TbpBY167A-Based Vaccine Can Protect Pigs against Glässer’s Disease Triggered by Glaesserella parasuis SV7 Expressing TbpB Cluster I
Abstract
:1. Introduction
2. Materials and Methods
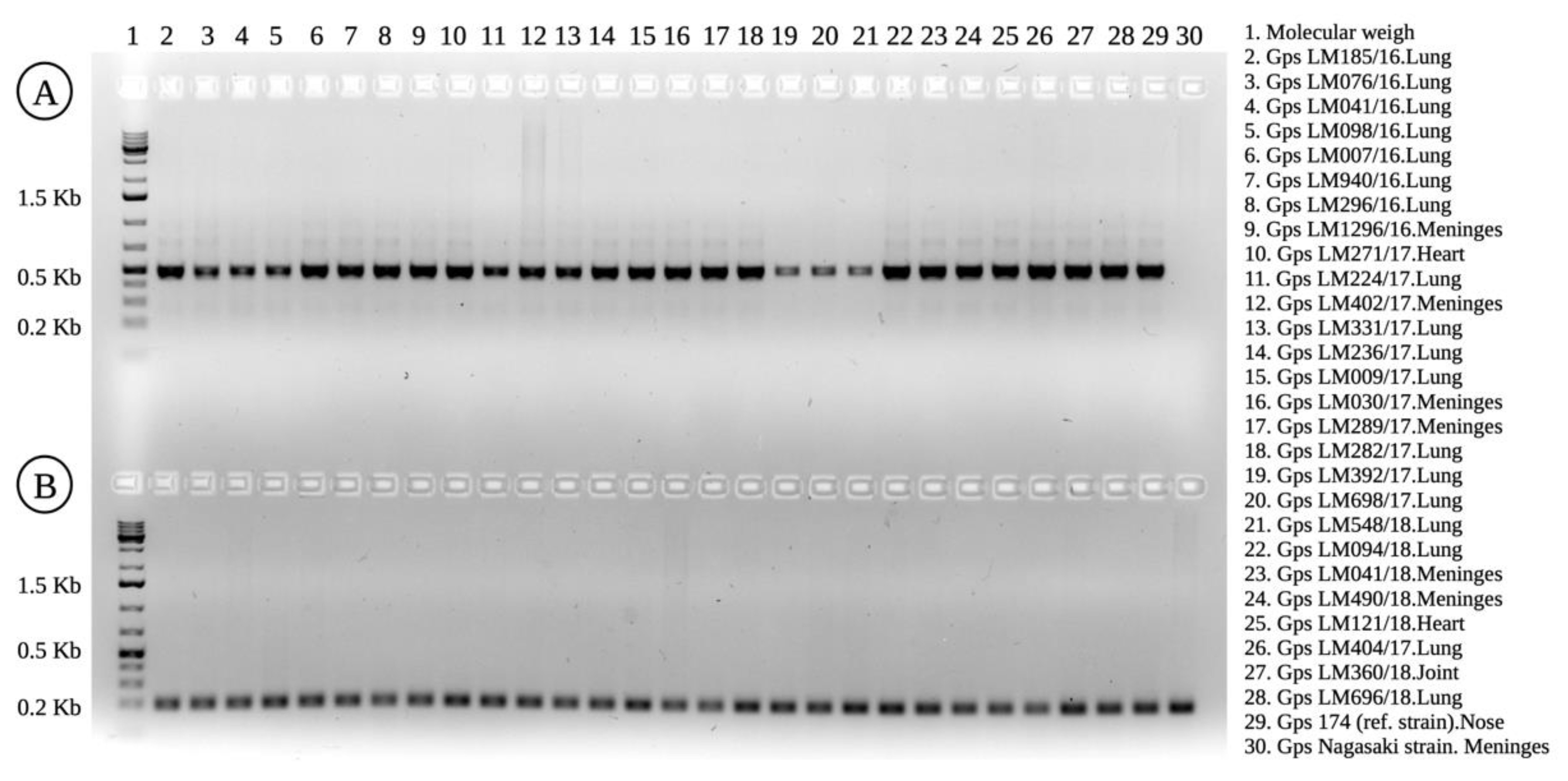
2.1. Glaesserella Parasuis Field Isolates
2.2. Virulence Prediction LS-PCR
2.3. TbpB Gene Amplification and Sequencing
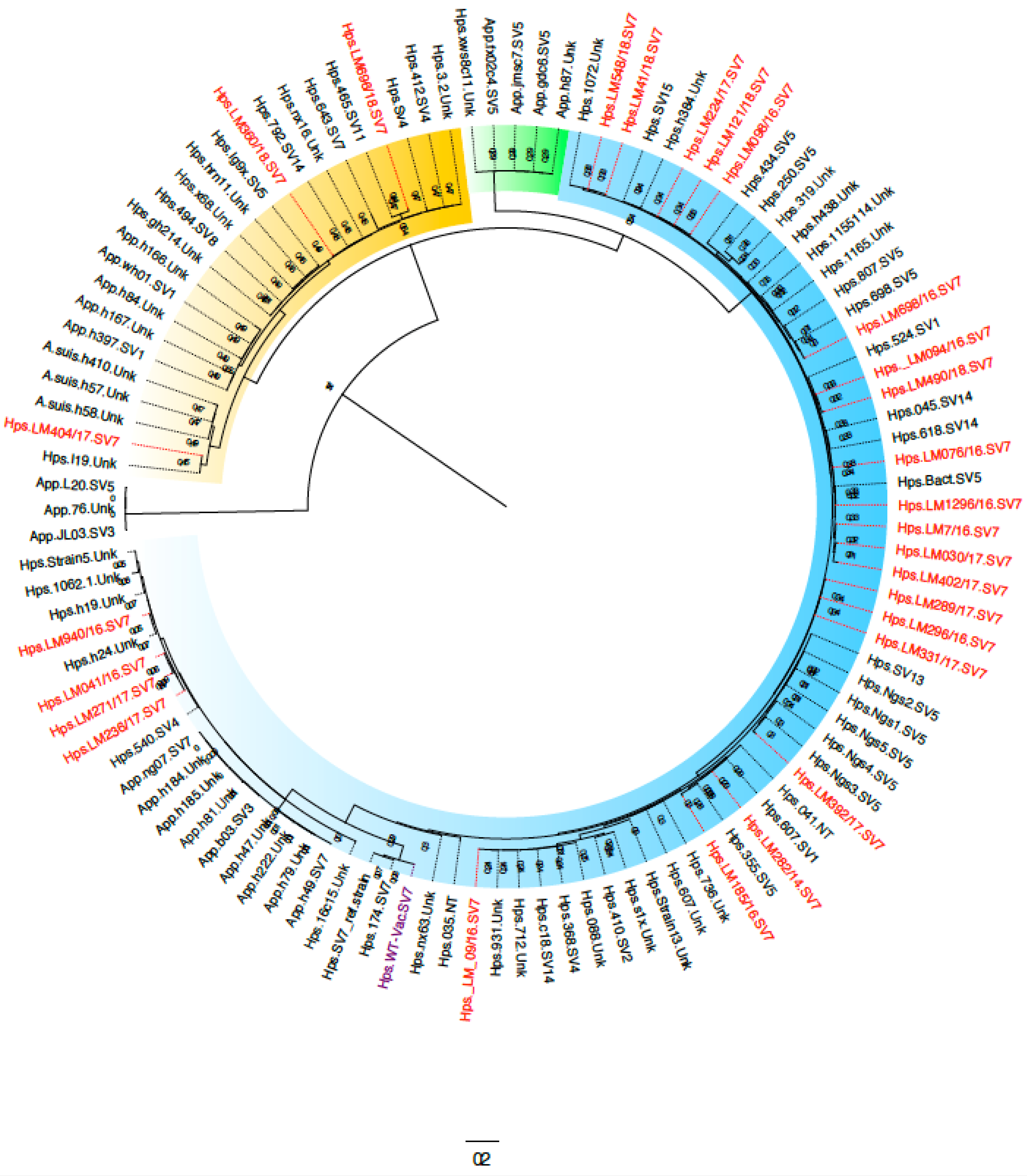
2.4. Phylogenetic Analysis
2.5. TbpB Expression Analysis on Glaesserella Parasuis
2.6. Capacity of Anti-TbpBY167A IgG in Recognizing Native TbpBs on Clinical Isolates of G. parasuis SV7
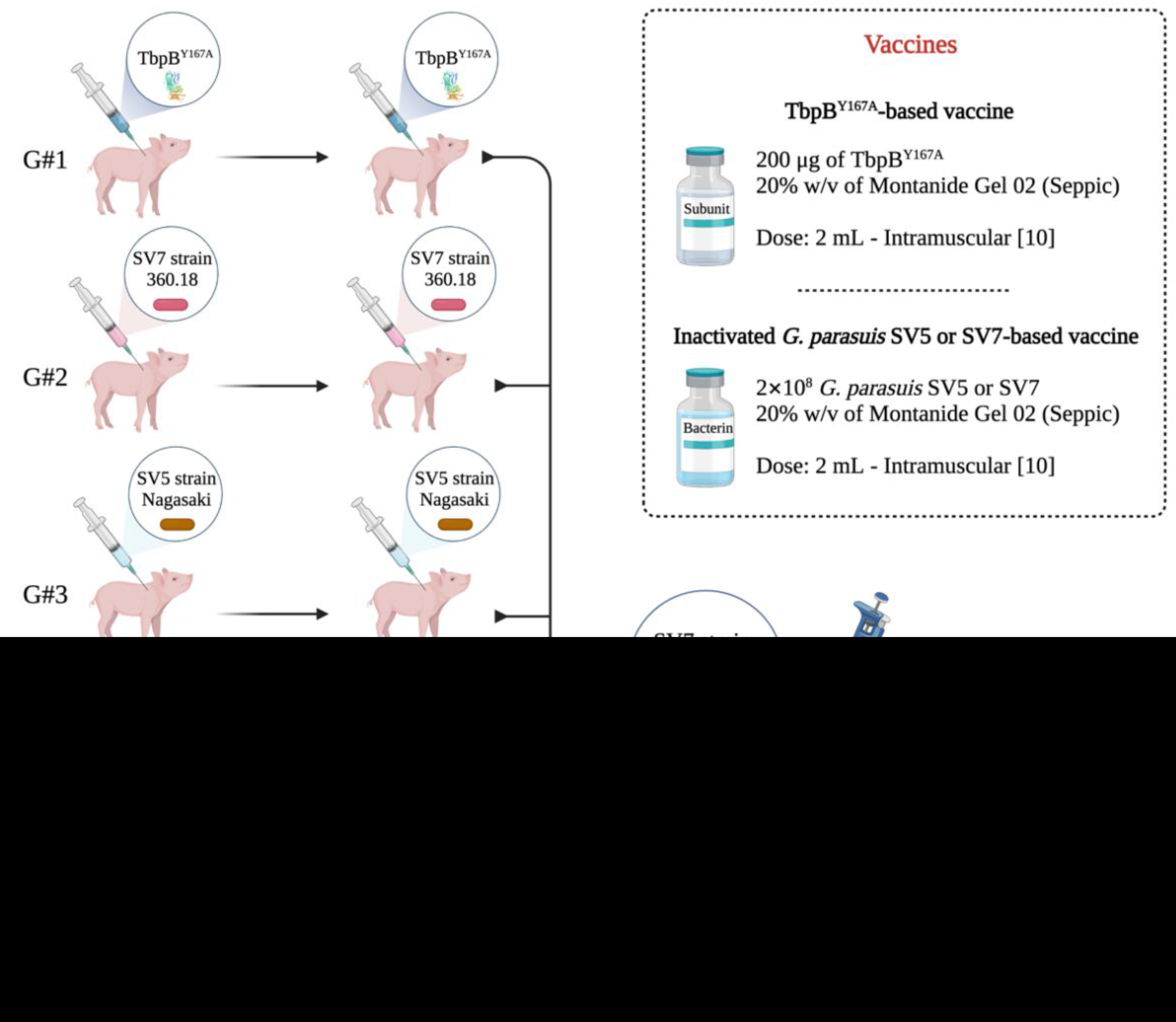
2.7. In Vivo Evaluation of the Protective Capacity of the TbpBY167A (Cluster III) against G. Parasuis SV7 (TbpB Cluster I)
2.8. Clinical Evaluation
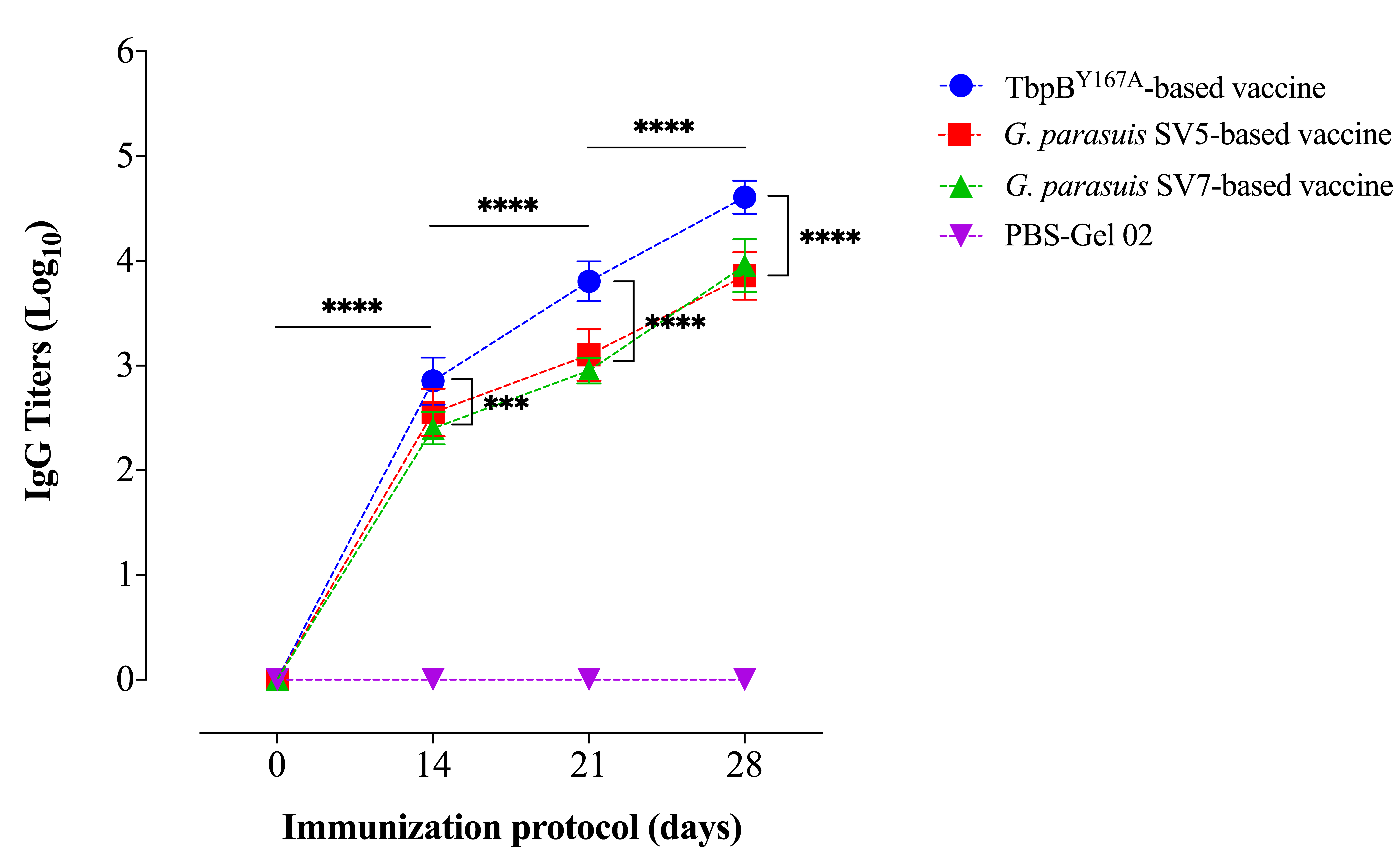
2.9. Vaccine Immunogenicity
2.10. Ethical Statement
2.11. Statistical Analysis
3. Results and Discussion
3.1. Characterization and Geographical Distribution of SV7 Clinical Isolates
3.2. Phylogenetical Analysis of TbpB from Glaesserella Parasuis SV7
3.3. Antigenicity Analysis
3.4. TbpBY167A Induces Protection against G. parasuis Strains That Express TbpB Cluster I
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rapp-Gabrielson, V.J.; Gabrielson, D.A. Prevalence of Haemophilus parasuis serovars among isolates from swine. Am. J. Vet. Res. 1992, 53, 659–664. [Google Scholar] [PubMed]
- Cerda-Cuellar, M.; Naranjo, J.F.; Verge, A.; Nofrarias, M.; Cortey, M.; Olvera, A.; Segales, J.; Aragon, V. Sow vaccination modulates the colonization of piglets by Haemophilus parasuis. Vet. Microbiol. 2010, 145, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Salmon, H.; Berri, M.; Gerdts, V.; Meurens, F. Humoral and cellular factors of maternal immunity in swine. Dev. Comp. Immunol. 2009, 33, 384–393. [Google Scholar] [CrossRef]
- Pomorska-Mol, M.; Markowska-Daniel, I.; Rachubik, J.; Pejsak, Z. Effect of maternal antibodies and pig age on the antibody response after vaccination against Glassers disease. Vet. Res. Commun. 2011, 35, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Dazzi, C.C.; Guizzo, J.A.; Prigol, S.R.; Kreutz, L.C.; Driemeier, D.; Chaudhuri, S.; Schryvers, A.B.; Frandoloso, R. New Pathological Lesions Developed in Pigs by a “Non-virulent” Strain of Glaesserella parasuis. Front. Vet. Sci. 2020, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Palzer, A.; Haedke, K.; Heinritzi, K.; Zoels, S.; Ladinig, A.; Ritzmann, M. Associations among Haemophilus parasuis, Mycoplasma hyorhinis, and porcine reproductive and respiratory syndrome virus infections in pigs with polyserositis. Can. Vet. J. 2015, 56, 285–287. [Google Scholar]
- Saade, G.; Deblanc, C.; Bougon, J.; Marois-Crehan, C.; Fablet, C.; Auray, G.; Belloc, C.; Leblanc-Maridor, M.; Gagnon, C.A.; Zhu, J.; et al. Coinfections and their molecular consequences in the porcine respiratory tract. Vet. Res. 2020, 51, 80. [Google Scholar] [CrossRef]
- Baltes, N.; Hennig-Pauka, I.; Gerlach, G.F. Both transferrin binding proteins are virulence factors in Actinobacillus pleuropneumoniae serotype 7 infection. FEMS Microbiol. Lett. 2002, 209, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Curran, D.M.; Adamiak, P.J.; Fegan, J.E.; Qian, C.; Yu, R.H.; Schryvers, A.B. Sequence and structural diversity of transferrin receptors in Gram-negative porcine pathogens. Vaccine 2015, 33, 5700–5707. [Google Scholar] [CrossRef]
- Barber, M.F.; Elde, N.C. Nutritional immunity. Escape from bacterial iron piracy through rapid evolution of transferrin. Science 2014, 346, 1362–1366. [Google Scholar] [CrossRef] [Green Version]
- Ostan, N.K.H.; Moraes, T.F.; Schryvers, A.B. Lactoferrin receptors in Gram-negative bacteria: An evolutionary perspective. Biochem. Cell Biol. 2021, 99, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Redfield, R.J.; Findlay, W.A.; Bosse, J.; Kroll, J.S.; Cameron, A.D.; Nash, J.H. Evolution of competence and DNA uptake specificity in the Pasteurellaceae. BMC Evol. Biol. 2006, 6, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraes, T.F.; Yu, R.H.; Strynadka, N.C.; Schryvers, A.B. Insights into the bacterial transferrin receptor: The structure of transferrin-binding protein B from Actinobacillus pleuropneumoniae. Mol. Cell 2009, 35, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Frandoloso, R.; Martinez-Martinez, S.; Calmettes, C.; Fegan, J.; Costa, E.; Curran, D.; Yu, R.H.; Gutierrez-Martin, C.B.; Rodriguez-Ferri, E.F.; Moraes, T.F.; et al. Nonbinding site-directed mutants of transferrin binding protein B exhibit enhanced immunogenicity and protective capabilities. Infect. Immun. 2015, 83, 1030–1038. [Google Scholar] [CrossRef] [Green Version]
- Noinaj, N.; Easley, N.C.; Oke, M.; Mizuno, N.; Gumbart, J.; Boura, E.; Steere, A.N.; Zak, O.; Aisen, P.; Tajkhorshid, E.; et al. Structural basis for iron piracy by pathogenic Neisseria. Nature 2012, 483, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Frandoloso, R.; Chaudhuri, S.; Frandoloso, G.C.P.; Yu, R.-H.; Schryvers, A.B. Proof of Concept for Prevention of Natural Colonization by Oral Needle-Free Administration of a Microparticle Vaccine. Front. Immunol. 2020, 11, 2744. [Google Scholar] [CrossRef]
- Guizzo, J.A.; Chaudhuri, S.; Prigol, S.R.; Yu, R.H.; Dazzi, C.C.; Balbinott, N.; Frandoloso, G.P.; Kreutz, L.C.; Frandoloso, R.; Schryvers, A.B. The amino acid selected for generating mutant TbpB antigens defective in binding transferrin can compromise the in vivo protective capacity. Sci. Rep. 2018, 8, 7372. [Google Scholar] [CrossRef]
- Rossi-Campos, A.; Anderson, C.; Gerlach, G.F.; Klashinsky, S.; Potter, A.A.; Willson, P.J. Immunization of pigs against Actinobacillus pleuropneumoniae with two recombinant protein preparations. Vaccine 1992, 10, 512–518. [Google Scholar] [CrossRef]
- Takahashi, K.; Naga, S.; Yagihashi, T.; Ikehata, T.; Nakano, Y.; Senna, K.; Maruyama, T.; Murofushi, J. A cross-protection experiment in pigs vaccinated with Haemophilus parasuis serovars 2 and 5 bacterins, and evaluation of a bivalent vaccine under laboratory and field conditions. J. Vet. Med. Sci. Jpn. Soc. Vet. Sci. 2001, 63, 487–491. [Google Scholar] [CrossRef] [Green Version]
- Miniats, O.P.; Smart, N.L.; Rosendal, S. Cross protection among Haemophilus parasuis strains in immunized gnotobiotic pigs. Can. J. Vet. Res. Rev. Can. Rech. Vet. 1991, 55, 37–41. [Google Scholar]
- Pires, E.J.; Balbinott, N.T.; Trevisan, G.L.; Machado, G.; Klein, S.C.; Rebelatto, R.; Gutiérrez-Martín, C.B.; Kreutz, L.C.; Schryvers, A.B.; Frandoloso, R. Molecular serotyping of clinical strains of Haemophilus (Glaesserella) parasuis brings new insights regarding Glässer’s disease outbreaks in Brazil. PeerJ 2019, 7, e6817. [Google Scholar] [CrossRef] [Green Version]
- Lacouture, S.; Rodriguez, E.; Strutzberg-Minder, K.; Gottschalk, M. Canada: Serotyping of Haemophilus parasuis field isolates from diseased pigs in Quebec by indirect hemagglutination assay and multiplex polymerase chain reaction (PCR). Can. Vet. J. 2017, 58, 802–804. [Google Scholar] [PubMed]
- Kielstein, P.; Rapp-Gabrielson, V.J. Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J. Clin. Microbiol. 1992, 30, 862–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galofre-Mila, N.; Correa-Fiz, F.; Lacouture, S.; Gottschalk, M.; Strutzberg-Minder, K.; Bensaid, A.; Pina-Pedrero, S.; Aragon, V. A robust PCR for the differentiation of potential virulent strains of Haemophilus parasuis. BMC Vet. Res. 2017, 13, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barasuol, B.M.; Guizzo, J.A.; Fegan, J.E.; Martinez-Martinez, S.; Rodriguez-Ferri, E.F.; Gutierrez-Martin, C.B.; Kreutz, L.C.; Schryvers, A.B.; Frandoloso, R. New insights about functional and cross-reactive properties of antibodies generated against recombinant TbpBs of Haemophilus parasuis. Sci. Rep. 2017, 7, 10377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turni, C.; Pyke, M.; Blackall, P.J. Validation of a real-time PCR for Haemophilus parasuis. J. Appl. Microbiol. 2010, 108, 1323–1331. [Google Scholar] [CrossRef]
- Stringer, O.W.; Bosse, J.T.; Lacouture, S.; Gottschalk, M.; Fodor, L.; Angen, O.; Velazquez, E.; Penny, P.; Lei, L.; Langford, P.R.; et al. Proposal of Actinobacillus pleuropneumoniae serovar 19, and reformulation of previous multiplex PCRs for capsule-specific typing of all known serovars. Vet. Microbiol. 2021, 255, 109021. [Google Scholar] [CrossRef]
- Townsend, K.M.; Boyce, J.D.; Chung, J.Y.; Frost, A.J.; Adler, B. Genetic organization of Pasteurella multocida cap Loci and development of a multiplex capsular PCR typing system. J. Clin. Microbiol. 2001, 39, 924–929. [Google Scholar] [CrossRef] [Green Version]
- Hozbor, D.; Fouque, F.; Guiso, N. Detection of Bordetella bronchiseptica by the polymerase chain reaction. Res. Microbiol. 1999, 150, 333–341. [Google Scholar]
- Kerdsin, A.; Akeda, Y.; Hatrongjit, R.; Detchawna, U.; Sekizaki, T.; Hamada, S.; Gottschalk, M.; Oishi, K. Streptococcus suis serotyping by a new multiplex PCR. J. Med. Microbiol. 2014, 63, 824–830. [Google Scholar] [CrossRef] [Green Version]
- Olvera, A.; Sibila, M.; Calsamiglia, M.; Segales, J.; Domingo, M. Comparison of porcine circovirus type 2 load in serum quantified by a real time PCR in postweaning multisystemic wasting syndrome and porcine dermatitis and nephropathy syndrome naturally affected pigs. J. Virol. Methods 2004, 117, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Martin de la Fuente, A.J.; Rodriguez-Ferri, E.F.; Frandoloso, R.; Martinez, S.; Tejerina, F.; Gutierrez-Martin, C.B. Systemic antibody response in colostrum-deprived pigs experimentally infected with Haemophilus parasuis. Res. Vet. Sci. 2009, 86, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Macedo, N.; Gottschalk, M.; Strutzberg-Minder, K.; Van, C.N.; Zhang, L.; Zou, G.; Zhou, R.; Marostica, T.; Clavijo, M.J.; Tucker, A.; et al. Molecular characterization of Glaesserella parasuis strains isolated from North America, Europe and Asia by serotyping PCR and LS-PCR. Vet. Res. 2021, 52, 68. [Google Scholar] [CrossRef] [PubMed]
- Schuwerk, L.; Hoeltig, D.; Waldmann, K.H.; Strutzberg-Minder, K.; Valentin-Weigand, P.; Rohde, J. Serotyping and pathotyping of Glaesserella parasuis isolated 2012-2019 in Germany comparing different PCR-based methods. Vet. Res. 2020, 51, 137. [Google Scholar] [CrossRef]
- Frandoloso, R.; Schryvers, A.B. Structure-based antigen design: Targeting transferrin receptors to prevent respiratory and systemic disease of swine. In Proceedings of the International Pig Veterinary Society Congress—IPVS2020, Rio de Janeiro, Brazil, 2–5 June 2020. [Google Scholar]
- Frandoloso, R.; Martinez, S.; Rodriguez-Ferri, E.F.; Garcia-Iglesias, M.J.; Perez-Martinez, C.; Martinez-Fernandez, B.; Gutierrez-Martin, C.B. Development and characterization of protective Haemophilus parasuis subunit vaccines based on native proteins with affinity to porcine transferrin and comparison with other subunit and commercial vaccines. Clin. Vaccine Immunol. 2011, 18, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Del Rio, M.L.; Gutierrez, C.B.; Rodriguez Ferri, E.F. Value of indirect hemagglutination and coagglutination tests for serotyping Haemophilus parasuis. J. Clin. Microbiol. 2003, 41, 880–882. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Liu, H.; Xue, Y.; Chen, K.; Liu, Z.; Xue, Q.; Wang, C. Analysis of efficacy obtained with a trivalent inactivated Haemophilus parasuis serovars 4, 5, and 12 vaccine and commercial vaccines against Glasser’s disease in piglets. Can. J. Vet. Res. 2017, 81, 22–27. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos Prigol, S.; Klein, R.; Chaudhuri, S.; Paraboni Frandoloso, G.; Guizzo, J.A.; Gutiérrez Martín, C.B.; Schryvers, A.B.; Kreutz, L.C.; Frandoloso, R. TbpBY167A-Based Vaccine Can Protect Pigs against Glässer’s Disease Triggered by Glaesserella parasuis SV7 Expressing TbpB Cluster I. Pathogens 2022, 11, 766. https://doi.org/10.3390/pathogens11070766
Ramos Prigol S, Klein R, Chaudhuri S, Paraboni Frandoloso G, Guizzo JA, Gutiérrez Martín CB, Schryvers AB, Kreutz LC, Frandoloso R. TbpBY167A-Based Vaccine Can Protect Pigs against Glässer’s Disease Triggered by Glaesserella parasuis SV7 Expressing TbpB Cluster I. Pathogens. 2022; 11(7):766. https://doi.org/10.3390/pathogens11070766
Chicago/Turabian StyleRamos Prigol, Simone, Rafaela Klein, Somshukla Chaudhuri, Gabriela Paraboni Frandoloso, João Antônio Guizzo, César Bernardo Gutiérrez Martín, Anthony Bernard Schryvers, Luiz Carlos Kreutz, and Rafael Frandoloso. 2022. "TbpBY167A-Based Vaccine Can Protect Pigs against Glässer’s Disease Triggered by Glaesserella parasuis SV7 Expressing TbpB Cluster I" Pathogens 11, no. 7: 766. https://doi.org/10.3390/pathogens11070766
APA StyleRamos Prigol, S., Klein, R., Chaudhuri, S., Paraboni Frandoloso, G., Guizzo, J. A., Gutiérrez Martín, C. B., Schryvers, A. B., Kreutz, L. C., & Frandoloso, R. (2022). TbpBY167A-Based Vaccine Can Protect Pigs against Glässer’s Disease Triggered by Glaesserella parasuis SV7 Expressing TbpB Cluster I. Pathogens, 11(7), 766. https://doi.org/10.3390/pathogens11070766






