Abstract
Glaesserella parasuis is the etiological agent of Glässer’s disease, which is associated with polyserositis and arthritis and has a significant impact on the economy of the pig production industry. For the optimal surveillance of this pathogen, as well as for the investigation of G. parasuis-associated diseases, it is crucial to identify G. parasuis at the serovar level. In this work, we designed and developed new high-resolution melting (HRM) approaches, namely, the species-specific GPS-HRM1 and two serovar-specific HRM assays (GPS-HRM2 and GPS-HRM3), and evaluated the sensitivity and specificity of the assays. The HRM assays demonstrated good sensitivity, with 12.5 fg–1.25 pg of input DNA for GPS-HRM1 and 125 fg–12.5 pg for GPS-HRM2 and GPS-HRM3, as well as a specificity of 100% for the identification of all recognized 15 G. parasuis serovars. Eighteen clinical isolates obtained between 2014 and 2022 in Switzerland were tested by applying the developed HRM assays, which revealed a heterogeneous distribution of serovars 2, 7, 4, 13, 1, and 14. The combination with virulence marker vtaA (virulence-associated trimeric autotransporters) allows for the prediction of potentially virulent strains. The assays are simple to execute and enable a reliable low-cost approach, thereby refining currently available diagnostic tools.
1. Introduction
After a detailed phylogenetic analysis, Haemophilus parasuis was renamed Glaesserella (G.) parasuis [1], which is a globally emerging pig pathogen and etiological agent of Glässer’s disease that has a significant impact on the economy of swine production [2]. G. parasuis is a Gram-negative bacterium belonging to the Pasteurellaceae family, and it can be found in many commercial swine farms as a colonizer of the nasal cavity of piglets, where it can be recovered two days post-natal [3]. On the one hand, G. parasuis can act as a porcine commensal bacterium of the upper respiratory tract with different degrees of virulence [4,5]; on the other hand, especially in weaner pigs, some virulent strains can lead to serious systemic diseases, such as polyserositis, polyarthritis, and meningitis [6,7]. The onset of Glässer’s disease generally occurs in piglets between 1 and 4 months of age due to the stress of weaning or relocation in combination with a decrease in maternal immunity [8,9]. Another factor that favors disease onset is the co-infection of G. parasuis and viruses, such as the highly pathogenic porcine reproductive and respiratory syndrome virus (PRRSV) and/or swine influenza virus [10,11]. Not only is G. parasuis responsible for Glässer’s disease, but it is also implicated in the complex of swine respiratory disease [12], either as a predisposing factor as a secondary pathogen, as a primary pathogen for bronchopneumonia [7,13], or as the causal agent of acute septicemia [14]. The clinical signs of acute Glässer’s disease comprise typically swollen joints with lameness, a high fever above 41.5 °C, abdominal breathing, trembling, paddling, and coughing [15]. Pigs with moderate clinical signs can progress to a chronic stage with a reduced growth rate and lameness, and they typically show rough hair [7]. Affected farms present a mortality rate associated with Glässer’s disease between 5 and 10% [9]. Several strains of G. parasuis can colonize piglets at once, and, thus, in a single farm, multiple strains of G. parasuis can be found [3]. Initially, it was thought that serovars would be an essential virulence marker [16]. However, various research groups have reported that no strict correlation between serovars and virulence can be observed [17,18,19,20,21].
There is a broad genetic variability within the G. parasuis species mediated through plasmids and/or transposons [22]. A phylogenic analysis reported the existence of two main lineages, namely, lineages I and II, revealing an association with serovars, countries, and multilocus sequence types. A G. parasuis strain from Switzerland was identified to be part of lineage I, which mainly comprises serovars 5, 12, and 14. Lineage II predominantly includes serovars 2 and 10 [22]. Recently published studies about the distribution of G. parasuis in North America, Europe, and Asia found that the geographic prevalence of molecular serovars varied slightly among different countries; however, due to the variable sizes of the examined samples, no differences in serovar prevalence could be determined [23]. Overall, serovars 1, 4, 5/12, and 7 were most often identified, whereas serovars 4 and 5/12 were widely distributed across all regions included in the study [23].
For disease control, it is important to identify the correct serovar and the virulence potential of isolates in order to differentiate between commensals and virulence-associated G. parasuis strains. In 2015, Howell et al. [24] published a multiplex serotyping PCR, which was developed using serovar-specific primers based on the capsule loci capable of differentiating 14 of the 15 known G. parasuis serovars without discrimination between serovars 5 and 12. Jia et al. [25] developed a multiplex PCR, which allows for differentiation between all recognized 15 serovars; however, this assay uses different annealing temperatures, causing the identification of the correct serovar to be time and work intensive. Besides these serotyping assays, other PCR assays have been developed to trace the virulence markers of G. parasuis. A group of specific virulence-associated genes, called virulence-associated trimeric autotransporters (vtaA) group 1-translocator, has been found to be related to virulent G. parasuis strains [26]. Furthermore, in vtaA genes, two different leader sequences have been identified, and an assay differentiating between putative virulent and non-virulent G. parasuis strains based on the leader sequence of the vtaA gene, called LS-PCR, has been developed [5].
It would be greatly advantageous to have a molecular tool that combines the identification of G. parasuis, determining its putative virulence, and, at the same time, having an accurate discrimination between all existing serovars. A high-resolution melting (HRM) analysis is a low-cost and rapid method based on PCR, and it examines the dissociation behavior of PCR amplicons. An incremental increase in temperature after PCR completion leads to the dissociation of double-stranded DNA into single-stranded molecules, thus resulting in a decline in fluorescence intensity. The melting temperature (Tm) for each amplicon is specific and depends on the amplicon sequence, its GC content, and its length [27].
In this work, a new high-resolution melting (HRM) approach was developed combining the identification, serotyping, and prediction of the putative virulence of G. parasuis isolates as a simple and cost-efficient molecular assay.
2. Results
2.1. HRM Assay
The designed primers, as indicated in Table 1, Table 2 and Table 3, were divided into two assays (GPS-HRM2 and GPS-HRM3) considering their distinctive Tm to obtain the best possible resolution power in order to enable the differentiation between all 15 serovars. GPS-HRM1 allows for the simultaneous identification of G. parasuis, using HPS_219690793 as a species-specific target and vtaA for differentiation between virulent and non-virulent G. parasuis isolates. All 15 G. parasuis reference strains amplified the gene HPS_219690793, and the subsequent HRM showed a melting curve with a Tm of 75.8 ± 0.2 for serovars 1, 2, 4, 5, 7, 11, 12, 13, 14, and 15, and a Tm of 75.7 ± 0.2 for serovars 3, 6, 8, 9, and 10, clearly identifying G. parasuis. Additionally, the non-virulent G. parasuis strains of serovars 3, 6, 8, 9, and 10 successfully amplified vtaA using primers AV1-F and NV1-R, represented by a melting curve with a Tm of 79.65 ± 0.3. Therefore, the group of putative virulent strains could clearly be differentiated from the non-virulent isolates, obtaining either one melting peak for the virulent group or two peaks for the non-virulent group (Figure 1).

Table 1.
GPS-HRM 1 primers for identification of Glaesserella parasuis using the species-specific gene marker HPS_219690793 and virulence marker vtaA for differentiation between virulent and non-virulent G. parasuis strains. Primer pair AV1-F and NV1-R targets the leader sequence of vtaA (virulence-associated trimeric autotransporter), associated with non-clinical (non-virulent) isolates.

Table 2.
GPS-HRM2 primers for identification of Glaesserella parasuis serovars 1, 2, 5, 7, 8, 10, and 15.

Table 3.
GPS-HRM3 primers for identification of Glaesserella parasuis serotypes 3, 4, 6, 9, 11, 12, 13, and 14.
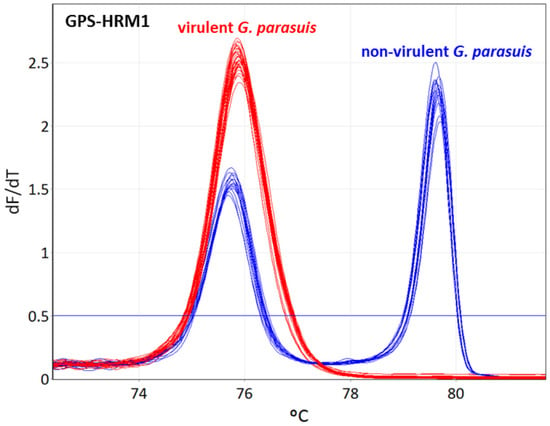
Figure 1.
Identification of Glaesserella parasuis and differentiation between virulent and non-virulent strains using high-resolution melting. GPS-HRM1 assay amplifies the species-specific gene HPS_219690793, identifying G. parasuis and vtaA for discrimination of virulent and non-virulent G. parasuis strains, respectively. G. parasuis serovars 1, 2, 4, 5, 7, 11, 12, 13, 14, and 15 are illustrated in red (virulent strains), whereas serovars 3, 6, 8, 9, and 10 are illustrated in blue (non-virulent strains).
All identified G. parasuis isolates obtained using GPS-HRM1 were further analyzed by simultaneously applying two different primer mixes (GPS-HRM2 and GPS-HRM3) in parallel. Combining the results of GPS-HRM2 and GPS-HRM3 enabled the differentiation of all 15 serovars of G. parasuis as a consequence of distinct melting curves for each serovar (Figure 2). For each serovar, a distinct Tm and its standard deviation are listed (Table 2 and Table 3). Generally, the estimated resolution power of GPS-HRM1 was 0.3 centigrade, whereas for both HRM assays GPS-HRM2/-HRM3, a difference of 0.15 centigrade was resolvable.
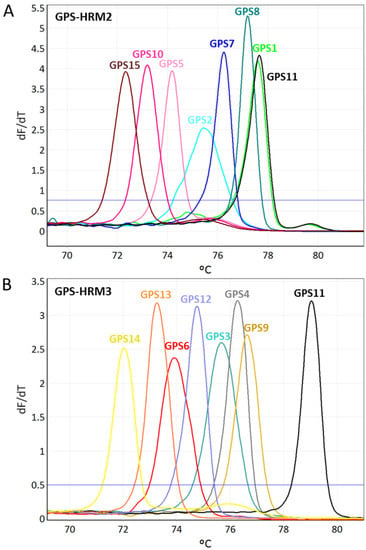
Figure 2.
Illustrations of high-resolution melting (HRM) assays GPS-HRM2 (A) and GPS-HMR3 (B), capable of identifying all recognized 15 serovars of Glaesserella parasuis. Using GPS-HRM2, six serovars can be differentiated, as well as one group consisting of G. parasuis serovar 1/11. GPS-HRM3 allows for differentiation of eight serovars and, thus, resolves the grouping of serovar 1/11 by separately assigning serovar 11 and uniquely identifying serovar 1.
The coefficient of variation (CV) of the variability of the assays obtained when using GPS-HRM1 to target HPS_219690793 resulted in CV% ≤ 0.07% for intra-assay variability and CV% ≤ 0.06% for inter-assay variability. The second target of GPS-HRM1 (vtaA) yielded CV% ≤ 0.05% for intra-assay variability and CV% ≤ 0.03% for inter-assay variability (Table S1). GPS-HRM2 and GPS-HRM3 revealed CV% ≤ 0.09% for intra-assay variability and CV% ≤ 0.14% for inter-assay variability, respectively. The low CV values obtained for each GPS-HRM assay suggest high reproducibility and robustness (Table S2).
2.2. Analytical Specificity
Analyzing the 27 different bacterial strains with the use of GPS-HRM1 for exclusivity testing did not give rise to any melting curves, and, thus, the negative results reveal a specificity of 100%. Moreover, all 15 G. parasuis reference strains did not show any cross-reactions among the different serovars, and this is illustrated as unique melting curves for each serovar (Figure 2).
2.3. Analytical Sensitivity
Standard curves were acquired by testing the tenfold dilution series using the genomic DNA of the 15 G. parasuis reference strains when performing GPS-HRM1, which amplified the G. parasuis-specific gene locus HPS_21969079 and vtaA associated with non-virulent isolates (Figure S1). GPS-HRM2 and GPS-HRM3 were performed by amplifying 15 different primer pairs specific to each serovar, which resulted in 15 different standard curves (Figure S2). The linear range of the standard curves obtained with GPS-HRM1 was between 50 and 5 × 106 GE for the 15 G. parasuis reference strains. At a confidence level of 95%, the following LODs were identified: 5 GE (12.5 fg) for G. parasuis serovar 15; 50 GE (125 fg) for G. parasuis serovars 1, 2, 5, 6, 7, 8, 9, 10, 11, 12, 13, and 14; and 500 GE (1.25 pg) for G. parasuis serovars 3 and 4. GPS-HRM1 revealed high correlation coefficients of R2 > 0.99 for the standard curves. In Figure S3, an example of a representative standard dilution series of G. parasuis illustrates the high sensitivity of G. parasuis when performing GPS-HRM1. The standard curve showed linearity across a broad range of DNA concentrations ranging from 5 to 5,000,000 GE.
GPS-HRM2 and GPS-HRM3 had a more variable linear range of standard curves than GPS-HRM1. LODs above the relevant confidence level of 95% were found to be 50 GE (125 fg of genomic DNA) for G. parasuis serovars 1, 2, and 3; 500 GE (1.25 pg of genomic DNA) for G. parasuis serovars 4, 5, 6, 7, 8, 9, 10, and 15; and 5000 GE (12.5 pg of genomic DNA) for G. parasuis serovars 11, 12, 13, and 14. The correlation coefficients of the standard curves were R2 > 0.95 (Table S3).
GPS-HRM1 had a LOD between 5 and 500 genome equivalents (GE) (12.5 fg–1.25 pg) of DNA and PCR efficiencies between 96 and 105%. GPS-HRM2 and GPS-HRM3 had LODs between 50 and 5000 GE (125 fg–12.5 pg) genomic DNA and PCR efficiencies between 92 and 105%.
2.4. Efficiency
The efficiency of GPS-HRM1 was between 96 and 107% for each of the 15 G. parasuis reference strains. The PCR efficiencies of GPS-HRM2 and GPS-HRM3 were between 92 and 105% (Figures S1 and S2, and Table S3).
2.5. Clinical Isolates
The Tm values obtained from the HRM assays testing the eighteen clinical isolates were compared to the Tm values obtained from the 15 G. parasuis reference strains. The eighteen clinical isolates were recognized to be G. parasuis by the species-specific primers. No HRM signal was obtained for the eighteen clinical isolates with the PCR when using primers detecting non-virulent G. parasuis isolates, thus revealing that the analyzed isolates were virulent. Using the HRM assays GPS-HRM2 and GPS-HRM3, the correct serovars of the eighteen isolates were unambiguously uniquely identified by referring to the Tm values (Figure 3). In total, 38.9% (n = 7) of the isolates were found to be G. parasuis serovar 2, 22.2% (n = 4) were found to be serovar 7, 16.7% (n = 3) were found to be serovar 4, 11.1% (n = 2) were found to be serovar 13, and 5.6% (n = 1) was found for both serovars 1 and 14.
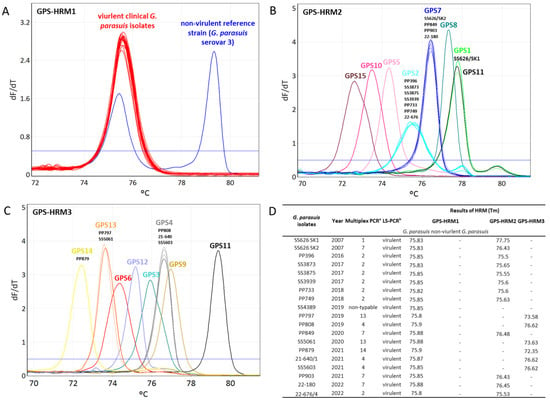
Figure 3.
Serotyping using a high-resolution melting (HRM) analysis of eighteen Swiss clinical Glaesserella parasuis isolates dating from 2007 to 2022. Representation of HRM melting curves using (A) GPS-HRM1, (B) GPS-HRM2, and (C) GPS-HRM3. (D) Results of melting temperature (Tm) acquired from GPS-HRM1, GPS-HRM2, and GPS-HRM3. GPS-HRM1 was performed using G. parasuis serovar 3 (SW114) as a positive control; in GPS-HRM2 and GPS-HRM3, all 15 G. parasuis reference strains were used as positive controls. a serovar determination by multiplex PCR [24]; b virulence prediction by leader sequence PCR [5].
3. Discussion
Due to the severe clinical signs caused by G. parasuis, it is essential to fight the pathogen appropriately. Besides strict hygienic measures, antimicrobial treatments and vaccines are widely used tools to combat Glässer’s disease [29]. The knowledge about globally increasing antimicrobial resistance underlines the importance of vaccination for the prevention of G. parasuis infection. The successful development of vaccines targeting certain virulent and prevalent strains requires a reliable and fast identification of predominant G. parasuis serovars in affected farms.
With the development of the novel HRM approaches, performing GPS-HRM1 allows for the efficient identification of G. parasuis and its potential virulence while performing GPS-HRM2 and GPS-HRM3 leads to the differentiation of G. parasuis serovars. The assays demonstrated a specificity of 100% for all 15 G. parasuis serovars, since none of the 15 reference strains resulted in non-specific signals. Using GPS-HRM1 to target HSP_219690793, G. parasuis showed a LOD of 5-500 GE (12.5–1250 fg) of input DNA, illustrating good sensitivity. In comparison to a previously standardized real-time PCR assay targeting infB, the parameters, such as the correlation coefficient and efficiency values, were in the same range [28]. The serotyping assays GPS-HRM2 and GPS-HRM3 only reached a detection limit of 50–5000 GE (0.125–12.5 pg) of input DNA and, therefore, did not achieve the same sensitivity as GPS-HRM1 because of the higher complexity of the PCR master mix, which comprised up to eight primer pairs.
Performing qPCR followed by an HRM analysis is a very convenient closed-tube procedure requiring few manipulation steps. In contrast to a conventional PCR, which includes the analysis of PCR products by capillary or agarose gel electrophoresis, the novel HRM assays can be executed more efficiently in a laboratory with no downstream processing of samples. Therefore, performing HRM is less time consuming, and, at the same time, the cost of resources is reduced. Additionally, data can easily be accessed and interpreted in comparison to the recognition of sometimes-difficult band patterns obtained with a conventional PCR. Considering the sensitivity of the HRM assays, genomic DNA between 12.5 fg and 12.5 pg was sufficient to obtain a result; this in contrast to a conventional PCR, which requires more genomic template DNA (typically in the nanogram range) to obtain a signal, further underlining the advantages of the developed assay.
It is important to mention that primers targeting the funB of G. parasuis serovar 1 cross-react with the genomic DNA of serovar 11; thus, serovars 1 and 11 result in the same melting peaks when using GPS-HRM2 (Figure 2A). However, an unambiguous identification can be acquired by performing GPS-HRM3 using serovar-11-specific primers targeting amtA. Combining the results of GPS-HRM2 and GPS-HMR3 allows for the unique assignment of serovar 11 and serovar 1, respectively (Figure 2B). Notably, new serovars can potentially be identified when obtaining a G. parasuis species-specific HRM signal using GPS-HRM1 in combination with the absence of a serovar-specific melting curve in GPS-HRM2 or GPS-HRM3.
For diagnostic purposes, the serotyping of G. parasuis is time consuming and needs a lot of resources. Consequently, in many routine diagnostic laboratories, serotyping is often not performed. However, it is of the outmost importance to identify the virulence and serovars of involved strains for the correct interpretation of their relevance. With the knowledge of implicated serovars, autogenous vaccines can be created, and disease spread can be eradicated. Furthermore, the novel HRM assays can help to overcome the extensive use of antibiotics. In the future, it is planned to evaluate this method for application with DNA directly extracted from tissue material or swabs.
As shown in the exclusivity study, the HRM assays allow for the differentiation of G. parasuis strains from their most important differential agents, such as Escherichia coli, Streptococcus suis, Mycoplasma hyorhinis, and Mycoplasma hyosynoviae.
The vaccines available in Switzerland are based on serovar 5 (Porcilis Glässer, MSD Animal Health GmbH, Lucerne, Switzerland). Furthermore, commercially available bacterin vaccines in other countries contain a combination of serovars 4 and 5 or serovars 1 and 6 [9]. However, cross-protection against other serovars is often reduced [30]. Since different serovars are found in one farm, or even in one animal, it would be favorable to identify and type relevant systemic isolates and to use those as an autogenous vaccine [9]. Therefore, these novel assays can help to reliably identify the appropriate serovar and can help to maximize suitable protection.
In Switzerland, serovar identification of the eighteen clinical G. parasuis isolates gathered between 2014 and 2022 illustrated a heterogenous distribution of serovars 2, 7, 4, 13, 1, and 14 in declining order at frequencies of 38.9–5.6% each (Table 4). However, no statement about the real prevalence can be made since the small number of eighteen isolates is not meaningful. It would be interesting to monitor the continuance of G. parasuis in the future in order to verify the observation that serovar 5/12, unlike in most other countries, is absent in Switzerland and, eventually, to determine the reason for the particular serovar distributions of G. parasuis.

Table 4.
Clinical isolates of Glaesserella parasuis used in the study.
4. Materials and Methods
4.1. Bacterial Strains and Clinical Isolates
For the development of novel HRM assays, the following G. parasuis reference strains of serovars 1–15 were examined: nr.4, SW140, SW114, SW124, Nagasaki, 13l, 174, C5, D74, H555, H465, H425, 84-17975, 84-22113, and 84-15995 (Table 5).

Table 5.
Glaesserella parasuis reference strains used for the development of high-resolution melting (HRM) assays. All reference strains were obtained from Judith Rodhe from the Institute of Microbiology in Hannover, Germany. V, virulent; NV, non-virulent.
Eighteen clinical isolates were collected between 2007 and 2022 from routine diagnostic submissions to the Section of Veterinary Bacteriology, University of Zurich (Table 4), and they were analyzed with the novel HRM assays. The non-virulent strains were either not among the samples obtained from diagnostic submissions or could not successfully be isolated.
Clinical samples were grown on solid chocolate agar (Thermo Fisher Diagnostics AG, Pratteln, Switzerland) for up to 48 h at 37 °C in 5% CO2.
4.2. HRM Conditions
Primers were designed with the sequences of G. parasuis retrieved from the NCBI databank, using gene loci based on previous reports, or, alternatively, primers were directly adapted from previous publications [5,24,28]. Primers targeting sequences HSP-219690793, glyC (serovar 3 specific), and wcwK (serovar 12 specific) were designed by taking into consideration the optimal distribution of the different Tms of the peaks. Briefly, the nucleotide sequences of the primers were shifted and sized within the target region in order to fit HRM criteria, such as optimizing the amplicon length to smaller than 160 basepairs. Primer sequences were selected for the assay whenever the interval of the Tms of the different serovars was clearly distinctive. The specificity testing of the primer sequences was performed using a BLAST analysis. All primers were manufactured by Microsynth (Balgach, Switzerland). The HRM assays were developed using a Rotor-Gene Q (Qiagen, Hilden, Germany). The extraction of genomic DNA was performed using InstaGene Matrix (Bio-Rad Laboratories, Richmond, VA, USA) according to the manufacturer’s instructions. DNA concentration was determined using spectrophotometry with a NanoDrop 2000c (Thermo Fisher Scientific, Waltham, MA, USA).
The PCR reaction was performed in a total reaction volume of 15 µL. The reaction mix included 1 µL of genomic DNA, 7.5 µL of Type-it HRM PCR Master Mix (2×) (Qiagen), the primers at the concentrations listed (Table 1, Table 2 and Table 3), and water. The following PCR thermocycling parameters were applied: denaturation at 95 °C for 5 min, 40 cycles at 95 °C for 10 s, annealing/extension either at 57.5 °C (GPS-HRM1) or at 61 °C (GPS-HRM 2 and GPS-HRM3) for 30 s; and a final cycle at 95 °C for 10 s and at 40 °C for 2 min. HRM ramping was performed from 62 °C to 95 °C, acquiring data of the fluorescence at 0.1 °C increments every 2 s in order to create melting plots specific to each serovar. As a positive control for the PCR, genomic DNA extracted from the reference strains was used in each HRM run. Distilled water was applied as a negative control in each HRM assay.
Using 15 reference strains (Table 5), the HRM assays were developed. The melting curves were analyzed using Rotor-Gene Q Software 2.3.1 (Qiagen). Using this software, a melt curve analysis was performed. The Tm of the obtained peaks was examined using a threshold of 0.5 dF/dT. Signals below the threshold of 0.5 dF/dT were not considered.
To assess the repeatability of GPS-HRM1, GPS-HRM2, and GPS-HRM3, 20 ng of genomic DNA of all 15 serovar reference strains was examined in triplicate, thereby evaluating the intra- and inter-assay variabilities of the obtained Tm of each HRM assay.
4.3. Analytical Specificity
An exclusivity panel of 27 bacterial isolates encompassing eleven different species was examined with the three assays GPS-HRM1, GPS-HRM2, and GPS-HRM3 to evaluate analytical specificity. The following strains comprising three groups were tested: Gram-negative coccobacilli, namely, Actinobacillus suis (n = 1), Pasteurella multocida (n = 3), Actinobacillus pleuropneumoniae (n = 3), and Bordetella bronchiseptica (n = 2); nasal commensal microorganisms, such as G. parasuis: Moraxella spp. (n = 1) and Neisseria animaloris (n = 1); and bacteria involved in causing lesions similar to those caused in Glässer’s disease, namely, Streptococcus suis (n = 5), Erysipelothrix rhusiopathiae (n = 1), Escherichia coli (n = 2), Mycoplasma hyorhinis (n = 4), and Mycoplasma hyosynoviae (n = 4).
4.4. Analytical Sensitivity
For the evaluation of the analytical sensitivity of the three HRM assays, the 15 G. parasuis reference strains were analyzed. With an estimation of the genome size of G. parasuis between 1.4 and 2.2 Mbp [19], one genome equivalent (GE) corresponded to approximately 2.5 fg of genomic DNA.
To determine the detection range and linearity of GPS-HRM1, GPS-HRM2, and GPS-HRM3 using the 15 G. parasuis reference stains, a triplicate testing of a tenfold standard dilution series was performed comprising genomic DNA of 12.5 ng (5 × 106 GE), 1.25 ng (5 × 105 GE), 125 pg (5 × 104 GE), 12.5 pg (5 × 103 GE), 1.25 pg (500 GE), 125 fg (50 GE), and 12.5 fg (5 GE). The correlation coefficient (R2) represents the linearity of each standard dilution series of the 15 G. parasuis serovars. For the determination of the limit of detection (LOD) in a confidence interval of 95%, the lowest concentration of each serovar was chosen, at which a melting curve for all triplicates with a threshold value greater than 0.5 dF/dT and a standard deviation of the crossing threshold (Ct) greater than 0.5 was obtained.
4.5. Efficiency
To calculate the PCR efficiency in the linear range of the dilution series, the following equation was used: efficiency = (101/-slope − 1) × 100. In order to calculate the slope of the standard curves, Ct values obtained from triplicate measurements of the dilution series (5 × 106 GE, 5 × 105 GE, 5 × 104 GE, 5 × 103 GE, 500 GE, 50 GE, and 5 GE) of the three HRM assays tested were plotted against GE. Using the formula mentioned above, the efficiency of each G. parasuis serovar was determined.
4.6. Clinical Isolates
Eighteen clinical isolates were further characterized as described in Section 4.1 by applying primer mixes for GPS-HRM1, GPS-HMR2, and GPS-HRM3. For the assignment of the serovars, the Tm received from each clinical isolate was compared with the corresponding Tm obtained from the 15 G. parasuis serovar reference strains.
5. Conclusion
The novel HRM assays, which address, at the same time, the identification of G. parasuis and its potential virulence (GPS-HRM1), as well as differentiation between all 15 serovars (GPS-HRM2 and GPS-HRM3), deliver a practical diagnostic method that can provide rapid insight into possible pathogenic stains. The assays can be used in diagnostic laboratories to obtain a broad picture about the prevalence of G. parasuis among pig farms, thereby opening new perspectives among the currently available diagnostic methods.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11070752/s1, Figure S1: Standard curves of tenfold dilution series (5 × 106—5 genome equivalents) acquired by the serotype-specific GPS-HRM1 assay for all reference Glaesserella parasuis strains are plotted in the linear dynamic range. PCR efficiencies between 96 and 105% with high correlation coefficients (R2 > 0.99) were obtained. Figure S2: Standard curves of tenfold dilution series (5 × 106—5 genome equivalents) acquired by the serotype-specific GPS-HRM2 and GPS-HRM3 assays for all reference Glaesserella parasuis strains are plotted in the linear dynamic range. PCR efficiencies between 92 and 105% with correlation coefficients (R2 > 0.95) were obtained. Figure S3: Identification of Glaesserella parasuis targeting species-specific gene HPS_219690793 (GPS-HRM1) demonstrated high sensitivity. Using a tenfold dilution series of genomic DNA of G. parasuis (as an example representation of G. parasuis serovar 15), GPS-HRM1 was performed using primers targeting HPS_219690793. The tenfold dilution series contained DNA quantities of 5 genome equivalent (GE) (violet), 50 GE (blue), 500 GE (light blue), 5000 GE (green), 50,000 GE (yellow), 500,000 GE (orange), and 5,000,000 GE (red). Illustration of the tenfold dilution series as (A) PCR amplification; (B) melting curves showing a limit of detection of 5 GE; (C) standard curve with great linearity between 5 and 5,000,000 GE of genomic DNA, including a high correlation coefficient (R2 > 0.99). Table S1: Inter- and intra-assay variabilities of GPS-HRM1. Table S2: Inter- and intra-assay variabilities of GPS-HRM2 and GPS-HRM3. Table S3: Efficiency and limit of detection (LOD) of high-resolution melting assays (GPS-HRM1, GPS-HRM2, and GPS-HRM3) testing all 15 Glaesserella parasuis serovars targeting HSP_219690793, vtaA, and 15 serovar-specific loci.
Author Contributions
Conceptualization, R.S., S.P. and S.S.; methodology, S.P. and S.S.; investigation, F.R., S.P. and S.S.; writing—original draft preparation, S.S.; writing—review and editing, R.S., S.P. and F.R.; project administration, R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Swiss Federal Food Safety and Veterinary Office under project number 1.21.06.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the supplementary material.
Acknowledgments
We are grateful to Judith Rohde from the Institute of Microbiology in Hannover for kindly providing all G. parasuis reference strains. We would like to thank all members of the diagnostic lab of the Section of Veterinary Bacteriology, Vetsuisse Faculty, University of Zurich, for collecting the clinical isolates.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dickerman, A.; Bandara, A.B.; Inzana, T.J. Phylogenomic analysis of Haemophilus parasuis and proposed reclassification to Glaesserella parasuis, gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Costa-Hurtado, M.; Barba-Vidal, E.; Maldonado, J.; Aragon, V. Update on Glässer’s disease: How to control the disease under restrictive use of antimicrobials. Vet. Microbiol. 2020, 242, 108595. [Google Scholar] [CrossRef]
- Cerdà-Cuéllar, M.; Naranjo, J.F.; Verge, A.; Nofrarías, M.; Cortey, M.; Olvera, A.; Segalés, J.; Aragon, V. Sow vaccination modulates the colonization of piglets by Haemophilus parasuis. Vet. Microbiol. 2010, 145, 315–320. [Google Scholar] [CrossRef]
- Olvera, A.; Cerdà-Cuéllar, M.; Aragon, V. Study of the population structure of Haemophilus parasuis by multilocus sequence typing. Microbiology 2006, 152, 3683–3690. [Google Scholar] [CrossRef] [PubMed]
- Galofré-Milà, N.; Correa-Fiz, F.; Lacouture, S.; Gottschalk, M.; Strutzberg-Minder, K.; Bensaid, A.; Pina-Pedrero, S.; Aragon, V. A robust PCR for the differentiation of potential virulent strains of Haemophilus parasuis. BMC Vet. Res. 2017, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Shibata, M.; Kajio, N.; Morozumi, T. Pathologic observations of pigs intranasally inoculated with serovar 1, 4 and 5 of Haemophilus parasuis using immunoperoxidase method. J. Vet. Med. Sci. 1994, 56, 639–644. [Google Scholar] [CrossRef]
- Little, T.W. Haemophilus infection in pigs. Vet. Rec. 1970, 87, 399–402. [Google Scholar] [CrossRef]
- Nielsen, R.; Danielsen, V. An outbreak of Glasser’s disease. Studies on etiology, serology and the effect of vaccination. Nord. Vet. Med. 1975, 27, 20–25. [Google Scholar]
- Aragon, V.; Segalés, J.; Tucker, A. Glässer’s Disease. In Diseases of Swine; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 844–853. [Google Scholar]
- Li, J.; Wang, S.; Li, C.; Wang, C.; Liu, Y.; Wang, G.; He, X.; Hu, L.; Liu, Y.; Cui, M.; et al. Secondary Haemophilus parasuis infection enhances highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) infection-mediated inflammatory responses. Vet. Microbiol. 2017, 204, 35–42. [Google Scholar] [CrossRef]
- Pomorska-Mol, M.; Dors, A.; Kwit, K.; Czyzewska-Dors, E.; Pejsak, Z. Coinfection modulates inflammatory responses, clinical outcome and pathogen load of H1N1 swine influenza virus and Haemophilus parasuis infections in pigs. BMC Vet. Res. 2017, 13, 376. [Google Scholar] [CrossRef]
- Choi, Y.K.; Goyal, S.M.; Joo, H.S. Retrospective analysis of etiologic agents associated with respiratory diseases in pigs. Can. Vet. J. 2003, 44, 735–737. [Google Scholar] [PubMed]
- Oliveira, S.; Pijoan, C. Haemophilus parasuis: New trends on diagnosis, epidemiology and control. Vet. Microbiol. 2004, 99, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Peet, R.L.; Fry, J.; Lloyd, J.; Henderson, J.; Curran, J.; Moir, D. Haemophilus parasuis septicaemia in pigs. Aust. Vet. J. 1983, 60, 187. [Google Scholar] [CrossRef] [PubMed]
- Vahle, J.L.; Haynes, J.S.; Andrews, J.J. Experimental reproduction of Haemophilus parasuis infection in swine: Clinical, bacteriological, and morphologic findings. J. Vet. Diagn. Investig. 1995, 7, 476–480. [Google Scholar] [CrossRef]
- Kielstein, P.; Rapp-Gabrielson, V.J. Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J. Clin. Microbiol. 1992, 30, 862–865. [Google Scholar] [CrossRef]
- Howell, K.J.; Weinert, L.A.; Chaudhuri, R.R.; Luan, S.L.; Peters, S.E.; Corander, J.; Harris, D.; Angen, Ø.; Aragon, V.; Bensaid, A.; et al. The use of genome wide association methods to investigate pathogenicity, population structure and serovar in Haemophilus parasuis. BMC Genom. 2014, 15, 1179. [Google Scholar] [CrossRef]
- Oliveira, S.; Blackall, P.J.; Pijoan, C. Characterization of the diversity of Haemophilus parasuis field isolates by use of serotyping and genotyping. Am. J. Vet. Res. 2003, 64, 435–442. [Google Scholar] [CrossRef]
- Brockmeier, S.L.; Register, K.B.; Kuehn, J.S.; Nicholson, T.L.; Loving, C.L.; Bayles, D.O.; Shore, S.M.; Phillips, G.J. Virulence and draft genome sequence overview of multiple strains of the swine pathogen Haemophilus parasuis. PLoS ONE 2014, 9, e103787. [Google Scholar] [CrossRef]
- Aragon, V.; Cerdà-Cuéllar, M.; Fraile, L.; Mombarg, M.; Nofrarías, M.; Olvera, A.; Sibila, M.; Solanes, D.; Segalés, J. Correlation between clinico-pathological outcome and typing of Haemophilus parasuis field strains. Vet. Microbiol. 2010, 142, 387–393. [Google Scholar] [CrossRef]
- Schuwerk, L.; Hoeltig, D.; Waldmann, K.H.; Strutzberg-Minder, K.; Valentin-Weigand, P.; Rohde, J. Serotyping and pathotyping of Glaesserella parasuis isolated 2012-2019 in Germany comparing different PCR-based methods. Vet. Res. 2020, 51, 137. [Google Scholar] [CrossRef]
- Wan, X.; Li, X.; Osmundson, T.; Li, C.; Yan, H. Whole-genome sequence analyses of Glaesserella parasuis isolates reveals extensive genomic variation and diverse antibiotic resistance determinants. PeerJ 2020, 8, e9293. [Google Scholar] [CrossRef] [PubMed]
- Macedo, N.; Gottschalk, M.; Strutzberg-Minder, K.; Van, C.N.; Zhang, L.; Zou, G.; Zhou, R.; Marostica, T.; Clavijo, M.J.; Tucker, A.; et al. Molecular characterization of Glaesserella parasuis strains isolated from North America, Europe and Asia by serotyping PCR and LS-PCR. Vet. Res. 2021, 52, 68. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.J.; Peters, S.E.; Wang, J.; Hernandez-Garcia, J.; Weinert, L.A.; Luan, S.L.; Chaudhuri, R.R.; Angen, Ø.; Aragon, V.; Williamson, S.M.; et al. Development of a multiplex PCR assay for rapid molecular serotyping of Haemophilus parasuis. J. Clin. Microbiol. 2015, 53, 3812–3821. [Google Scholar] [CrossRef]
- Jia, A.; Zhou, R.; Fan, H.; Yang, K.; Zhang, J.; Xu, Y.; Wang, G.; Liao, M. Development of serotype-specific PCR assays for typing of Haemophilus parasuis isolates circulating in Southern China. J. Clin. Microbiol. 2017, 55, 3249–3257. [Google Scholar] [CrossRef]
- Olvera, A.; Pina, S.; Macedo, N.; Oliveira, S.; Aragon, V.; Bensaid, A. Identification of potentially virulent strains of Haemophilus parasuis using a multiplex PCR for virulence-associated autotransporters (vtaA). Vet. J. 2012, 191, 213–218. [Google Scholar] [CrossRef]
- Vossen, R.H.; Aten, E.; Roos, A.; den Dunnen, J.T. High-resolution melting analysis (HRMA): More than just sequence variant screening. Hum. Mutat. 2009, 30, 860–866. [Google Scholar] [CrossRef]
- Cui, Y.; Guo, F.; Cai, X.; Cao, X.; Guo, J.; Wang, H.; Yang, B.; Zhou, H.; Su, X.; Blackall, P.J.; et al. Ct value-based real time PCR serotyping of Glaesserella parasuis. Vet. Microbiol. 2021, 254, 109011. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, H.; Xue, Y.; Chen, K.; Liu, Z.; Xue, Q.; Wang, C. Analysis of efficacy obtained with a trivalent inactivated Haemophilus parasuis serovars 4, 5, and 12 vaccine and commercial vaccines against Glässer’s disease in piglets. Can. J. Vet. Res. 2017, 81, 22–27. [Google Scholar] [PubMed]
- Macedo, N.; Cheeran, M.C.; Rovira, A.; Holtcamp, A.; Torremorell, M. Effect of enrofloxacin on Haemophilus parasuis infection, disease and immune response. Vet. Microbiol. 2017, 199, 91–99. [Google Scholar] [CrossRef]
- Lacouture, S.; Rodriguez, E.; Strutzberg-Minder, K.; Gottschalk, M. Canada: Serotyping of Haemophilus parasuis field isolates from diseased pigs in Quebec by indirect hemagglutination assay and multiplex polymerase chain reaction (PCR). Can. Vet. J. 2017, 58, 802–804. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).