peetermans, W.E.; Viegi, G.; Welte, T. Risk factors for community-acquired pneumonia in adults in Europe: A literature review. Thorax 2013, 68, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Di Pasquale, M.F.; Sotgiu, G.; Gramegna, A.; Radovanovic, D.; Terraneo, S.; Reyes, L.F.; Rupp, J.; González Del Castillo, J.; Blasi, F.; Aliberti, S.A.; et al. Prevalence and Etiology of Community-acquired Pneumonia in Immunocompromised Patients. Clin. Infect. Dis. 2019, 68, 1482–1493. [Google Scholar] [CrossRef] [Green Version]
- Cantón, R.; Barberán, J.; Linares, M.; Molero, J.M.; Rodríguez-González-Moro, J.M.; Salavert, M.; González Del Castillo, J. Decalogue for the selection of oral antibiotics for lower respiratory tract infections. Rev. Esp. Quim. 2022, 35, 16–29. [Google Scholar] [CrossRef]
- Lauderdale, T.L.; Chang, F.Y.; Ben, R.J.; Yin, H.C.; Ni, Y.H.; Tsai, J.W.; Cheng, S.H.; Wang, J.T.; Liu, Y.C.; Cheng, Y.W.; et al. Etiology of community acquired pneumonia among adult patients requiring hospitalization in Taiwan. Respir. Med. 2005, 99, 1079–1086. [Google Scholar] [CrossRef] [Green Version]
- Wójkowska-Mach, J.; Gryglewska, B.; Romaniszyn, D.; Natkaniec, J.; Pobiega, M.; Adamski, P.; Grodzicki, T.; Kubicz, D.; Heczko, P.B. Age and other risk factors of pneumonia among residents of Polish long-term care facilities. Int. J. Infect. Dis. 2013, 17, e37–e43. [Google Scholar] [CrossRef] [Green Version]
- Williams, S.; Gousen, S.; DeFrances, C. National Hospital Care Survey Demonstration Projects: Pneumonia Inpatient Hospitalizations and Emergency Department Visits. Natl. Health Stat Rep. 2018, 116, 1–11. [Google Scholar]
- Meena, D.S.; Kumar, D. Candida Pneumonia: An Innocent Bystander or a Silent Killer? Med. Princ. Pract. 2022, 31, 98–102. [Google Scholar] [CrossRef]
- Aliberti, S.; Dela Cruz, C.S.; Amati, F.; Sotgiu, G.; Restrepo, M.I. Community-acquired pneumonia. Lancet 2021, 398, 906–919. [Google Scholar] [CrossRef]
- Houck, P.M.; Bratzler, D.W.; Nsa, W.; Ma, A.; Bartlett., J.G. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch. Intern. Med. 2004, 164, 637–644. [Google Scholar] [CrossRef]
- Rodrigo, C.; McKeever, T.M.; Woodhead, M.; Welham, S.; Lim, W.S.; British Thoracic Society. Admission via the emergency department in relation to mortality of adults hospitalised with community-acquired pneumonia: An analysis of the British Thoracic Society national community-acquired pneumonia audit. Emerg. Med. J. 2015, 32, 55–59. [Google Scholar] [CrossRef]
- Marti, C.; John, G.; Genné, D.; Prendki, V.; Rutschmann, O.T.; Stirnemann, J.; Garin, N. Time to antibiotics administration and outcome in community-acquired pneumonia: Secondary analysis of a randomized controlled trial. Eur. J. Intern. Med. 2017, 43, 58–61. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).