Practical Validation of United States Centers for Disease Control and Prevention Assays for the Detection of Human Respiratory Syncytial Virus in Pediatric Inpatients in Japan
Abstract
:1. Introduction
2. Results
2.1. Assay Conditions
2.2. Evaluation of the US-CDC Assays
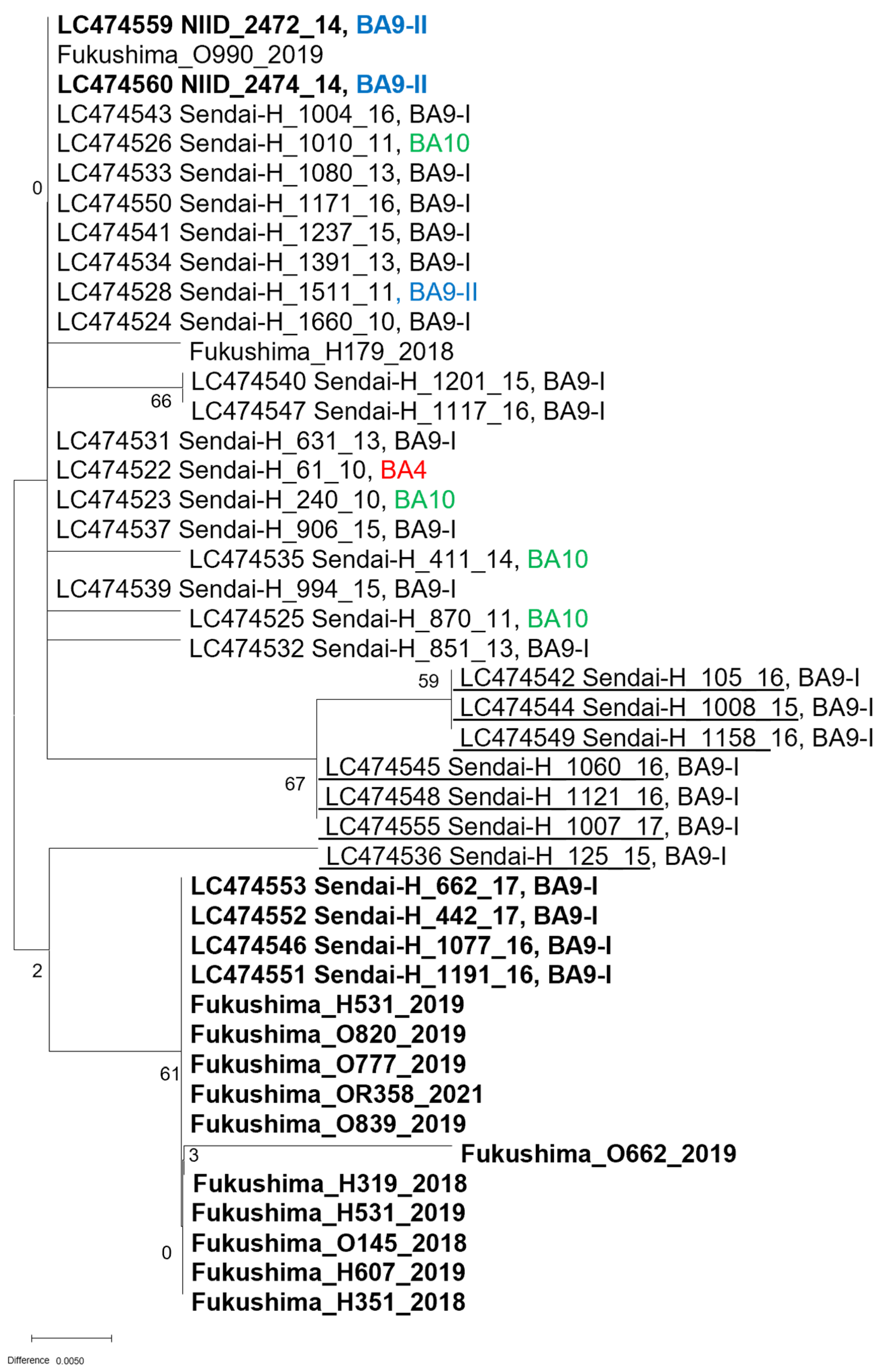
2.3. Sequencing of the Pan-RSV Assay Target Region
2.4. Analysis of Read Coverage of the RSV Isolates
3. Discussion
4. Materials and Methods
4.1. Clinical Specimens
4.2. Real-Time RT-PCR for Detection of RSV
4.3. Evaluation of Primer/Probe Mismatches with the Pan-RSV and Duplex Assays
4.4. Sequencing Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chin, J.; Magoffin, R.L.; Shearer, L.A.; Schieble, J.H.; Lennette, E.H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am. J. Epidemiol. 1969, 89, 449–463. [Google Scholar] [CrossRef]
- Alwan, W.H.; Kozlowska, W.J.; Openshaw, P.J. Distinct types of lung disease caused by functional subsets of antiviral T cells. J. Exp. Med. 1994, 179, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Bradding, P.; Walls, A.F.; Holgate, S.T. The role of the mast cell in the pathophysiology of asthma. J. Allergy Clin. Immunol. 2006, 117, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Global Influenza Programme (GIP), WHO Informal Consultation on Surveillance of RSV on the Global Influenza Surveillance and Response System (GISRS) Platform, Meeting Report. Available online: https://www.who.int/publications/i/item/who-informal-consultation-on-surveillance-of-rsv-on-the-global-influenza-surveillance-and-response-system-(gisrs)-platform (accessed on 27 January 2022).
- Hirve, S.; Crawford, N.; Palekar, R.; Zhang, W. Clinical characteristics, predictors, and performance of case definition-Interim results from the WHO global respiratory syncytial virus surveillance pilot. Influenza Other Respir. Viruses 2020, 14, 647–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. WHO Surveillance Case Definitions for ILI and SARI. Available online: https://www.who.int/teams/global-influenza-programme/surveillance-and-monitoring/case-definitions-for-ili-and-sari (accessed on 22 April 2022).
- Fry, A.M.; Chittaganpitch, M.; Baggett, H.C.; Peret, T.C.; Dare, R.K.; Sawatwong, P.; Thamthitiwat, S.; Areerat, P.; Sanasuttipun, W.; Fischer, J.; et al. The burden of hospitalized lower respiratory tract infection due to respiratory syncytial virus in rural Thailand. PLoS ONE 2010, 5, e15098. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Piedra, P.A.; Avadhanula, V.; Durigon, E.L.; Machablishvili, A.; Lopez, M.R.; Thornburg, N.J.; Peret, T.C.T. Duplex real-time RT-PCR assay for detection and subgroup-specific identification of human respiratory syncytial virus. J. Virol. Methods 2019, 271, 113676. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Information for the Molecular Detection of Influenza Viruses. Available online: https://cdn.who.int/media/docs/default-source/influenza/molecular-detention-of-influenza-viruses/protocols_influenza_virus_detection_feb_2021.pdf?sfvrsn=df7d268a_5 (accessed on 28 April 2022).
- Shirato, K.; Nao, N.; Kawase, M.; Kageyama, T. An Ultra-Rapid Real-Time RT-PCR Method Using PCR1100 for Detecting Human Orthopneumovirus. Jpn. J. Infect. Dis. 2020, 73, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Kitai, Y.; Sato, K.; Shirato, K.; Ohmiya, S.; Watanabe, O.; Kisu, T.; Ota, R.; Takeda, M.; Kawakami, K.; Nishimura, H. Variation in Thermal Stability among Respiratory Syncytial Virus Clinical Isolates under Non-freezing Conditions. Viruses 2022, 14, 679. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, M.; Kume, Y.; Suwa, R.; Kawase, M.; Ono, T.; Chishiki, M.; Norito, S.; Sato, M.; Sakuma, H.; Suzuki, S.; et al. Thirteen Nearly Complete Genome Sequences of Human Bocavirus 1 Isolated from Pediatric Inpatients in Fukushima, Japan. Microbiol. Resour. Announc. 2022, 11, e0102721. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Kawase, M.; Matsuyama, S. Wild-type human coronaviruses prefer cell-surface TMPRSS2 to endosomal cathepsins for cell entry. Virology 2018, 517, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Agoti, C.N.; Otieno, J.R.; Munywoki, P.K.; Mwihuri, A.G.; Cane, P.A.; Nokes, D.J.; Kellam, P.; Cotten, M. Local evolutionary patterns of human respiratory syncytial virus derived from whole-genome sequencing. J. Virol. 2015, 89, 3444–3454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Who Strategy for Global Respiratory Syncytial Virus Surveillance Project Based on the Influenza Platform. Available online: https://www.who.int/publications/i/item/who-strategy-for-global-respiratory-syncytial-virus-surveillance-project-based-on-the-influenza-platform (accessed on 14 February 2022).
- Peret, T.C.; Hall, C.B.; Schnabel, K.C.; Golub, J.A.; Anderson, L.J. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J. Gen. Virol. 1998, 79, 2221–2229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peret, T.C.; Hall, C.B.; Hammond, G.W.; Piedra, P.A.; Storch, G.A.; Sullender, W.M.; Tsou, C.; Anderson, L.J. Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in North America. J. Infect. Dis. 2000, 181, 1891–1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Beato, R.; Martinez, I.; Franci, C.; Real, F.X.; Garcia-Barreno, B.; Melero, J.A. Host cell effect upon glycosylation and antigenicity of human respiratory syncytial virus G glycoprotein. Virology 1996, 221, 301–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Shean, R.C.; Makhsous, N.; Stoddard, G.D.; Lin, M.J.; Greninger, A.L. VAPiD: A lightweight cross-platform viral annotation pipeline and identification tool to facilitate virus genome submissions to NCBI GenBank. BMC Bioinform. 2019, 20, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Year Registered | All | 2010–2015 | 2016–2022 | ||
|---|---|---|---|---|---|
| Total number | 2935 | 1069 | 1185 | ||
| Pan-RSV | Forward | Number of mismatched sequences | 60 (2.0%) | 4 (0.37%) | 6 (0.5%) |
| (Within 10 nucleotides from the 3′-end) | 57 (1.9%) | 3 (0.28%) | 6 (0.5%) | ||
| Reverse | Number of mismatched sequences | 1457 (49.7%) | 456 (42.7%) | 703 (59.3%) | |
| (Within 10 nucleotides from the 3′-end) | 275 (9.4%) | 10 (0.9%) | 257 (21.7%) | ||
| Probe | Number of mismatched sequences | 1579 (53.8%) | 623 (58.3%) | 544 (45.9%) | |
| Duplex | Forward | Number of mismatched sequences | N.D. * | N.D. | 25 (2.1%) |
| (Within 10 nucleotides from the 3′-end) | N.D. | N.D. | 18 (1.5%) | ||
| Reverse | Number of mismatched sequences | N.D. | N.D. | 34 (2.9%) | |
| (Within 10 nucleotides from the 3′-end) | N.D. | N.D. | 3 (0.3%) |
| Sequence Pattern | Forward | Probe | Reverse | Number/Total Reads |
|---|---|---|---|---|
| (5′ → 3′) | (3′ ← 5′) | (3′ ← 5′) | ||
| GGCAAATATGGAAACATACGTGAA | AGCTTCACGAAGGCTCCACATACACAG | CTGTTCARTACAATGTCCTAGAAAAAGA | ||
| 1 | - * | AACTTCACGAGGGCTCCACATACACAG | - | 9/10 |
| 2 | - | AACTTCACGAGGGCTCCACATACACAG | CTGTTCAATACAATGTCCTGGAAAAAGA | 1/10 |
| Sequence Pattern | Forward | Probe | Reverse | Number/Total Reads |
|---|---|---|---|---|
| (5′ → 3′) | (3′ ← 5′) | (3′ ← 5′) | ||
| GGCAAATATGGAAACATACGTGAA | AGCTTCACGAAGGCTCCACATACACAG | CTGTTCARTACAATGTCCTAGAAAAAGA | ||
| 1 | - * | - | CTGTTCAGTACAATGTTCTAGAAAAAGA | 20/78 |
| 2 | GGCAAATATGGAAACACACGTGAA | - | CTGTTCAGTACAATGTTCTAGAAAAAGA | 1/78 |
| 3 | - | - | CTGTTCAGTACAATGTTATAGAAAAAGA | 2/78 |
| 4 | - | - | CTGTCCAGTACAATGTTCTAGAAAAAGA | 46/78 |
| 5 | - | - | CTGTTCAGTACAATGTTCTAGAAAAGGA | 2/78 |
| 6 | - | - | CTGTCCAGTACAATGTTCTGGAGAAAGA | 1/78 |
| 7 | - | AGCTTCACGAAGACTCCACATACACAG | CTGTTCAGTACAATGTTCTAGAAAAAGA | 1/78 |
| 8 | - | AGCTTCACGAAGACTCCACACAAACAG | CTGTTCAGTACAATGTTCTAGAAAAAGA | 3/78 |
| 9 | - | AGCTTCACGAAGGCTCCACACAAACAG | CTGTTCAGTACAATGTTCTAGAAAAAGA | 2/78 |
| Sequence Name | Mismatch(es) | Analytical Sensitivity (Copies) | p-Value (vs. 1391/13) |
|---|---|---|---|
| RSV/B/Sendai/1391/13 | Forward primer (CTGTTCAGTACAATGTTCTAGAAAAAGA) | 1.1 ± 0.6 | |
| RSV/B/Sendai/125/15 | Probe (AGCTTCACGAAGACTCCACATACACAG) | 1.7 ± 1.6 | 0.374 |
| RSV/B/Sendai/1008/15 | Probe (AGCTTCACGAAGACTCCACACAAACAG) | 0.8 ± 0.0 | 0.500 |
| RSV/B/Sendai/1060/16 | Probe (AGCTTCACGAAGGCTCCACACAAACAG) | 2.0 ± 2.6 | 0.296 |
| RSV/B/Sendai/442/17 | Forward primer (CTGTCCAGTACAATGTTCTAGAAAAAGA) | 1.4 ± 1.3 | 0.515 |
| Average Coverage for the Targeted Region | ||||||
|---|---|---|---|---|---|---|
| Isolate Name | Subgroup | Pan-RSV | Duplex Assay | mRNA for M | mRNA for N | Run ID * |
| Fukushima_OR343_2021 | A | 37 | 515 | 63914 | 99287 | DRR353553 |
| Fukushima_OR371_2021 | A | 94 | 629 | 93792 | 110272 | DRR353554 |
| Fukushima_OR358_2021 | B | 52 | 1391 | 95320 | 87481 | DRR353555 |
| Fukushima_OR379_2021 | B | 82 | 2841 | 186829 | 236367 | DRR353556 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwa, R.; Kume, Y.; Kawase, M.; Chishiki, M.; Ono, T.; Norito, S.; Sato, K.; Okamoto, M.; Kumaki, S.; Nagai, Y.; et al. Practical Validation of United States Centers for Disease Control and Prevention Assays for the Detection of Human Respiratory Syncytial Virus in Pediatric Inpatients in Japan. Pathogens 2022, 11, 754. https://doi.org/10.3390/pathogens11070754
Suwa R, Kume Y, Kawase M, Chishiki M, Ono T, Norito S, Sato K, Okamoto M, Kumaki S, Nagai Y, et al. Practical Validation of United States Centers for Disease Control and Prevention Assays for the Detection of Human Respiratory Syncytial Virus in Pediatric Inpatients in Japan. Pathogens. 2022; 11(7):754. https://doi.org/10.3390/pathogens11070754
Chicago/Turabian StyleSuwa, Reiko, Yohei Kume, Miyuki Kawase, Mina Chishiki, Takashi Ono, Sakurako Norito, Ko Sato, Michiko Okamoto, Satoru Kumaki, Yukio Nagai, and et al. 2022. "Practical Validation of United States Centers for Disease Control and Prevention Assays for the Detection of Human Respiratory Syncytial Virus in Pediatric Inpatients in Japan" Pathogens 11, no. 7: 754. https://doi.org/10.3390/pathogens11070754
APA StyleSuwa, R., Kume, Y., Kawase, M., Chishiki, M., Ono, T., Norito, S., Sato, K., Okamoto, M., Kumaki, S., Nagai, Y., Hosoya, M., Takeda, M., Nishimura, H., Hashimoto, K., & Shirato, K. (2022). Practical Validation of United States Centers for Disease Control and Prevention Assays for the Detection of Human Respiratory Syncytial Virus in Pediatric Inpatients in Japan. Pathogens, 11(7), 754. https://doi.org/10.3390/pathogens11070754







