Pfkelch13 Plasmodium falciparum Mutations in Huambo, Angola
Abstract
1. Introduction
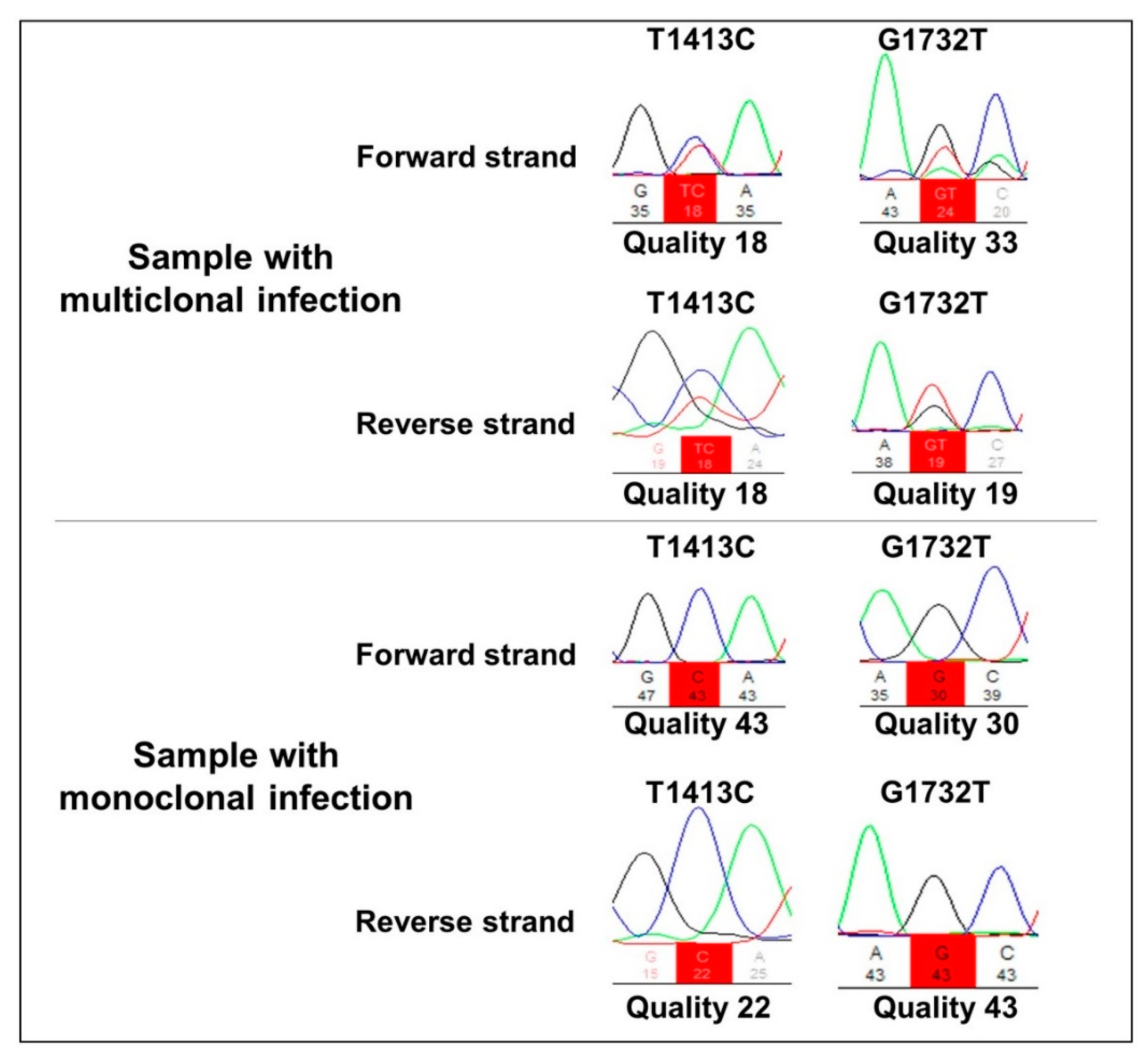
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population and Sampling
4.2. Ethical Aspects
4.3. Malaria Diagnosis
4.4. DNA Extraction, Amplification, and Sequencing
4.5. Sequence Analyses of Polymorphisms
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brasil, P.; Zalis, M.G.; de Pina-Costa, A.; Siqueira, A.M.; Júnior, C.B.; Silva, S.; Areas, A.L.L.; Pelajo-Machado, M.; de Alvarenga, D.A.M.; da Silva Santelli, A.C.F.; et al. Outbreak of human malaria caused by Plasmodium simium in the Atlantic Forest in Rio de Janeiro: A molecular epidemiological investigation. Lancet Glob. Health 2017, 5, e1038–e1046. [Google Scholar] [CrossRef]
- Singh, B.; Kim, S.L.; Matusop, A.; Radhakrishnan, A.; Shamsul, S.S.; Cox-Singh, J.; Thomas, A.; Conway, D.J. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 2004, 363, 1017–1024. [Google Scholar] [CrossRef]
- Ta, T.H.; Hisam, S.; Lanza, M.; Jiram, A.I.; Ismail, N.; Rubio, J.M. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar. J. 2014, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021/ (accessed on 2 April 2022).
- Sherrard-Smith, E.; Hogan, A.B.; Hamlet, A.; Watson, O.J.; Whittaker, C.; Winskill, P.; Ali, F.; Mohammad, A.B.; Uhomoibhi, P.; Maikore, I.; et al. The potential public health consequences of COVID-19 on malaria in Africa. Nat. Med. 2020, 26, 1411–1416. [Google Scholar] [CrossRef]
- United Nations Organization. Malaria Contaminated 228 Million and Killed 405,000 People Last Year. 2019. Available online: https://news.un.org/pt/story/2019/12/1696561 (accessed on 22 October 2020).
- Meibalan, E.; Marti, M. Biology of Malaria Transmission. Cold Spring Harb. Perspect. 2017, 7, a025452. [Google Scholar] [CrossRef] [PubMed]
- Fairhurst, R.M.; Dondorp, A.M. Artemisinin-Resistant Plasmodium falciparum Malaria. Microbiol. Spectr. 2016, 4, 409–429. [Google Scholar] [CrossRef] [PubMed]
- Miotto, O.; Sekihara, M.; Tachibana, S.I.; Yamauchi, M.; Pearson, R.D.; Amato, R.; Gonçalves, S.; Mehra, S.; Noviyanti, R.; Marfurt, J.; et al. Emergence of artemisinin-resistant Plasmodium falciparum with kelch13 C580Y mutations on the island of New Guinea. PLoS Pathog. 2020, 16, 1009133. [Google Scholar] [CrossRef]
- Ariey, F.; Witkowski, B.; Amaratunga, C.; Beghain, J.; Langlois, A.C.; Khim, N.; Kim, S.; Duru, V.; Bouchier, C.; Ma, L.; et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014, 505, 50–55. [Google Scholar] [CrossRef]
- Ménard, D.; Khim, N.; Beghain, J.; Adegnika, A.A.; Shafiul-Alam, M.; Amodu, O.; Rahim-Awab, G.; Barnadas, C.; Berry, A.; Boum, Y.; et al. A Worldwide Map of Plasmodium falciparum K13-Propeller Polymorphisms. N. Engl. J. Med. 2016, 374, 2453–2464. [Google Scholar] [CrossRef]
- Coppée, R.; Jeffares, D.C.; Miteva, M.A.; Sabbagh, A.; Clain, J. Comparative structural and evolutionary analyses predict functional sites in the artemisinin resistance malaria protein K13. Sci. Rep. 2019, 9, 10675. [Google Scholar] [CrossRef]
- Dondorp, A.M.; Nosten, F.; Yi, P.; Das, D.; Phyo, A.P.; Tarning, J.; Lwin, K.M.; Ariey, F.; Hanpithakpong, W.; Lee, S.J. Artemisinin Resistance in Plasmodium falciparum Malaria. N. Engl. J. Med. 2009, 361, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Escobar, C.; Pateira, S.; Lobo, E.; Lobo, L.; Teodosio, R.; Dias, F.; Fernandes, N.; Arez, A.P.; Varandas, L.; Nogueira, F. Polymorphisms in Plasmodium falciparum K13-Propeller in Angola and Mozambique after the Introduction of the ACTs. PLoS ONE 2015, 10, e0119215. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.M.; Parobek, C.M.; DeConti, D.K.; Kayentao, K.; Coulibaly, S.O.; Greenwood, B.M.; Tagbor, H.; Williams, J.; Bojang, K.; Njie, F.; et al. Absence of Putative Artemisinin Resistance Mutations Among Plasmodium falciparum in Sub-Saharan Africa: A Molecular Epidemiologic Study. J. Infect Dis. 2015, 211, 680–688. [Google Scholar] [CrossRef]
- Kamau, E.; Campino, S.; Amenga-Etego, L.; Drury, E.; Ishengoma, D.; Johnson, K.; Mumba, D.; Kekre, M.; Yavo, W.; Mead, D.; et al. K13-propeller polymorphisms in Plasmodium falciparum parasites from sub-Saharan Africa. J. Infect. Dis. 2015, 211, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yang, C.; Li, S.; Zhao, Y.; Liu, Y.; Qian, D.; Wang, H.; Lu, D.; Zhang, H.; Huang, F. Molecular Surveillance of Drug Resistance of Plasmodium falciparum Isolates Imported from Angola in Henan Province, China. Antimicrob. Agents Chemother. 2019, 63, e00552-19. [Google Scholar] [CrossRef]
- Huang, B.; Deng, C.; Yang, T.; Xue, L.; Wang, Q.; Huang, S.; Su, X.Z.; Liu, Y.; Zheng, S.; Guan, Y.; et al. Polymorphisms of the artemisinin-resistant marker (K13) in Plasmodium falciparum parasite populations of Grande Comore Island 10 years after artemisinin combination therapy. Parasit. Vectors 2015, 8, 634. [Google Scholar] [CrossRef]
- Maïga-Ascofaré, O.; May, J. Is the A578S Single-Nucleotide Polymorphism inK13-propeller Marker of Emerging Resistance to Artemisinin Among Plasmodium falciparum in Africa? J. Infect. Dis. 2016, 213, 165–166. [Google Scholar] [CrossRef]
- Bergmann, C.; Van Loon, W.; Habarugira, F.; Tacoli, C.; Jäger, J.C.; Savelsberg, D.; Nshimiyimana, F.; Rwamugem, E.; Mbarushimana, D.; Ndoli, J.; et al. Increase in Kelch 13 Polymorphisms in Plasmodium falciparum, Southern Rwanda. Emerg. Infect. Dis. 2021, 27, 294–296. [Google Scholar] [CrossRef]
- Uwimana, A.; Legrand, E.; Stokes, B.H.; Ndikumana, J.L.M.; Warsame, M.; Umulisa, N.; Ngamije, D.; Munyaneza, T.; Mazarati, J.B.; Munguti, K.; et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat. Med. 2020, 26, 1602–1608. [Google Scholar] [CrossRef]
- World Health Organization. Artemisinin Resistance and Artemisinin-Based Combination Therapy Efficacy: Status Report; World Health Organization: Geneva, Switzerland, 2018. Available online: https://apps.who.int/iris/handle/10665/274362 (accessed on 7 April 2022).
- Stokes, B.H.; Dhingra, S.K.; Rubiano, K.; Mok, S.; Straimer, J.; Gnädig, N.F.; Deni, I.; Schindler, K.A.; Bath, J.R.; Ward, K.E.; et al. Plasmodium falciparum K13 mutations in Africa and Asia impact artemisinin resistance and parasite fitness. Elife 2021, 10, 66277. [Google Scholar] [CrossRef]
- Balikagala, B.; Fukada, N.; Ikeda, M.; Katuro, O.T.; Tachibana, S.; Yamauchi, M.; Opio, W.; Emoto, S.; Anywar, D.A.; Kimura, E.; et al. Evidence of Arthemisinin-Resistant Malaria in Africa. N. Engl. J. Med. 2021, 385, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Paloque, L.; Coppée, R.; Stokes, B.H.; Gnädig, N.F.; Niaré, K.; Augereau, J.M.; Fidock, D.A.; Clain, J.; Benoit-Vical, F. Mutation in the Plasmodium falciparum BTB/POZ Domain of K13 Protein Confers Artemisinin Resistance. Antimicrob. Agents Chemother. 2022, 66, e0132021. [Google Scholar] [CrossRef] [PubMed]
- Plucinski, M.M.; Ferreira, M.; Ferreira, C.M.; Burns, J.; Gaparayi, P.; João, L.; da Costa, O.; Gill, P.; Samutondo, C.; Quivinja, J.; et al. Evaluating malaria case management at public health facilities in two provinces in Angola. Malar. J. 2017, 16, 186. [Google Scholar] [CrossRef]
- Zalis, M.G.; Ferreira-Da-Cruz, M.F.; Balthazar-Guedes, H.C.; Banic, D.M.; Alecrim, W.; Souza, J.M.; Druilhe, P.; Daniel-Ribeiro, C.T. Malaria diagnosis: Standardization of a polymerase chain reaction for the detection of Plasmodium falciparum parasites in individuals with low-grade parasitemia. Parasitol. Res. 1996, 82, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.R.; Lavigne, A.; Peterka, C.L.; Brasil, P.; Ménard, D.; Daniel-Ribeiro, C.T.; Ferreira-Da-Cruz, M.F. Absence of K13 Polymorphism in Plasmodium falciparum from Brazilian Areas Where the Parasite Is Endemic. Antimicrob. Agents Chemother. 2018, 62, e00354-18. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Weckx, S.; Del-Favero, J.; Rademakers, R.; Claes, L.; Cruts, M.; De Jonghe, P.; Van Broeckhoven, C.; De Rijk, P. novoSNP, a novel computational tool for sequence variation discovery. Genome Res. 2005, 15, 436–442. [Google Scholar] [CrossRef]
| Haplotypes | DNA Target Sequence | Frequency |
|---|---|---|
| T0 1 | FGNLCRTMAYVGATVPGNRIPVERMVPRAMCDEEQSIA | 66 (85%) |
| T1 2 | FGNLCRTMAYVGATVPGNRIPVERMVPRAMCDEEQSIA | 10 (13%) |
| T2 3 | FGNLCRTMAYVGATVPGNRIPVERMVPRSMCDEEQSIA | 2 (2%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, A.B.B.; de Abreu-Fernandes, R.; Neto, Z.; Jandondo, D.; Almeida-de-Oliveira, N.K.; de Lavigne Mello, A.R.; Morais, J.; Daniel-Ribeiro, C.T.; Menard, D.; Ferreira-da-Cruz, M.d.F. Pfkelch13 Plasmodium falciparum Mutations in Huambo, Angola. Pathogens 2022, 11, 554. https://doi.org/10.3390/pathogens11050554
Rodrigues ABB, de Abreu-Fernandes R, Neto Z, Jandondo D, Almeida-de-Oliveira NK, de Lavigne Mello AR, Morais J, Daniel-Ribeiro CT, Menard D, Ferreira-da-Cruz MdF. Pfkelch13 Plasmodium falciparum Mutations in Huambo, Angola. Pathogens. 2022; 11(5):554. https://doi.org/10.3390/pathogens11050554
Chicago/Turabian StyleRodrigues, Ana Beatriz Batista, Rebecca de Abreu-Fernandes, Zoraima Neto, Domingos Jandondo, Natália Ketrin Almeida-de-Oliveira, Aline Rosa de Lavigne Mello, Joana Morais, Cláudio Tadeu Daniel-Ribeiro, Didier Menard, and Maria de Fátima Ferreira-da-Cruz. 2022. "Pfkelch13 Plasmodium falciparum Mutations in Huambo, Angola" Pathogens 11, no. 5: 554. https://doi.org/10.3390/pathogens11050554
APA StyleRodrigues, A. B. B., de Abreu-Fernandes, R., Neto, Z., Jandondo, D., Almeida-de-Oliveira, N. K., de Lavigne Mello, A. R., Morais, J., Daniel-Ribeiro, C. T., Menard, D., & Ferreira-da-Cruz, M. d. F. (2022). Pfkelch13 Plasmodium falciparum Mutations in Huambo, Angola. Pathogens, 11(5), 554. https://doi.org/10.3390/pathogens11050554







