First Expert Elicitation of Knowledge on Drivers of Emergence of Bovine Besnoitiosis in Europe
Abstract
1. Introduction
2. Results
2.1. Response Rate and Field of Expertise Mobilised by the Experts
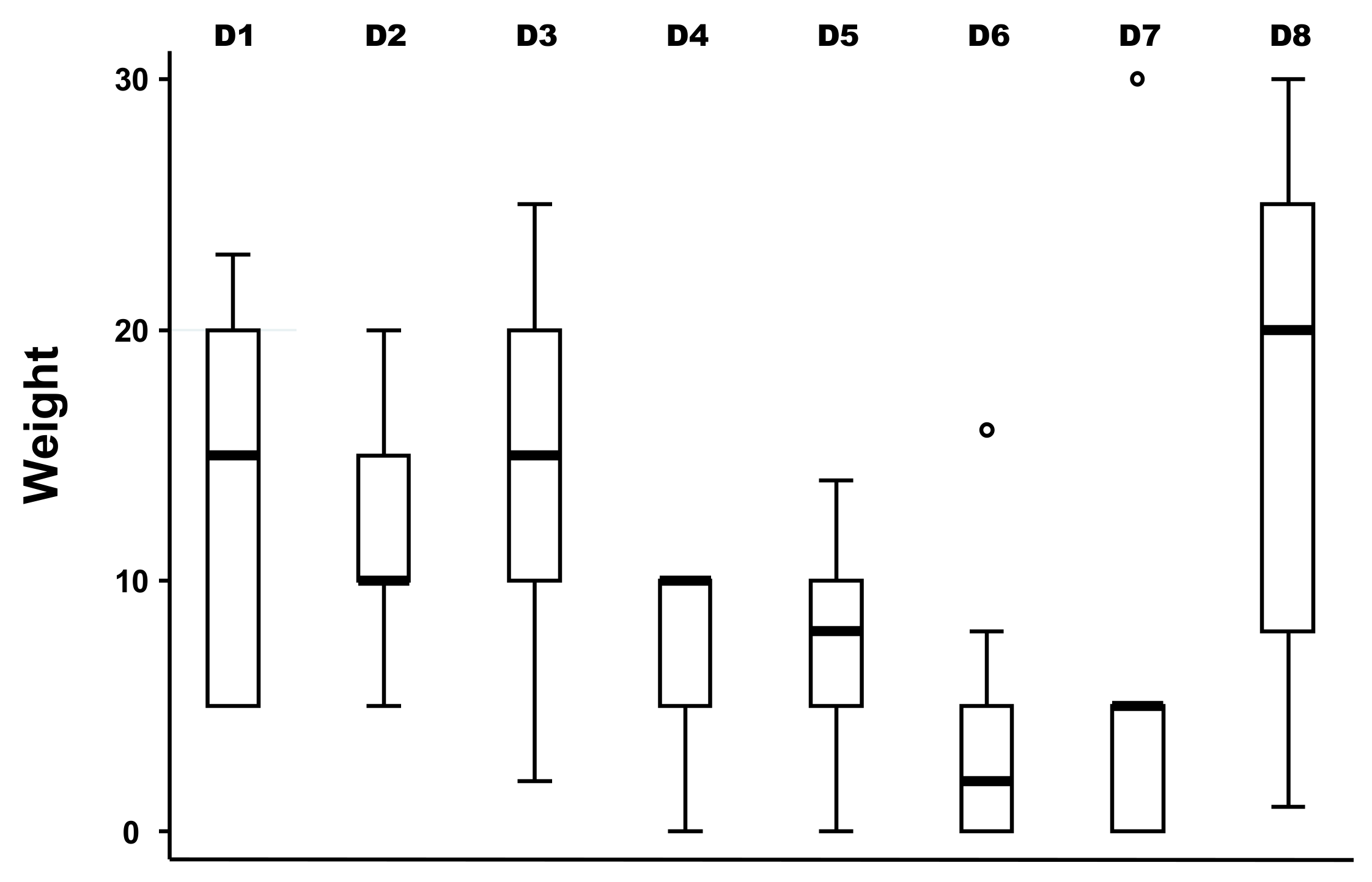
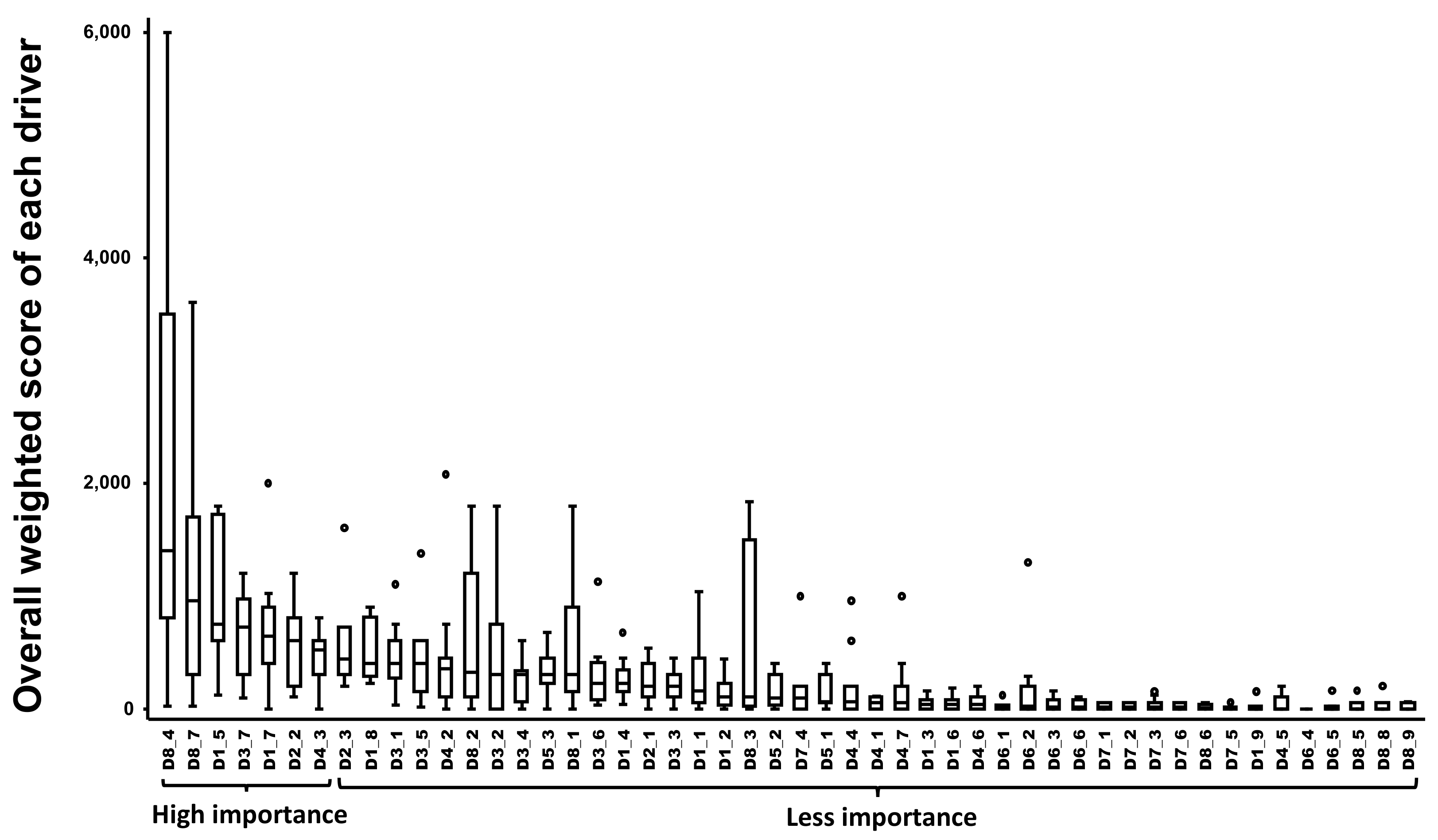
2.2. Estimating the Overall Weighted Score and Ranking of Drivers of Bovine Besnoitiosis in Cattle
2.3. Cluster Analysis
2.4. Sensitivity Analysis of the Impact of Experts on the Final Ranking of Bovine Besnoitiosis Top Drivers of Emergence in Cattle
3. Discussion
4. Materials and Methods
4.1. Species Included
4.2. Questionnaire Design
4.3. Expert Elicitation on Drivers Used to Assess the Emergence of Bovine Besnoitiosis in Europe
4.4. Scoring and Weighting System
4.5. Calculation of an Overall Weighted Score for Each Driver and Ranking Process
4.6. Cluster Analysis
4.7. Sensitivity Analysis to Test the Robustness of the Expert Elicitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Profile of Experts Involved in the Elicitation of Knowledge (N = 11)
| Last Name | First Name | Gender | Institution | Country | Field of Expertise |
|---|---|---|---|---|---|
| Álvarez-García | Gema | Female | Complutense University of Madrid | Spain | Animal health: Parasitology and parasitic diseases |
| Alzieu | Jean-Pierre | Male | Veterinary Laboratory of the department of Ariège | France | Animal parasitology |
| Delooz | Laurent | Male | Regional Association for Animal Registration and Health | Belgium | Animal disease epidemiology |
| Evrard | Julien | Male | Regional Association for Animal Registration and Health | Belgium | Animal disease project management |
| Gentile | Arcangelo | Male | Department of Veterinary Medical Sciences, University of Bologna | Italy | Bovine internal medicine |
| Houtain | Jean-Yves | Male | ARSIA | Belgium | Animal disease management |
| Jacquiet | Philippe | Male | Ecole Nationale Vétérinaire de Toulouse | France | Parasitology, parasitic diseases, applied zoology and tropical parasitology |
| Gazzonis | Alessia Libera | Female | Università degli Studi di Milano | Italy | Veterinary parasitology, parasitic diseases |
| Liénard | Emmanuel | Male | Ecole Nationale Vétérinaire de Toulouse | France | Parasitology, parasitic diseases and applied zoology |
| Schares | Gedeon | Male | Friedrich-Loeffler-Institut | Germany | Animal health |
| Villa | Luca | Male | Università degli Studi di Milano | Italy | Veterinary parasitology, parasitic diseases |
Appendix B. Domains with Each Defined Driver and Their Respective Defined Scores
| Domain D1. Disease/Pathogen Characteristics | ||
|---|---|---|
| D1_1 | Current Knowledge of the Pathogen. | |
| Score 0 | ||
| Score 1 | Very high: deep scientific knowledge on the pathogen, extensive scientific literature available on its biology (transmission mode, knowledge on vector(s), infectivity, etc.) | |
| Score 2 | High: detailed scientific knowledge on the pathogen but conflicting scientific results; some elements of the pathogen’s biology are still not elucidated | |
| Score 3 | Moderate: limited scientific knowledge on the pathogen agent because it is still under characterization; pathogen recently discovered/isolated but belonging to a well-known and studied family of pathogens; the pathogen is characterized by multiple variants not characterized yet | |
| Score 4 | Low: lack of scientific knowledge on the pathogen (multiplication, infectivity, incubation period, transmission mode, etc.); pathogen agent recently discovered and emerging | |
| D1_2 | The current species specificity of the causing agent of the disease | |
| Score 0 | ||
| Score 1 | Low: Only one host is involved belonging to the same family. e.g., only cattle, small ruminants, swine | |
| Score 2 | Medium: two species involved | |
| Score 3 | High: three species involved | |
| Score 4 | Very high: affects more than 3 types of families | |
| D1_3 | Genetic variability of the infectious agent | |
| Score 0 | Negligible: The infectious agent is genetically stable | |
| Score 1 | Low: The genetic variability is low therefore it has a low effect in the (re)emergence of the pathogen | |
| Score 2 | Medium: The pathogen can be considered with a medium genetic variability | |
| Score 3 | High: The pathogen is considered with a high genetic variability | |
| Score 4 | Very high: Very high genetic instability (e.g., high mutation rate, re-assortment and recombination). Potentially the three phenomena can characterise the pathogen’s evolution | |
| D1_4 | Transmission of the agent in relation of the possible spread of the epidemic or pandemic (i.e. ease/speed of spread) | |
| Score 0 | ||
| Score 1 | Low: Low and slow transmission within groups of animals. Between a group of animals only if an infected animal is introduced, close contact | |
| Score 2 | Medium: Medium ease/speed transmission within the group of animals and between groups of animals | |
| Score 3 | High: Fast transmission within a group of animals. In a short period of time all animals of the group are infected. Adjacent groups become infected fast | |
| Score 4 | Very High: Very fast and high transmission within the groups of animals and between groups of animals. A complete area is infected in a very short period of time | |
| D1_5 | Risk of showing no clinical signs and silent spread during infection and post infection | |
| Score 0 | Null: Silent spread is not part of the pathogen’s characteristics | |
| Score 1 | Low: Very short incubation period and signs of infections easily detected/recognised. | |
| Score 2 | Moderate: Very short incubation period and signs of infection are NOT easily detected/recognised | |
| Score 3 | Medium: Long incubation period, clinical signs are not characteristics and therefore specific diagnosis is necessary to detect infection. | |
| Score 4 | Very high: Long incubation period. Disease/infection shows not clinical symptoms during the infectious period. Chronic shedder | |
| D1_6 | Wildlife reservoir and potential spread from it | |
| Score 0 | Null: no known wildlife reservoir. Disease has never been reported in wildlife species | |
| Score 1 | Low: few clinical cases have been reported in wildlife and no transmission to livestock has ever been documented | |
| Score 2 | Moderate: wildlife is a reservoir of the disease but only accidental spillovers to livestock have been reported | |
| Score 3 | High: wildlife is a reservoir of the pathogen/disease but certain environmental conditions (e.g. floods, farms crossing the farmland-bush division, etc) have to occur for the pathogen/disease to (re)emerge in livestock | |
| Score 4 | Very high: Disease establishes itself in wildlife as a reservoir and very hard to eradicate it from wildlife. Livestock easily gets infected with the contact with wildlife | |
| D1_7 | Existence of vectors (vertebrate and invertebrate, e.g., mosquitoes, bats, rodents, ticks, midges, culicoides) and potential spread | |
| Score 0 | Null: No known vector | |
| Score 1 | Low: only one type of vector is present in the country but it’s role in the transmission is presumed low (has not been assessed to date) | |
| Score 2 | Moderate: only one type of vector exists in the country and has only been suspected as source and spread of disease | |
| Score 3 | High: only one competent vector is present and can carry and spread the disease | |
| Score 4 | Very high: more than one type of vector can carry and spread the disease and are found spread in most of the territory | |
| D1_8 | Transmission of the pathogen | |
| Score 0 | ||
| Score 1 | Low: Animals only are infected by direct close contact with other infected animals and vertical transmission | |
| Score 2 | Moderate: transmission by direct and indirect contact only (e.g., through vehicles, clothes, instruments) or non-flying vector (e.g., ticks) | |
| Score 3 | High: Exclusively vector transmission by flying vectors (e.g., culicoides, mosquitoes) | |
| Score 4 | Very high: more than three modes of transmission and/or airborne transmission | |
| D1_9 | Environmental persistence | |
| Score 0 | Null: pathogen does not survive in the environment | |
| Score 1 | Low: only anecdotal isolation of the pathogen from the environment has been recorded | |
| Score 2 | Moderate: The survival of the agent in the environment is limited (only temporary) and it’s dependent on certain environmental conditions such as humidity, temperature, rainfall, etc. | |
| Score 3 | High: The survival of the agent in the environment is limited (only temporary) and NOT dependent on certain environmental conditions such as humidity, temperature, rainfall, etc. | |
| Score 4 | Very high: agent naturally surviving in the environment (soil, water) and organic materials were it has a long term-survival | |
| Number of drivers = 9, hence 90 points to be distributed within this domain for the intra-domain weighing | ||
| Domain D2. Distance to Europe | ||
| D2_1 | Current incidence (cases)/prevalence of the disease in the world | |
| Score 0 | ||
| Score 1 | Pathogen has been reported only in the countries of the Australasia (Australia, New Zealand, New Guinea and Neighbouring Pacific Islands) region | |
| Score 2 | Disease was reported in countries of the Americas, Caribbean and Asia (excluding the Russian Federation) | |
| Score 3 | Disease was reported/present in the African continent | |
| Score 4 | Disease was reported in countries of the Mediterranean Basin, Middle East and the Russian Federation | |
| D2_2 | European geographic proximity of the pathogen/disease to Europe | |
| Score 0 | ||
| Score 1 | Disease has never been present in Europe | |
| Score 2 | Disease has been reported in Europe in the past but is currently exotic. | |
| Score 3 | Disease is currently present in at least one European country which is NOT bordering your country | |
| Score 4 | Diseases is currently present in at least one of the countries bordering your country | |
| D2_3 | To your knowledge when was the disease last reported in Europe | |
| Score 0 | More than 20 years ago | |
| Score 1 | More than 10 years ago | |
| Score 2 | More than 5 years ago | |
| Score 3 | More than 1 year ago | |
| Score 4 | Currently present in Europe | |
| Number of drivers = 3, hence 30 points to be distributed within this domain for the intra-domain weighing | ||
| Domain D3. Ability to Monitor, Treat and Control the Disease | ||
| D3_1 | Ability of preventive/control measures to stop the disease from entering the country or spreading (containment of the epidemic/pandemic), excluding treatment, vaccination and vector(s)/reservoir(s) control | |
| Score 0 | ||
| Score 1 | Very High: Sanitary certificate; effective traceability of animals and by-products; effective disinfection measures; no contact between domestic and wild animals; effective biosecurity measures | |
| Score 2 | High: No sanitary certificate; effective traceability of animals and by-products; effective disinfection measures; limited or incomplete possibilities to restrict contacts between domestic and wild animals; effective biosecurity measures | |
| Score 3 | Low: No sanitary certificate; incomplete traceability of animals and by-products; ineffective disinfection measures; incomplete restriction of contacts between domestic and wild animals; ineffective biosecurity measures | |
| Score 4 | Very low: No sanitary certificate; no traceability of animals and by-products; ineffective disinfection measures; impossibility to restrict contact between farms or between domestic and wild animals; biosecurity measures totally ineffective | |
| D3_2 | Vaccine availability | |
| Score 0 | ||
| Score 1 | Very high: Commercialized vaccine available on a global scale (worldwide) | |
| Score 2 | High:Local/mono-species vaccine available at a regional/national scale and/or for a targeted species (not systematically available for a global fight plan) | |
| Score 3 | Low:Experimental vaccine, not commercialized to date; severe adverse reaction when applied; limited protector effect | |
| Score 4 | Very low:Absence; no vaccine available on the market for a use in the species considered in the study, no experimental vaccine either | |
| D3_3 | Control of reservoir(s) and/or vector(s) | |
| Score 0 | Null: No vector-borne transmission and/or no reservoir(s) known to date | |
| Score 1 | Very high: Effective. Limited reservoir(s) with limited geographical repartition, easy-to-identify; high scientific knowledge on vector(s)/reservoir(s); effective fighting measures | |
| Score 2 | High: Limited reservoir(s)/vector(s) with limited geographical repartition; easy-to-identify, high scientific knowledge on vector(s)/reservoir(s); effective fighting measures but not applicable at a large scale; limited fighting measures | |
| Score 3 | Low: Numerous reservoirs vectors identified with limited geographical repartition; hard to identify. Lack of scientific knowledge on vector(s)/reservoir(s). Fighting measures are poorly effective—resistances and/or negative impact on environment; | |
| Score 4 | Very low: Numerous Vector(s)/reservoir(s)identified with wide geographic distribution; hard to identify, absence of scientific knowledge on vector(s)/reservoir(s); no effective fighting measure against vector(s) (no active molecule, resistance to measures applied) | |
| D3_4 | Availability and quality of diagnostic tools in your country | |
| Score 0 | ||
| Score 1 | Very High: Field test(s) available and easy to use, with highly discriminating sensitivity and specificity | |
| Score 2 | High: Tests used in local/regional laboratories by not in the field | |
| Score 3 | Low: tests only used in specialized laboratories/national reference laboratory | |
| Score 4 | Very Low: no diagnostic tools available to date | |
| D3_5 | Disease is currently under surveillance overseas (OIE, EU) | |
| Score 0 | ||
| Score 1 | Very high: Generalized surveillance implemented by all EU Member States and worldwide surveillance (i.e. OIE reported) | |
| Score 2 | High: Surveillance of the pathogen only EU member states | |
| Score 3 | Low: Surveillance only in some EU member states (because they had cases of the disease) and only in some non-EU countries (not a disease reported in any international organisations) | |
| Score 4 | Very low:Absence of surveillance of the pathogen in all EU member countries and worldwide | |
| D3_6 | Eradication experience in other countries and/or your country | |
| Score 0 | ||
| Score 1 | Very high: Previous experience on eradication has been applied, fast and successfully | |
| Score 2 | High: Previous experience on eradicating the disease but with some setbacks in the process | |
| Score 3 | Low: Knowledge on eradication procedures but have never had to implement an eradication program in your country | |
| Score 4 | Very low: It is a novel disease, first time countries are faced with a new disease to eradicate | |
| D3_7 | Detection of emergence—e.g., difficulties for the farmer/veterinarian to declare the disease or clinical signs not so evident | |
| Score 0 | ||
| Score 1 | Very high: Disease is easily detected with clinically signs and farmers are aware of the disease and willing to notify it as soon as possible it | |
| Score 2 | High: Disease is easily detected by the clinical signs but farmers don’t have sufficient knowledge/awareness nor interest to notify it | |
| Score 3 | Moderate: Disease is not as easily detect by the clinical signs and farmers don’t have sufficient knowledge/awareness nor interest to notify | |
| Score 4 | Low: The infected animal does not show any pathognomonic clinical sign(s); farmer is reluctant to declare/notify any abnormality | |
| Number of drivers = 7, hence 70 points to be distributed within this domain for the intra-domain weighing | ||
| DOMAIN D4. Farm/European characteristics. | ||
| D4_1 | Mono species farms—One single farmed animal (e.g., only bovines) or multi species farms (farms with more than one species e.g., goats and bovines in the same farm/land/premises) | |
| Score 0 | ||
| Score 1 | Negligible: the type of farm does not influence in any form (re)emergence of the disease among the livestock population | |
| Score 2 | Low: mono or multi species farm has a low effect on the risk of disease to emerge or re-emerge | |
| Score 3 | Moderate: the type or types of farmed animals has a moderate effect on the emergence of the disease in your country | |
| Score 4 | High: the type of farmed animals has a high influence for the disease to emerge and spread in your country | |
| D4_2 | Farm demography/management: such as type of dairy or beef (cattle) production. For pigs—reproduction, fattening, finishing farm or both | |
| Score 0 | ||
| Score 1 | Negligible: population demography does not influence in any form the (re)emergence of the disease among the livestock population | |
| Score 2 | Low: the demographic population of the farm is a low influencing factor for disease (re)emergence. e.g., Disease only clinically affects only one age strata (i.e.) new-borns, therefore adults are immune to it | |
| Score 3 | Moderate: the demographic of the population has a moderate effect on the (re)emergence of the disease, as it can (re)emerge in more than one type of demography but other conditioning factors have to occur in conjunction | |
| Score 4 | High: the type of demographic of the farm has a high effect on the (re)emergence of the disease as it can (re)emerge in different types of farmed animals and all types of age groups | |
| D4_3 | Animal density of farms. Extensive (small holders with a few animals) versus intensive farming | |
| Score 0 | ||
| Score 1 | Negligible: animal farm density is not a risk factor for the disease to emerge in your country | |
| Score 2 | Low: farm density (extensive or intensive) of animals has a low effect on the pathogen’s/disease (re)emergence | |
| Score 3 | Moderate: farm density of animals in the farm (extensive v/s intensive) has a moderate effect on the emergence of pathogen/disease | |
| Score 4 | High: farm density of animals has a high effect on the (re)emergence of pathogen/disease | |
| D4_4 | Feeding practices of farms | |
| Score 0 | ||
| Score 1 | Negligible: Feeding practices have a negligible effect on the (re)emergence of the pathogen/disease | |
| Score 2 | Low: Feeding practices have a low effect on the (re)emergence of the pathogen/disease | |
| Score 3 | Moderate: Feeding practices have a moderate effect on the (re)emergence of the pathogen/disease | |
| Score 4 | High: Feeding practices have a high effect on the (re)emergence of the pathogen/disease | |
| D4_5 | Human movements among premises—Veterinarians or farm staff | |
| Score 0 | ||
| Score 1 | Negligible: disease is spread by other means | |
| Score 2 | Low: movement of human staff has a low effect on the introduction or spread of the disease | |
| Score 3 | Moderate: movement of human staff has a moderate effect on the introduction or spread of the disease | |
| Score 4 | High: movement of human staff has a high effect on the introduction or spread of the disease | |
| D4_6 | Proximity of livestock farm to wildlife and wildlife reservoirs of disease e.g., contact with wild or feral birds and animals which have been scavenging on landfill sites that contain contaminated animal products | |
| Score 0 | ||
| Score 1 | Negligible: Disease (re)emergence from wildlife and wildlife reservoir never reported | |
| Score 2 | Low: Disease (re)emergence from wildlife and wildlife reservoir rarely reported | |
| Score 3 | Moderate: Disease (re)emergence from wildlife and wildlife reservoir is documented regularly | |
| Score 4 | High: wildlife is a reservoir for the disease and the main source of infection for livestock | |
| D4_7 | Changes of land use, e.g., field fragmentation, creation of barriers, landfill sites | |
| Score 0 | ||
| Score 1 | Negligible: Changes in land use have a negligible effect on the (re)emergence of pathogen/disease | |
| Score 2 | Low: changes in land use have a low effect on the (re)emergence of the disease/pathogen but need other factors (e.g., land use changes combined with higher winter temperatures) | |
| Score 3 | Moderate: land use changes increases the availability of vectors or increases the pathogen’s survival. Also empty land can create a suitable environment for certain wildlife carrying the disease (e.g., migratory birds) | |
| Score 4 | High: land use changes are one of the main drivers for pathogen or its vectors | |
| Number of drivers = 7, hence 70 points to be distributed within this domain for the intra-domain weighing | ||
| Domain D5. Changes in Climatic Conditions | ||
| D5_1 | Influence of annual rainfall in the survival and transmission of the pathogen/disease | |
| Score 0 | ||
| Score 1 | Negligible: Pathogen survival and mode of transmission of the disease are not influenced by increased rainfall | |
| Score 2 | Low: pathogen survival and mode of transmission of the disease are slightly influenced by increased rainfall | |
| Score 3 | Moderate: pathogen survival and mode of transmission of the disease are moderately influenced by increased rainfall | |
| Score 4 | High: pathogen survival and mode of transmission of the disease are highly influenced by increased rainfall | |
| D5_2 | Influence of annual humidity in the survival and transmission of the pathogen/disease | |
| Score 0 | ||
| Score 1 | Negligible: Pathogen survival and mode of transmission of the disease are not influenced by increased humidity | |
| Score 2 | Low: pathogen survival and mode of transmission of the disease are slightly influenced by increased humidity | |
| Score 3 | Moderate: pathogen survival and mode of transmission of the disease are moderately influenced by increased humidity | |
| Score 4 | High: pathogen survival and mode of transmission of the disease are highly influenced by increased humidity | |
| D5_3 | Influence of annual temperature in the survival and transmission of the pathogen/disease | |
| Score 0 | ||
| Score 1 | Negligible: Pathogen survival and mode of transmission of the disease are not influenced by increased temperature | |
| Score 2 | Low: pathogen survival and mode of transmission of the disease are slightly influenced by increased temperature | |
| Score 3 | Moderate: pathogen survival and mode of transmission of the disease are moderately influenced by increased temperature | |
| Score 4 | High: pathogen survival and mode of transmission of the disease are highly influenced by increased temperature | |
| Number of drivers = 3, hence 30 points to be distributed within this domain for the intra-domain weighing | ||
| Domain D6. Wildlife Interface | ||
| D6_1 | Potential roles of zoo’s in the (re)emergence of the pathogen | |
| Score 0 | ||
| Score 1 | Negligible: The disease can be present in zoo animals, but it is not known to have been transmitted from zoo animals to livestock | |
| Score 2 | Low: The disease can enter a zoo (e.g., with introduction of an infected exotic animal) but only accidental transmissions of the disease from zoo animals to livestock have been reported. Hence, zoos have a low effect on the (re)emergence of the disease in livestock of your country | |
| Score 3 | Moderate: The disease can enter a zoo and be present in zoo animals but it needs a vector (biological/mechanical) for its transmission into livestock. Therefore, zoos have a moderate effect on the (re)emergence of the disease your country | |
| Score 4 | High: Disease can be introduced to a zoo via an infected imported animal, zoo animals can carry the disease that can easily jump to livestock animals | |
| D6_2 | The rural(farm)-wildlife interface | |
| Score 0 | ||
| Score 1 | Negligible: the disease has never (re)emerged from the narrowing of the farm-wild interface | |
| Score 2 | Low: the disease has a low probability to (re)emerge via the livestock farm-forest interface. The disease has been known to (re)emerge from the wild bush but very rarely | |
| Score 3 | Moderate: the disease has a moderate probability of (re)emergence via the farm/wildlife interface. Barriers ( natural or artificial) are needed to keep the disease/pathogen (re)emerging in livestock | |
| Score 4 | High: there is a high probability for the disease to (re)emerge via the farm/forest interface. Barriers (natural or artificial) separating farms from natural forests are ineffective | |
| D6_3 | Increase of autochthons (indigenous animal) wild mammals in Europe and neighbouring countries | |
| Score 0 | Not applicable: disease has not been reported in wildlife | |
| Score 1 | Negligible: the increase the autochthonous mammals population does not affect the risk of the diseases to (re)emergence | |
| Score 2 | Low: The slight increase of autochthonous mammals can slightly increase the probably of the disease emerging | |
| Score 3 | Moderate: The increase of wild mammals has been associated with the re-emergence of the disease | |
| Score 4 | High: The increase of wild mammals is the only factor associated with outbreaks of the disease in livestock | |
| D6_4 | Increase in endemic/migrating populations of wild birds | |
| Score 0 | Not applicable: Wild/migrating birds are not a reservoir of the disease | |
| Score 1 | Negligible: there is a negligible probability of disease (re)emerging in livestock because of an increase in populations of endemic/migrating wild birds. | |
| Score 2 | Low: there is a low probability of the disease (re)emerging and spreading through increased populations of endemic/migrating wild birds. Disease has spread from the endemic/migrating wild birds but only accidentally or under exceptional circumstances | |
| Score 3 | Moderate: there is a moderate probability of disease being introduced and spread through increased populations of endemic/migrating wild birds. They are hosts and in close contact with domestic livestock (i.e., poultry farms) may spread the disease | |
| Score 4 | High: there is a high probability for a disease to (re)emerge through increased populations of wild/migrating birds. These are hosts or reservoirs of the disease | |
| D6_5 | Hunting Activities: hunted animals can be brought back to where livestock is present | |
| Score 0 | ||
| Score 1 | Negligible: The risk of the disease/pathogen of (re)emerging in livestock due to hunting activities is practically null | |
| Score 2 | Low: disease is present in hunted wildlife and birds and only accidental cases have been reported in livestock that have (re)emerged because of hunting. The risk of the disease/pathogen of (re)emerging in livestock due to hunting activities is practically null | |
| Score 3 | Moderate: disease is present in hunted wildlife and birds but a certain control is established by the hunter | |
| Score 4 | High: disease is present in hunted wildlife and birds and hunting is one of the main modes of transmission of the disease to livestock | |
| D6_6 | Transboundary movements of terrestrial wildlife from other countries | |
| Score 0 | Not applicable: Disease is not carried by terrestrial wildlife | |
| Score 1 | Negligible: (re)emergence of the disease by terrestrial movements of wildlife has only been suspected but never confirmed | |
| Score 2 | Low: There is a low probability for the disease to (re)emerge and spread through transboundary movements of terrestrial wildlife | |
| Score 3 | Moderate: There is a moderate probability for the disease to (re)emerge and spread through transboundary movements of terrestrial wildlife | |
| Score 4 | High: There is a high probability for the disease to (re)emerge and spread through transboundary movements of terrestrial wildlife. These are host and may spread/carry the disease along | |
| Number of drivers = 6, hence 60 points to be distributed within this domain for the intra-domain weighing | ||
| Domain D7. Human Activities | ||
| D7_1 | In- and out- people movements linked to tourism | |
| Score 0 | ||
| Score 1 | Negligible: the movement of tourism is a negligible driver on the emergence or re-emergence of the disease | |
| Score 2 | Low: tourism increase has a low driver of the (re)emergence of the disease | |
| Score 3 | Moderate: tourism increase has a moderate driver for the (re)emergence of the disease. Biosecurity measures are enough to stop the entering of the pathogen | |
| Score 4 | High: tourist movement is a high driver on the (re)emergence of a disease. Tourists are highly likely to bring the disease into your country in their belongings and biosecurity measures are insufficient to stop the pathogen | |
| D7_2 | Human Immigration | |
| Score 0 | ||
| Score 1 | Negligible: the immigration movements are a negligible driver of the disease (re)emergence in your country | |
| Score 2 | Low: the immigration movements are a low driver of the disease (re)emergence in your country | |
| Score 3 | Moderate: the disease is currently present in countries where more immigrants come from and pathogen highly likely to enter through, clothes, shoes and or possession, but the current biosecurity measures in place are able to prevent the emergence of the disease in your country | |
| Score 4 | High: the immigration movement has a high effect as a driver on the emergence or re-emergence of disease in your country. Disease is highly likely to emerge using this route as biosecurity measures are not enough to avoid emergence of the disease | |
| D7_3 | Transport movements: more specifically commercial flights, commercial transport by ships, cars or military (excluding transport vehicles of live animals) | |
| Score 0 | ||
| Score 1 | Negligible: the role of commercial movements as a driver on the (re)emergence of the disease in your country is negligible | |
| Score 2 | Low: the role of commercial movements as a driver on the (re)emergence of the disease in your country is low. It is easily preventable by implementing biosecurity measures | |
| Score 3 | Moderate: the role of commercial movements as a driver on the (re)emergence of a disease in your country is moderate. Disease can be prevented if biosecurity measures are tightened | |
| Score 4 | High: the role of commercial movements as a driver on the (re)emergence of a disease in your country is high. Disease is hard to control via the current biosecurity measures | |
| D7_4 | Transport vehicles of live animals | |
| Score 0 | ||
| Score 1 | Negligible: the role of transport vehicles of live animals as a driver for the (re)emergence of the disease in your country is negligible | |
| Score 2 | Low: the role of transport vehicles of live animals as a driver for the (re)emergence of the disease in your country is low | |
| Score 3 | Moderate: the role of transport vehicles of live animals as a driver for (re)emergence of the disease in your country is moderate | |
| Score 4 | High: the role of transport vehicles of live animals as a driver for (re)emergence of the disease in your country is high | |
| D7_5 | Bioterrorism potential | |
| Score 0 | ||
| Score 1 | Negligible: the role of bioterrorism as a driver for a disease to (re)emerge is negligible: agent is available but difficult to handle or has a low potential of spread or generates few economic consequences | |
| Score 2 | Low: the role of bioterrorism as a driver for a disease to (re)emerge is low: agent is available and easy to handle by professionals and labs but has a low spread | |
| Score 3 | Moderate: the role of bioterrorism as a driver for a disease to (re)emerge is moderate: agent available and easy to handle by professionals and labs and rapidly spreads | |
| Score 4 | High: the role of bioterrorism as a driver for a disease to (re)emerge is high: Agent is available and easy to handle by individuals and rapidly spreads | |
| D7_6 | Inadvertent release of an exotic infectious agent from a containment facility e.g., Laboratory | |
| Score 0 | ||
| Score 1 | Negligible: the pathogen is not currently present in any laboratory | |
| Score 2 | Low: the pathogen is present in a containment facility but its release is very unlikely as it is very easily contained | |
| Score 3 | Moderate: the pathogen is present in a containment facility and its release can occur as not easily contained | |
| Score 4 | High: pathogen is handled in a risk 3 or 4 laboratory (BSL3 or BSL4) in the country. It can leave the facility if the correct biosecurity measures are not implemented correctly and easily spread to livestock | |
| Number of drivers = 6, hence 60 points to be distributed within this domain for the intra-domain weighing | ||
| Domain D8. Economic and Trade Activities | ||
| D8_1 | Decrease of resources allocated to the disease surveillance | |
| Score 0 | ||
| Score 1 | Negligible: resources allocated to the disease surveillance have no effect on the (re)emergence of the disease in your country Disease has never been under surveillance | |
| Score 2 | Low: resources allocated to the disease surveillance have a low effect on the (re)emergence of the disease in your country Disease has been under surveillance in the past and no change has happened after surveillance has been stopped | |
| Score 3 | Medium: resources allocated to the disease surveillance have a moderate effect on the (re)emergence of the disease in your country Disease is under passive surveillance (reported only when observed) but with no need to further increase its surveillance | |
| Score 4 | High: resources allocated to the disease surveillance have a high effect on the (re)emergence of the disease in your country Disease needs to be under active and passive surveillance as its (re)emergence can easily occur, therefore if its surveillance decreases it’s highly likely to (re)emerge | |
| D8_2 | Modification of the disease status (i.e., reportable disease becoming not reportable) or change in screening frequency due to a reduced national budget | |
| Score 0 | ||
| Score 1 | Negligible: modification of the disease status due to a reduced national budget has a negligible effect on the (re) emergence of the disease in your country | |
| Score 2 | Low: modification of the disease status due to a reduced national budget has a low effect on the (re) emergence of the disease in your country | |
| Score 3 | Moderate: modification of the disease status due to a reduced national budget has a moderate effect on the (re) emergence of the disease in your country | |
| Score 4 | High: modification of the disease status due to a reduced national budget has a high effect on the (re) emergence of the disease in your country | |
| D8_3 | Decrease of resources allocated to the implementation of biosecurity measures at border controls (e.g., harbors or airports) | |
| Score 0 | ||
| Score 1 | Negligible: decreasing the resources allocated to the implementation of biosecurity measures has a negligible effect on the (re)emergence of the disease in your country. Disease has never been detected in the past in a harbor or airport | |
| Score 2 | Low: decreasing the resources allocated to the implementation of biosecurity measures has a low effect on the (re)emergence of the disease in your country. The disease has been suspected to have entered other countries because of deficient biosecurity at border controls | |
| Score 3 | Medium: decreasing the resources allocated to the implementation of biosecurity measures has a moderate effect on the (re)emergence of the disease in your country. The disease has been introduced in other countries because of deficient biosecurity at border controls | |
| Score 4 | High: decreasing the resources allocated to the implementation of biosecurity measures highly increases the risk of (re)emergence of the disease in your country. In the past, the disease has been introduced in other countries and in your country because of deficient biosecurity at border controls | |
| D8_4 | Most likely influence of (il)legal movements of live animals (livestock, pets, horses etc) from neighbouring/European Union member states (MS) for the disease to (re)emerge in your country | |
| Score 0 | ||
| Score 1 | Negligible: (il)legal movements of live animals (livestock, pets, horses etc) from neighbouring/European Union MS have a negligible influence on the pathogen/disease (re)emergence in your country | |
| Score 2 | Low: (il)legal movements (livestock, pets, horses etc) from neighbouring/European Union MS have a low influence on the pathogen/disease (re)emergence in your country | |
| Score 3 | Moderate: (il)legal movements (livestock, pets, horses etc) from neighbouring/European Union MS have a moderate influence on the pathogen/disease (re)emergence in your country | |
| Score 4 | High: (il)legal movements (livestock, pets, horses etc.) from neighbouring/European Union MS have a high influence on the pathogen/disease (re)emergence in your country | |
| D8_5 | Most likely influence of (il)legal movements of pets from Third countries for the disease to (re)emerge in Europe | |
| Score 0 | ||
| Score 1 | Negligible: increased (il)legal imports of animal subproducts such as skin, meat and edible products from EU member states have a negligible influence on the pathogen/disease (re)emergence in your country | |
| Score 2 | Low: increased (il)legal imports of animal subproducts such as skin, meat and edible products from EU member states have a low influence on the pathogen/disease (re)emergence in your country | |
| Score 3 | Moderate: increased (il)legal imports of animal subproducts such as skin, meat and edible products from EU member states have a moderate influence on the pathogen/disease (re)emergence in your country | |
| Score 4 | High: increased (il)legal imports of animal subproducts such as skin, meat and edible products from EU member states have a high influence on the pathogen/disease (re)emergence in your country | |
| D8_6 | Most likely influence of increased (il)legal imports of non-animal products such as tires, wood, furniture from EU member states for the disease/pathogen to (re)emerge in your country | |
| Score 0 | ||
| Score 1 | Negligible: (il)legal movements of live animals (livestock, pets, horses etc) from Third countries have a negligible influence on the pathogen/disease (re)emergence in your country | |
| Score 2 | Low: (il)legal movements of live animals (livestock, pets, horses etc) from Third countries have a low influence on the pathogen/disease (re)emergence in your country | |
| Score 3 | Moderate: (il)legal movements of live animals (livestock, pets, horses etc) from Third countries have a moderate influence on the pathogen/disease (re)emergence in your country | |
| Score 4 | High: (il)legal movements of live animals (livestock, pets, horses etc) from Third countries have a high influence on the pathogen/disease (re)emergence in your country. | |
| D8_7 | Most likely influence of (il)legal movements of live animals (livestock, pets, horses etc) from Third countries for the disease to (re)emerge in your country | |
| Score 0 | ||
| Score 1 | Negligible: (il)legal movements of live animals (livestock, pets, horses etc) from Third countries have a negligible influence on the pathogen/disease (re)emergence in your country | |
| Score 2 | Low: (il)legal movements of live animals (livestock, pets, horses etc) from Third countries have a low influence on the pathogen/disease (re)emergence in your country | |
| Score 3 | Moderate: (il)legal movements of live animals (livestock, pets, horses etc) from Third countries have a moderate influence on the pathogen/disease (re)emergence in your country | |
| Score 4 | High: (il)legal movements of live animals (livestock, pets, horses etc) from Third countries have a high influence on the pathogen/disease (re)emergence in your country | |
| D8_8 | Most likely influence of increased imports of animal sub-products such as skin, meat and edible products from Third countries, for the disease to (re)emerge in your country | |
| Score 0 | ||
| Score 1 | Negligible: Increased imports of animal subproducts such as skin, meat and edible products from Third countries have a negligible influence on the pathogen/disease (re)emergence in your country | |
| Score 2 | Low: Increased imports of animal subproducts such as skin, meat and edible products from Third countries have a low influence on the pathogen/disease (re)emergence in your country | |
| Score 3 | Moderate: Increased imports of animal subproducts such as skin, meat and edible products from Third countries have a moderate influence on the pathogen/disease (re)emergence in your country | |
| Score 4 | High: Increased imports of animal subproducts such as skin, meat and edible products from Third countries have a high influence on the pathogen/disease (re)emergence in your country | |
| D8_9 | Most likely influence of increased (il)legal imports of non-animal products such as tires, wood, furniture from Third countries, for the disease to (re)emerge in your country | |
| Score 0 | ||
| Score 1 | Negligible: increased (il)legal imports of non-animal products such as tires, wood, furniture from Third countries have a negligible influence on the pathogen/disease (re)emergence in your country | |
| Score 2 | Low: increased (il)legal imports of non-animal products such as tires, wood, furniture from Third countries have a low influence on the pathogen/disease (re)emergence in your country | |
| Score 3 | Moderate: increased (il)legal imports of non-animal products such as tires, wood, furniture from Third countries have a moderate influence on the pathogen/disease (re)emergence in your country | |
| Score 4 | High: increased (il)legal imports of non-animal products such as tires, wood, furniture from Third countries have a high influence on the pathogen/disease (re)emergence in your country | |
| Number of drivers = 9, hence 90 points to be distributed within this domain for the intra-domain weighing | ||
Appendix C

- Disease/pathogen characteristics: 9 criteria
- Distance to Europe (spatial-temporal scales): 3 criteria
- Ability to monitor, treat and control the disease: 7 criteria
- Farm/European characteristic: 7 criteria
- Climatic conditions: 3 criteria
- Wildlife interface: 6 criteria
- Human activities: 6 criteria
- Economy and trade activities: 9 criteria
- Intra-category weighing
- 1)
- Score and balance each driver within each category of drivers:
- ■
- Please give a score according to what you estimate is the importance of each driver in the (re)-emergence of specific disease(s).
- ■
- After the scoring, please balance each driver for each category of drivers. Balancing the criteria will rely on the distribution of points between the different proposed criteria under each category. The total number of points to be distributed among the drivers is specified for each category (each spreadsheet). e.g., category pathogen characteristics total 90 points; distance of outbreaks (spatial-temporal scales) a total of 30 points to be distributed.
- 2)
- Intra-category weighing: The last step of the process will consist in the distribution of 80 points between the 8 categories of criteria (Pathogen characteristics, distance of outbreaks, etc.). This is on the 9th spreadsheet. The distribution will depend on which is believed to be the strongest category of drivers.
References
- Grisez, C.; Bottari, L.; Prévot, F.; Alzieu, J.-P.; Liénard, E.; Corbière, F.; Rameil, M.; Desclaux, X.; Lacz, C.; Boulon, C.; et al. Real-time PCR on skin biopsies for super-spreaders’ detection in bovine besnoitiosis. Parasites Vectors 2020, 13, 529. [Google Scholar] [CrossRef]
- Watt, B. Bovine Besnoitiosis, an Emerging Disease. Available online: https://www.ava.com.au/library-resources/library/ava-scientific-journals/acv/2013/bovine-bsnoitiosis-an-emerging-disease/Issue%2068%20Watt.pdf (accessed on 13 December 2021).
- Dubey, J.; Sreekumar, C.; Donovan, T.; Rozmanec, M.; Rosenthal, B.; Vianna, M.; Davis, W.; Belden, J. Redescription of Besnoitia bennetti (Protozoa: Apicomplexa) from the donkey (Equus asinus). Int. J. Parasitol. 2005, 35, 659–672. [Google Scholar] [CrossRef] [PubMed]
- McCully, R.M.; Basson, P.A.; van Niekerk, J.W.; Bigalke, R.D. Observations on Besnoitia cysts in the cardiovascular system of some wild antelopes and domestic cattle. Onderstepoort J. Vet. Res. 1966, 33, 245–276. [Google Scholar]
- Arnal, M.C.; Gutiérrez-Expósito, D.; Martínez-Durán, D.; Regidor-Cerrillo, J.; Revilla, M.; de Luco, D.F.; Jiménez-Meléndez, A.; Ortega-Mora, L.M.; Álvarez-García, G. Systemic Besnoitiosis in a Juvenile Roe Deer (Capreolus capreolus). Transbound. Emerg. Dis. 2016, 64, e8–e14. [Google Scholar] [CrossRef] [PubMed]
- Cortes, H.; Leitao, A.; Vidal, R.; Vila-Viçosa, M.J.; Ferreira, M.L.; Caeiro, V.; Hjerpe, C.A. Besnoitiosis in bulls in Portugal. Vet. Rec. 2005, 157, 262–264. [Google Scholar] [CrossRef] [PubMed]
- González-Barrio, D.; Köster, P.C.; Habela, M.A.; Martín-Pérez, M.; Fernández-García, J.L.; Balseiro, A.; Barral, M.; Nájera, F.; Figueiredo, A.M.; Palacios, M.J.; et al. Molecular survey of Besnoitia spp. (Apicomplexa) in faeces from European wild mesocarnivores in Spain. Transbound. Emerg. Dis. 2021, 68, 3156–3166. [Google Scholar] [CrossRef] [PubMed]
- Alzieu, J.P.; Cortes, H.; Gottstein, B.; Jacquiet, P.; Dorchies, P.; Schelcher, F.; L’hostis, M. La besnoitiose bovine: Actualités épidémiologiques et diagnostiques. Bull. G.T.V. Hors Série Parasitol. Bov. Nouv. Approch. 2007, 40, 41–49. [Google Scholar]
- Liénard, E.; Salem, A.; Grisez, C.; Prévot, F.; Bergeaud, J.; Franc, M.; Gottstein, B.; Alzieu, J.; Lagalisse, Y.; Jacquiet, P. A longitudinal study of Besnoitia besnoiti infections and seasonal abundance of Stomoxys calcitrans in a dairy cattle farm of southwest France. Vet. Parasitol. 2010, 177, 20–27. [Google Scholar] [CrossRef]
- Liénard, E.; Salem, A.; Jacquiet, P.; Grisez, C.; Prevot, F.; Blanchard, B.; Bouhsira, E.; Franc, M. Development of a protocol testing the ability of Stomoxys calcitrans (Linnaeus, 1758) (Diptera: Muscidae) to transmit Besnoitia besnoiti (Henry, 1913) (Apicomplexa: Sarcocystidae). Parasitol. Res. 2013, 112, 479–486. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bigalke, R.D. New concepts on the epidemiological features of bovine besnoitiosis as determined by laboratory and field investigations. Onderstepoort J. Vet. Res. 1968, 35, 3–137. [Google Scholar]
- Sharif, S.; Jacquiet, P.; Prévot, F.; Grisez, C.; Bouhsira, E.; Franc, M.; Liénard, E. Assessment of persistence of Besnoitia besnoiti (Henry, 1913) bradyzoites in Stomoxys calcitrans (Diptera: Muscidae). Rev. Med. Vet. 2017, 168, 197–203. [Google Scholar]
- Alzieu, J.P.; Jacquiet, P.; Boulon, C.; Méjan, F.; Desclaux, X.; Prévot, F.; Franc, M.; Rameil, M.; Grisez, C.; Malaveille, R.; et al. La besnoitiose bovine: Actualités physio-pathogéniques, cliniques et épidémiologiques. Bull. Des. GTV 2016, 84, 67–78. [Google Scholar]
- Gutierrez-Exposito, D.; Ferre, I.; Ortega-Mora, L.-M.; Álvarez-García, G. Advances in the diagnosis of bovine besnoitiosis: Current options and applications for control. Int. J. Parasitol. 2017, 47, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-García, G.; García-Lunar, P.; Gutiérrez-Expósito, D.; Shkap, V.; Ortega-Mora, L.M. Dynamics of Besnoitia besnoiti infection in cattle. Parasitology 2014, 141, 1419–1435. [Google Scholar] [CrossRef]
- Villa, L.; Gazzonis, A.L.; Zanzani, S.A.; Perlotti, C.; Sironi, G.; Manfredi, M.T. Bovine besnoitiosis in an endemically infected dairy cattle herd in Italy: Serological and clinical observations, risk factors, and effects on reproductive and productive performances. Parasitol. Res. 2019, 118, 3459–3468. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific statement on Bovine besnoitiosis. EFSA J. 2010, 8, 15. [Google Scholar]
- Salem, A.; Bouhsira, E.; Liénard, E.; Bousquet-Mélou, A.; Jacquiet, P.; Franc, M. Susceptibility of two European strains of Stomoxys calcitrans (L.) to cypermethrin, deltamethrin, fenvalerate, lambda-cyhalothrin, permethrin and phoxim. J. Appl. Res. Vet. Med. Vet. Solut. 2012, 10, 249–257. [Google Scholar]
- Tainchum, K.; Shukri, S.; Duvallet, G.; Etienne, L.; Jacquiet, P. Phenotypic susceptibility to pyrethroids and organophosphate of wild Stomoxys calcitrans (Diptera: Muscidae) populations in southwestern France. Parasitol. Res. 2018, 117, 4027–4032. [Google Scholar] [CrossRef] [PubMed]
- Mullens, B.A. Chapter 16: Horse flies and deer flies (Tabanidae). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Academic Press: London, UK, 2019; pp. 327–343. [Google Scholar] [CrossRef]
- Duvallet, G.; Boireau, P. Other vector-borne parasitic diseases: Animal helminthiases, bovine besnoitiosis and malaria. Rev. Sci. Tech. Off. Int. Epiz. 2015, 34, 651–658. [Google Scholar]
- Álvarez-García, G.; Frey, C.; Mora, L.M.O.; Schares, G.R.M. A century of bovine besnoitiosis: An unknown disease re-emerging in Europe. Trends Parasitol. 2013, 29, 407–415. [Google Scholar] [CrossRef]
- Mutinelli, F.; Schiavon, E.; Ceglie, L.; Fasolato, M.; Natale, A.; Rampin, F.; Carminato, A. Bovine besnoitiosis in imported cattle in Italy. Vet. Parasitol. 2010, 178, 198. [Google Scholar] [CrossRef] [PubMed]
- Gentile, A.; Militerno, G.; Schares, G.R.M.; Nanni, A.; Testoni, S.; Bassi, P.; Gollnick, N. Evidence for bovine besnoitiosis being endemic in Italy—First in vitro isolation of Besnoitia besnoiti from cattle born in Italy. Vet. Parasitol. 2012, 184, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Anastácio, C.; Bexiga, R.; Nolasco, S.; Zúquete, S.; Delgado, I.L.S.; Nunes, T.; Leitão, A. Impact of Endemic Besnoitiosis on the Performance of a Dairy Cattle Herd. Animals 2022, 12, 1291. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, H.; Klimpel, S.; Schein, E.; Heydorn, A.O.; Al-Quraishy, S.; Selmair, J. Another African disease in Central Europa: Besnoitiosis of cattle. I. Light and electron microscopical study. Parasitol. Res. 2008, 104, 861–868. [Google Scholar] [CrossRef]
- Lesser, M.; Braun, U.; Deplazes, P.; Gottstein, B.; Hilbe, M.; Basso, W. First cases of besnoitiosis in cattle in Switzerland. Schweiz. Arch. Tierheilkd. 2012, 154, 469–474. [Google Scholar] [CrossRef]
- Cortes, H.; Leitão, A.; Gottstein, B.; Hemphill, A. A review on bovine besnoitiosis: A disease with economic impact in herd health management, caused by Besnoitia besnoiti (Franco and Borges). Parasitology 2014, 141, 1406–1417. [Google Scholar] [CrossRef]
- Hornok, S.; Fedák, A.; Baska, F.; Hofmann-Lehmann, R.; Basso, W. Bovine besnoitiosis emerging in Central-Eastern Europe, Hungary. Parasites Vectors 2014, 7, 20. [Google Scholar] [CrossRef][Green Version]
- Ryan, E.G.; Lee, A.; Carty, C.; O’Shaughnessy, J.; Kelly, P.; Cassidy, J.P.; Sheehan, M.; Johnson, A.; de Waal, T. Bovine besnoitiosis (Besnoitia besnoiti) in an Irish dairy herd. Vet. Rec. 2016, 178, 608. [Google Scholar] [CrossRef]
- Vanhoudt, A.; Pardon, B.; De Schutter, P.; Bosseler, L.; Sarre, C.; Vercruysse, J.; Deprez, P. First confirmed case of bovine besnoitiosis in an imported bull in Belgium. Vlaams Diergeneeskd. Tijdschr. 2015, 84, 205–211. [Google Scholar] [CrossRef]
- Delooz, L.; Evrard, J.; Mpouam, S.E.; Saegerman, C. Emergence of Besnoitia besnoiti in Belgium. Pathogens 2021, 10, 1529. [Google Scholar] [CrossRef]
- Bianchini, J.; Humblet, M.; Cargnel, M.; Van Der Stede, Y.; Koenen, F.; De Clercq, K.; Saegerman, C. Prioritization of livestock transboundary diseases in Belgium using a multicriteria decision analysis tool based on drivers of emergence. Transbound. Emerg. Dis. 2019, 67, 344–376. [Google Scholar] [CrossRef]
- Saegerman, C.; Bianchini, J.; Snoeck, C.J.; Moreno, A.; Chiapponi, C.; Zohari, S.; Ducatez, M.F. First expert elicitation of knowledge on drivers of emergence of influenza D in Europe. Transbound. Emerg. Dis. 2020, 68, 3349–3359. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.; Sánchez, J.; Revie, C.W. Multi-Criteria Decision Analysis Tools for Prioritising Emerging or Re-Emerging Infectious Diseases Associated with Climate Change in Canada. PLoS ONE 2013, 8, e68338. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Best Practices in Ranking Emerging Infectious Disease Threats. A Literature Review. European Centre for Disease Prevention and Control. 2015. Available online: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/emerging-infectious-disease-threats-best-practices-ranking.pdf (accessed on 1 June 2022).
- Avila, L.N.; Gonçalves, V.S.P.; Perez, A.M. Risk of Introduction of Bovine Tuberculosis (TB) Into TB-Free Herds in Southern Bahia, Brazil, Associated with Movement of Live Cattle. Front. Vet. Sci. 2018, 5, 230. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Alcrudo, D.; Falco, J.R.; Raizman, E.; Dietze, K. Transboundary spread of pig diseases: The role of international trade and travel. BMC Vet. Res. 2019, 15, 64. [Google Scholar] [CrossRef]
- Saegerman, C.; Bertagnoli, S.; Meyer, G.; Ganière, J.-P.; Caufour, P.; De Clercq, K.; Jacquiet, P.; Fournié, G.; Hautefeuille, C.; Etore, F.; et al. Risk of introduction of lumpy skin disease in France by the import of vectors in animal trucks. PLoS ONE 2018, 13, e0198506. [Google Scholar] [CrossRef]
- Saegerman, C.; Bertagnoli, S.; Meyer, G.; Ganière, J.-P.; Caufour, P.; De Clercq, K.; Jacquiet, P.; Hautefeuille, C.; Etore, F.; Casal, J.; et al. Risk of introduction of Lumpy Skin Disease into France through imports of cattle. Transbound. Emerg. Dis. 2019, 66, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, N.S.; Scharr, J.C.; Schares, S.; Bärwald, A.; Langenmayer, M.C. Naturally acquired bovine besnoitiosis: Disease frequency, risk and outcome in an endemically infected beef herd. Transbound. Emerg. Dis. 2018, 65, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.B. Stable Fly (Stomoxys calcitrans, Muscidae). Encycl. Infect. Immun. 2022, 2, 903–913. [Google Scholar] [CrossRef]
- Frey, C.; Gutiérrez-Expósito, D.; Ortega-Mora, L.; Benavides, J.; Marcén, J.; Castillo, J.; Casasús, I.; Sanz, A.; García-Lunar, P.; Esteban-Gil, A.; et al. Chronic bovine besnoitiosis: Intra-organ parasite distribution, parasite loads and parasite-associated lesions in subclinical cases. Vet. Parasitol. 2013, 197, 95–103. [Google Scholar] [CrossRef]
- Jacquiet, P.; Liénard, E.; Franc, M. Bovine besnoitiosis: Epidemiological and clinical aspects. Vet. Parasitol. 2010, 174, 30–36. [Google Scholar] [CrossRef] [PubMed]
- García-Lunar, P.; Ortega-Mora, L.-M.; Schares, G.; Gollnick, N.S.; Jacquiet, P.; Grisez, C.; Prevot, F.; Frey, C.F.; Gottstein, B.; Álvarez-García, G. An Inter-Laboratory Comparative Study of Serological Tools Employed in the Diagnosis of Besnoitia besnoiti Infection in Bovines. Transbound. Emerg. Dis. 2012, 60, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Schares, G.; Maksimov, A.; Basso, W.; Moré, G.; Dubey, J.; Rosenthal, B.; Majzoub, M.; Rostaher, A.; Selmair, J.; Langenmayer, M.; et al. Quantitative real time polymerase chain reaction assays for the sensitive detection of Besnoitia besnoiti infection in cattle. Vet. Parasitol. 2011, 178, 208–216. [Google Scholar] [CrossRef] [PubMed]
- OIE. Terrestrial Animal Health Code. Criteria for the Inclusion of Diseases, Infections and Infestations in the OIE list. Chapter 1.2. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahc/2018/en_chapitre_criteria_diseases.htm#chapitre_criteria_diseases (accessed on 12 December 2021).
- Waap, H.; Nunes, T.; Cortes, H.; Leitão, A.; Vaz, Y. Prevalence and geographic distribution of Besnoitia besnoiti infection in cattle herds in Portugal. Parasitol. Res. 2014, 113, 3703–3711. [Google Scholar] [CrossRef] [PubMed]
- Waap, H.; Nunes, T.; Vaz, Y.; Leitão, A. Serological survey of Toxoplasma gondii and Besnoitia besnoiti in a wildlife conservation area in southern Portugal. Vet. Parasitol. Reg. Stud. Rep. 2016, 3–4, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Exposito, D.; Arnal, M.C.; Martínez-Durán, D.; Cerrillo, J.R.; Revilla, M.; de Luco, D.L.F.; Jiménez-Meléndez, A.; Bernal, R.C.; Habela, M.A.; García-Bocanegra, I.; et al. The role of wild ruminants as reservoirs of Besnoitia besnoiti infection in cattle. Vet. Parasitol. 2016, 223, 7–13. [Google Scholar] [CrossRef]
- Humblet, M.-F.; Losson, B.; Saegerman, C. Integrated Management of Blood-Feeding Arthropods in Veterinary Teaching Facilities—Part 1: Overview of Haematophagous Arthropods of Interest in North-Western Europe. Rev. Sci. Tech. 2020, 39, 737–756. [Google Scholar] [CrossRef]
- Humblet, M.-F.; Losson, B.; Saegerman, C. Integrated Management of Blood-Feeding Arthropods in Veterinary Teaching Facilities—Part 2: Overview of Control Methods against Adults and Immature Stages. Rev. Sci. Tech. 2020, 39, 757–777. [Google Scholar] [CrossRef]
- Humblet, M.-F.; Losson, B.; Saegerman, C. Integrated Management of Blood-Feeding Arthropods in Veterinary Teaching Facilities—Part 3: Proposal for a Coherent and Affordable Control Plan. Rev. Sci. Tech. 2020, 39, 779–793. [Google Scholar] [CrossRef]
- Gore, S.M. Biostatistics and the Medical Research Council. MRC News 1987, 35, 19–20. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saegerman, C.; Evrard, J.; Houtain, J.-Y.; Alzieu, J.-P.; Bianchini, J.; Mpouam, S.E.; Schares, G.; Liénard, E.; Jacquiet, P.; Villa, L.; et al. First Expert Elicitation of Knowledge on Drivers of Emergence of Bovine Besnoitiosis in Europe. Pathogens 2022, 11, 753. https://doi.org/10.3390/pathogens11070753
Saegerman C, Evrard J, Houtain J-Y, Alzieu J-P, Bianchini J, Mpouam SE, Schares G, Liénard E, Jacquiet P, Villa L, et al. First Expert Elicitation of Knowledge on Drivers of Emergence of Bovine Besnoitiosis in Europe. Pathogens. 2022; 11(7):753. https://doi.org/10.3390/pathogens11070753
Chicago/Turabian StyleSaegerman, Claude, Julien Evrard, Jean-Yves Houtain, Jean-Pierre Alzieu, Juana Bianchini, Serge Eugène Mpouam, Gereon Schares, Emmanuel Liénard, Philippe Jacquiet, Luca Villa, and et al. 2022. "First Expert Elicitation of Knowledge on Drivers of Emergence of Bovine Besnoitiosis in Europe" Pathogens 11, no. 7: 753. https://doi.org/10.3390/pathogens11070753
APA StyleSaegerman, C., Evrard, J., Houtain, J.-Y., Alzieu, J.-P., Bianchini, J., Mpouam, S. E., Schares, G., Liénard, E., Jacquiet, P., Villa, L., Álvarez-García, G., Gazzonis, A. L., Gentile, A., & Delooz, L. (2022). First Expert Elicitation of Knowledge on Drivers of Emergence of Bovine Besnoitiosis in Europe. Pathogens, 11(7), 753. https://doi.org/10.3390/pathogens11070753










