The Multifaceted Manifestations of Multisystem Inflammatory Syndrome during the SARS-CoV-2 Pandemic
Abstract
:1. Introduction
2. Coronavirus Infection
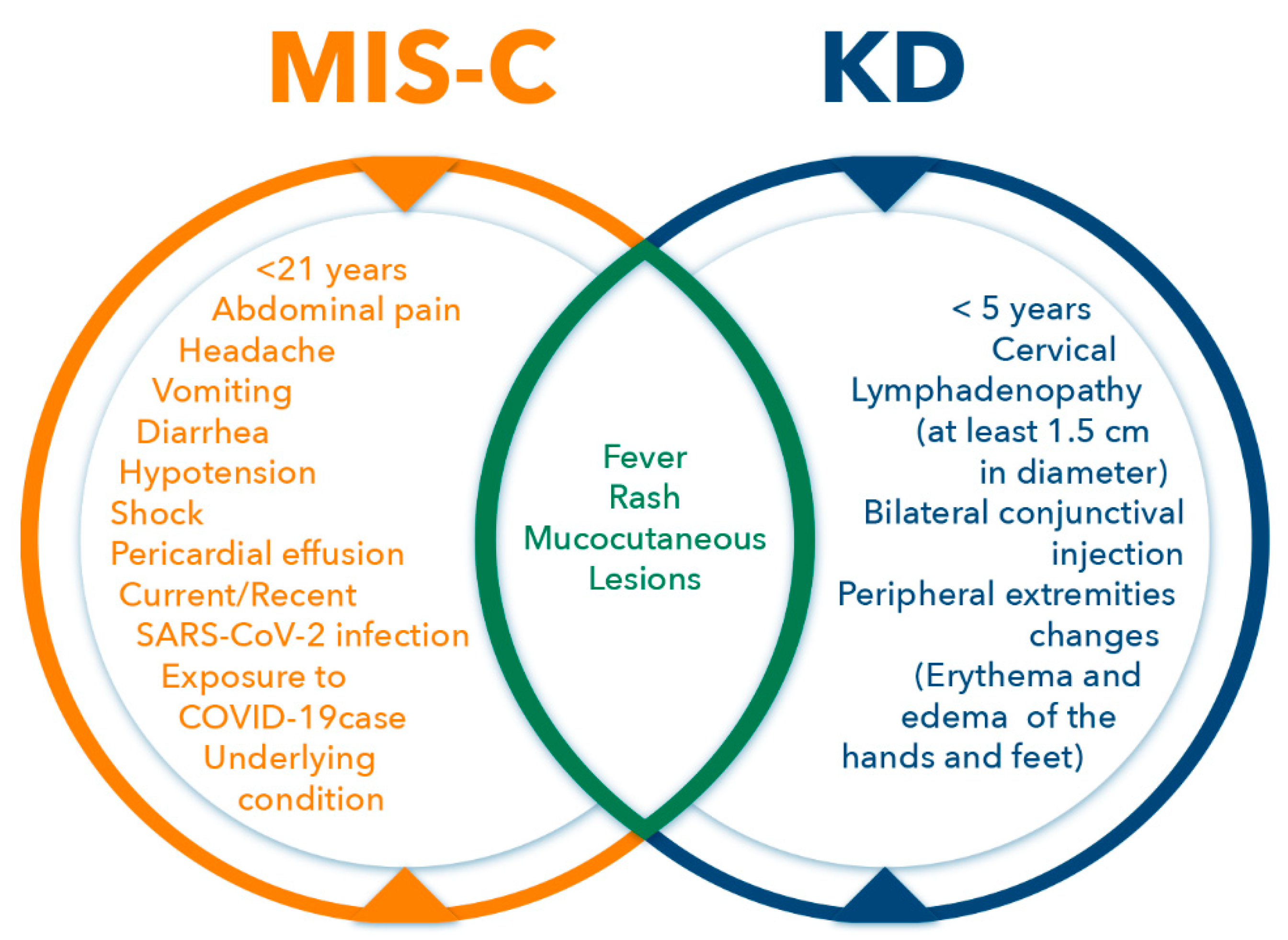
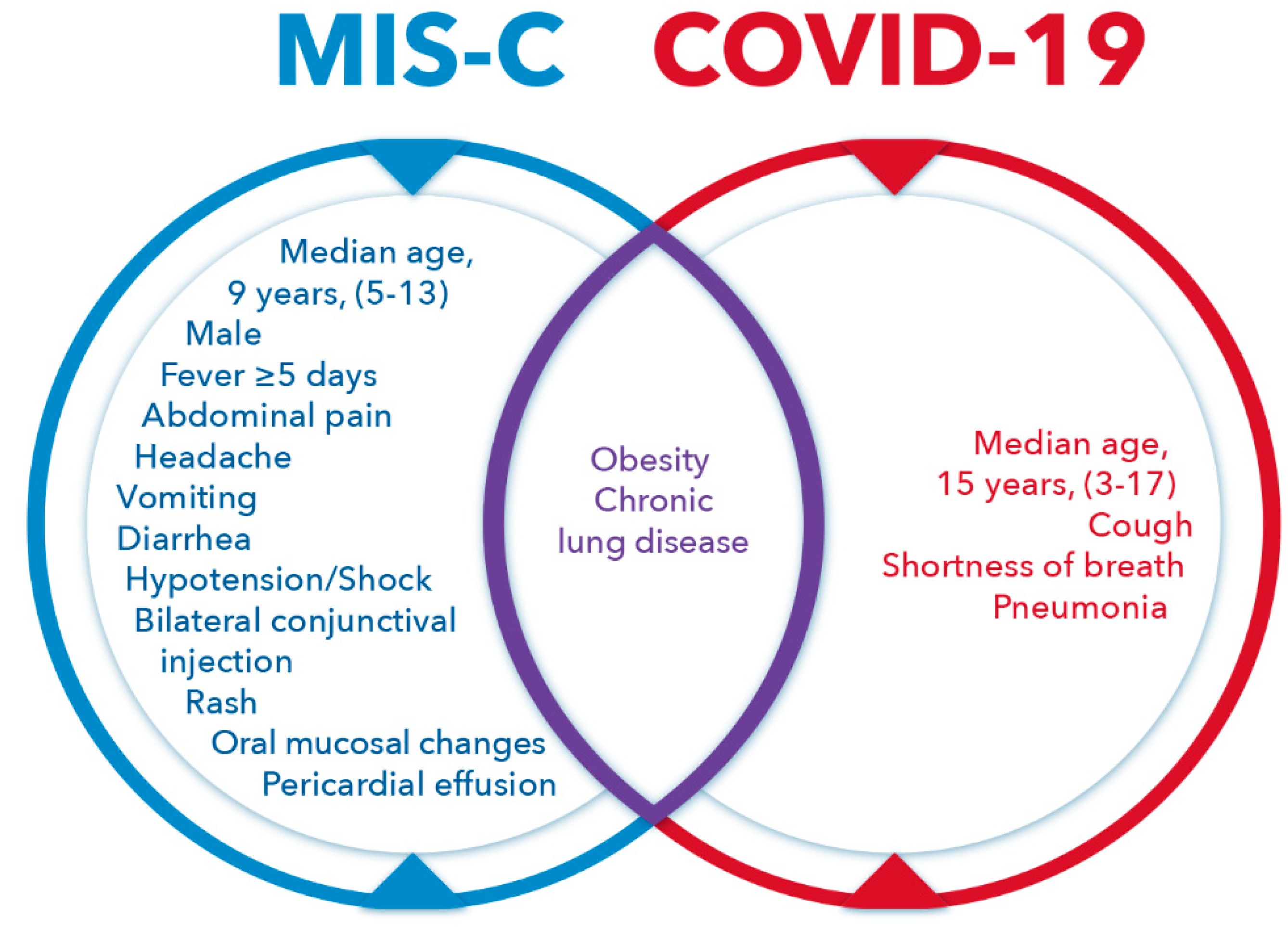
3. Multisystem Inflammatory Syndrome in Children (MIS-C)
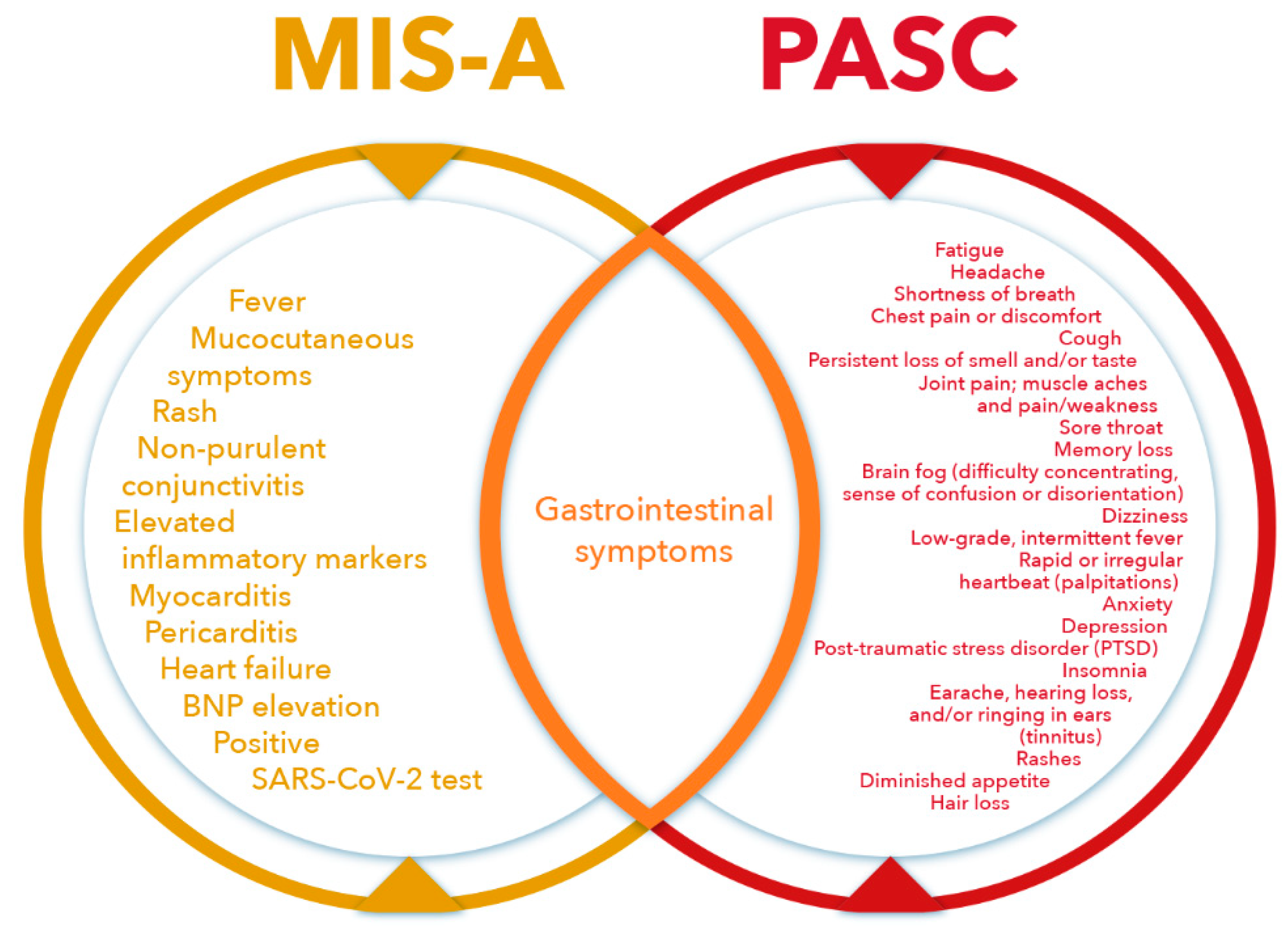
4. Multisystem Inflammatory Syndrome in Adults (MIS-A)
5. MIS-A and MIS-C after Vaccination
6. Postacute Sequelae of SARS-CoV-2 Infection
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Bradley, B.T.; Maioli, H.; Johnston, R.; Chaudhry, I.; Fink, S.L.; Xu, H.; Najafian, B.; Deutsch, G.; Lacy, J.M.; Williams, T.; et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: A case series. Lancet 2020, 396, 320–332. [Google Scholar] [CrossRef]
- Zhao, F.; Ma, Q.; Yue, Q.; Chen, H. SARS-CoV-2 Infection and Lung Regeneration. Clin. Microbiol. Rev. 2022, 35, e0018821. [Google Scholar] [CrossRef]
- Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021, 27, 28–33. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Killerby, M.E.; Biggs, H.M.; Haynes, A.; Dahl, R.M.; Mustaquim, D.; Gerber, S.I.; Watson, J.T. Human coronavirus circulation in the United States 2014-2017. J. Clin. Virol. 2018, 101, 52–56. [Google Scholar] [CrossRef]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, X.; Nair, H. Global Seasonality of Human Seasonal Coronaviruses: A Clue for Postpandemic Circulating Season of Severe Acute Respiratory Syndrome Coronavirus 2? J. Infect. Dis. 2020, 222, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Hui, D.; Wu, A.; Chan, P.; Cameron, P.; Joynt, G.M.; Ahuja, A.; Yung, M.Y.; Leung, C.B.; To, K.F.; et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003, 348, 1986–1994. [Google Scholar] [CrossRef]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.M.; Balkhy, H.H.; Hayden, F.G.; Bouchama, A.; Luke, T.; Baillie, J.K.; Al-Omari, A.; Hajeer, A.H.; Senga, M.; Denison, M.R.; et al. Middle East Respiratory Syndrome. N. Engl. J. Med. 2017, 376, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Hui, D.S.; Perlman, S. Middle East respiratory syndrome. Lancet 2015, 386, 995–1007. [Google Scholar] [CrossRef] [Green Version]
- Badawi, A.; Ryoo, S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): A systematic review and meta-analysis. Int. J. Infect. Dis. 2016, 49, 129–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Liang, W.; Jiang, M.; Guan, W.; Zhan, C.; Wang, T.; Tang, C.; Sang, L.; Liu, J.; Ni, Z.; et al. Risk Factors of Fatal Outcome in Hospitalized Subjects With Coronavirus Disease 2019 From a Nationwide Analysis in China. Chest 2020, 158, 97–105. [Google Scholar] [CrossRef]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; The Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.; Lai, S.T.; Poon, L.L.; Guan, Y.; Yam, L.Y.; Lim, W.; Nicholls, J.; Yee, W.K.; Yan, W.W.; Cheung, M.T.; et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 2003, 361, 1319–1325. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.W.; Xu, Y.M.; Lau, A.T.Y. Angiotensin-converting enzyme 2: The old door for new severe acute respiratory syndrome coronavirus 2 infection. Rev. Med. Virol. 2020, 30, e2122. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Sudre, C.H.; Lee, K.A.; Lochlainn, M.N.; Varsavsky, T.; Murray, B.; Graham, M.S.; Menni, C.; Modat, M.; Bowyer, R.C.E.; Nguyen, L.H.; et al. Symptom clusters in COVID-19: A potential clinical prediction tool from the COVID Symptom Study app. Sci. Adv. 2021, 7, eabd4177. [Google Scholar] [CrossRef]
- Koelle, K.; Martin, M.A.; Antia, R.; Lopman, B.; Dean, N.E. The changing epidemiology of SARS-CoV-2. Science 2022, 375, 1116–1121. [Google Scholar] [CrossRef]
- Madhi, S.A.; Kwatra, G.; Myers, J.E.; Jassat, W.; Dhar, N.; Mukendi, C.K.; Nana, A.J.; Blumberg, L.; Welch, R.; Ngorima-Mabhena, N.; et al. Population Immunity and Covid-19 Severity with Omicron Variant in South Africa. N. Engl. J. Med. 2022, 386, 1314–1326. [Google Scholar] [CrossRef]
- Bhalala, U.S.; Gist, K.M.; Tripathi, S.; Boman, K.; Kumar, V.K.; Retford, L.; Chiotos, K.; Blatz, A.M.; Dapul, H.; Verma, S.; et al. Characterization and Outcomes of Hospitalized Children With Coronavirus Disease 2019: A Report From a Multicenter, Viral Infection and Respiratory Illness Universal Study (Coronavirus Disease 2019) Registry. Crit. Care Med. 2022, 50, e40–e51. [Google Scholar] [CrossRef]
- Shi, D.S.; Whitaker, M.; Marks, K.J.; Anglin, O.; Milucky, J.; Patel, K.; Pham, H.; Chai, S.J.; Kawasaki, B.; Meek, J.; et al. Hospitalizations of Children Aged 5-11 Years with Laboratory-Confirmed COVID-19—COVID-NET, 14 States, March 2020-February 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 574–581. [Google Scholar] [CrossRef]
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef]
- Whittaker, E.; Bamford, A.; Kenny, J.; Kaforou, M.; Jones, C.E.; Shah, P.; Ramnarayan, P.; Fraisse, A.; Miller, O.; Davies, P.; et al. Clinical Characteristics of 58 Children with a Pediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2. JAMA 2020, 324, 259–269. [Google Scholar] [CrossRef]
- Davies, P.; Evans, C.; Kanthimathinathan, H.K.; Lillie, J.; Brierley, J.; Waters, G.; Johnson, M.; Griffiths, B.; du Pre, P.; Mohammad, Z.; et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: A multicentre observational study. Lancet Child Adolesc. Health 2020, 4, 669–677. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Dufort, E.M.; Koumans, E.H.; Chow, E.J.; Rosenthal, E.M.; Muse, A.; Rowlands, J.; Barranco, M.A.; Maxted, A.M.; Rosenberg, E.S.; Easton, D.; et al. Multisystem Inflammatory Syndrome in Children in New York State. N. Engl. J. Med. 2020, 383, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Godfred-Cato, S.; Bryant, B.; Leung, J.; Oster, M.E.; Conklin, L.; Abrams, J.; Roguski, K.; Wallace, B.; Prezzato, E.; Koumans, E.H.; et al. COVID-19-Associated Multisystem Inflammatory Syndrome in Children—United States, March–July 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.B.; Gilani, Z.; Godfred-Cato, S.; Belay, E.D.; Feldstein, L.R.; Patel, M.M.; Randolph, A.G.; Newhams, M.; Thomas, D.; Magleby, R.; et al. Incidence of Multisystem Inflammatory Syndrome in Children among US Persons Infected with SARS-CoV-2. JAMA Netw. Open 2021, 4, e2116420. [Google Scholar] [CrossRef]
- Belhadjer, Z.; Meot, M.; Bajolle, F.; Khraiche, D.; Legendre, A.; Abakka, S.; Auriau, J.; Grimaud, M.; Oualha, M.; Beghetti, M.; et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation 2020, 142, 429–436. [Google Scholar] [CrossRef]
- DeBiasi, R.L.; Harahsheh, A.S.; Srinivasalu, H.; Krishnan, A.; Sharron, M.P.; Parikh, K.; Smith, K.; Bell, M.; Michael, D.; Delaney, M.; et al. Multisystem Inflammatory Syndrome of Children: Subphenotypes, Risk Factors, Biomarkers, Cytokine Profiles, and Viral Sequencing. J. Pediatr. 2021, 237, 125–135.e18. [Google Scholar] [CrossRef]
- Matsubara, D.; Chang, J.; Kauffman, H.L.; Wang, Y.; Nadaraj, S.; Patel, C.; Paridon, S.M.; Fogel, M.A.; Quartermain, M.D.; Banerjee, A. Longitudinal Assessment of Cardiac Outcomes of Multisystem Inflammatory Syndrome in Children Associated with COVID-19 Infections. J. Am. Heart Assoc. 2022, 11, e023251. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kosaki, F.; Okawa, S.; Shigematsu, I.; Yanagawa, H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics 1974, 54, 271–276. [Google Scholar] [CrossRef]
- Shackelford, P.G.; Strauss, A.W. Kawasaki syndrome. N. Engl. J. Med. 1991, 324, 1664–1666. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T. Kawasaki disease. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2006, 82, 59–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef]
- Fujiwara, H.; Hamashima, Y. Pathology of the heart in Kawasaki disease. Pediatrics 1978, 61, 100–107. [Google Scholar] [CrossRef]
- Godfred-Cato, S.; Abrams, J.; Balachandran, N.; Jaggi, P.; Jones, K.; Rostad, C.; Lu, A.; Fan, L.; Jabbar, A.; Anderson, E.; et al. Distinguishing Multisystem Inflammatory Syndrome in Children from COVID-19, Kawasaki Disease and Toxic Shock Syndrome. Pediatr. Infect. Dis. J. 2022, 41, 315–323. [Google Scholar] [CrossRef] [PubMed]
- LaRovere, K.L.; Riggs, B.J.; Poussaint, T.Y.; Young, C.C.; Newhams, M.M.; Maamari, M.; Walker, T.C.; Singh, A.R.; Dapul, H.; Hobbs, C.V.; et al. Neurologic Involvement in Children and Adolescents Hospitalized in the United States for COVID-19 or Multisystem Inflammatory Syndrome. JAMA Neurol. 2021, 78, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Radia, T.; Williams, N.; Agrawal, P.; Harman, K.; Weale, J.; Cook, J.; Gupta, A. Multi-system inflammatory syndrome in children & adolescents (MIS-C): A systematic review of clinical features and presentation. Paediatr. Respir. Rev. 2021, 38, 51–57. [Google Scholar] [CrossRef]
- Zhao, Y.; Yin, L.; Patel, J.; Tang, L.; Huang, Y. The inflammatory markers of multisystem inflammatory syndrome in children (MIS-C) and adolescents associated with COVID-19: A meta-analysis. J. Med. Virol. 2021, 93, 4358–4369. [Google Scholar] [CrossRef]
- Ahmed, M.; Advani, S.; Moreira, A.; Zoretic, S.; Martinez, J.; Chorath, K.; Acosta, S.; Naqvi, R.; Burmeister-Morton, F.; Burmeister, F.; et al. Multisystem inflammatory syndrome in children: A systematic review. EClinicalMedicine 2020, 26, 100527. [Google Scholar] [CrossRef]
- Zambrano, L.D.; Newhams, M.M.; Olson, S.M.; Halasa, N.B.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Kamidani, S.; Tarquinio, K.M.; Maddux, A.B.; et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA Vaccination Against Multisystem Inflammatory Syndrome in Children Among Persons Aged 12-18 Years—United States, July-December 2021. Morb. Mortal. Wkly. Rep. 2022, 71, 52–58. [Google Scholar] [CrossRef]
- Shaigany, S.; Gnirke, M.; Guttmann, A.; Chong, H.; Meehan, S.; Raabe, V.; Louie, E.; Solitar, B.; Femia, A. An adult with Kawasaki-like multisystem inflammatory syndrome associated with COVID-19. Lancet 2020, 396, e8–e10. [Google Scholar] [CrossRef]
- Morris, S.B.; Schwartz, N.G.; Patel, P.; Abbo, L.; Beauchamps, L.; Balan, S.; Lee, E.H.; Paneth-Pollak, R.; Geevarughese, A.; Lash, M.K.; et al. Case Series of Multisystem Inflammatory Syndrome in Adults Associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Davogustto, G.E.; Clark, D.E.; Hardison, E.; Yanis, A.H.; Lowery, B.D.; Halasa, N.B.; Wells, Q.S. Characteristics Associated with Multisystem Inflammatory Syndrome among Adults with SARS-CoV-2 Infection. JAMA Netw. Open 2021, 4, e2110323. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; DeCuir, J.; Abrams, J.; Campbell, A.P.; Godfred-Cato, S.; Belay, E.D. Clinical Characteristics of Multisystem Inflammatory Syndrome in Adults: A Systematic Review. JAMA Netw. Open 2021, 4, e2126456. [Google Scholar] [CrossRef] [PubMed]
- Belay, E.D.; Godfred Cato, S.; Rao, A.K.; Abrams, J.; Wilson, W.W.; Lim, S.; Newton-Cheh, C.; Melgar, M.; DeCuir, J.; Webb, B.; et al. Multisystem Inflammatory Syndrome in Adults after SARS-CoV-2 infection and COVID-19 vaccination. Clin. Infect. Dis. 2021, ciab936. [Google Scholar] [CrossRef]
- Salzman, M.B.; Huang, C.W.; O’Brien, C.M.; Castillo, R.D. Multisystem Inflammatory Syndrome after SARS-CoV-2 Infection and COVID-19 Vaccination. Emerg. Infect. Dis. 2021, 27, 1944–1948. [Google Scholar] [CrossRef]
- Nune, A.; Iyengar, K.P.; Goddard, C.; Ahmed, A.E. Multisystem inflammatory syndrome in an adult following the SARS-CoV-2 vaccine (MIS-V). BMJ Case Rep. 2021, 14, e243888. [Google Scholar] [CrossRef]
- Grome, H.N.; Threlkeld, M.; Threlkeld, S.; Newman, C.; Martines, R.B.; Reagan-Steiner, S.; Whitt, M.A.; Gomes-Solecki, M.; Nair, N.; Fill, M.M.; et al. Fatal Multisystem Inflammatory Syndrome in Adult after SARS-CoV-2 Natural Infection and COVID-19 Vaccination. Emerg. Infect. Dis. 2021, 27, 2914–2918. [Google Scholar] [CrossRef]
- Yalcinkaya, R.; Oz, F.N.; Polat, M.; Ucan, B.; Teke, T.A.; Kaman, A.; Ozdem, S.; Savas Sen, Z.; Cinni, R.G.; Tanir, G. A Case of Multisystem Inflammatory Syndrome in a 12-year-old Male after COVID-19 mRNA Vaccine. Pediatr. Infect. Dis. J. 2021, 41, e87–e89. [Google Scholar] [CrossRef]
- Buchhorn, R.; Meyer, C.; Schulze-Forster, K.; Junker, J.; Heidecke, H. Autoantibody Release in Children after Corona Virus mRNA Vaccination: A Risk Factor of Multisystem Inflammatory Syndrome? Vaccines 2021, 9, 1353. [Google Scholar] [CrossRef]
- Carfi, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients after Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Logue, J.K.; Franko, N.M.; McCulloch, D.J.; McDonald, D.; Magedson, A.; Wolf, C.R.; Chu, H.Y. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw. Open 2021, 4, e210830. [Google Scholar] [CrossRef] [PubMed]
- Ayoubkhani, D.; Khunti, K.; Nafilyan, V.; Maddox, T.; Humberstone, B.; Diamond, I.; Banerjee, A. Post-covid syndrome in individuals admitted to hospital with covid-19: Retrospective cohort study. BMJ 2021, 372, n693. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Barnett, J.; Brill, S.E.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; Hillman, T.E.; Jacob, J.; Jarvis, H.C.; et al. ‘Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021, 76, 396–398. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Hirschtick, J.L.; Titus, A.R.; Slocum, E.; Power, L.E.; Hirschtick, R.E.; Elliott, M.R.; McKane, P.; Fleischer, N.L. Population-based estimates of post-acute sequelae of SARS-CoV-2 infection (PASC) prevalence and characteristics. Clin. Infect. Dis. 2021, 73, 2055–2064. [Google Scholar] [CrossRef]
- Xie, Y.; Bowe, B.; Al-Aly, Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat. Commun. 2021, 12, 6571. [Google Scholar] [CrossRef]
- Bai, F.; Tomasoni, D.; Falcinella, C.; Barbanotti, D.; Castoldi, R.; Mule, G.; Augello, M.; Mondatore, D.; Allegrini, M.; Cona, A.; et al. Female gender is associated with long COVID syndrome: A prospective cohort study. Clin. Microbiol. Infect. 2021, 28, 611.e9–611.e16. [Google Scholar] [CrossRef]
- Bell, M.L.; Catalfamo, C.J.; Farland, L.V.; Ernst, K.C.; Jacobs, E.T.; Klimentidis, Y.C.; Jehn, M.; Pogreba-Brown, K. Post-acute sequelae of COVID-19 in a non-hospitalized cohort: Results from the Arizona CoVHORT. PLoS ONE 2021, 16, e0254347. [Google Scholar] [CrossRef]
- LaVergne, S.M.; Stromberg, S.; Baxter, B.A.; Webb, T.L.; Dutt, T.S.; Berry, K.; Tipton, M.; Haberman, J.; Massey, B.R.; McFann, K.; et al. A longitudinal SARS-CoV-2 biorepository for COVID-19 survivors with and without post-acute sequelae. BMC Infect. Dis. 2021, 21, 677. [Google Scholar] [CrossRef]
- Clark, D.E.; Dendy, J.M.; Li, D.L.; Crum, K.; Dixon, D.; George-Durrett, K.; Parikh, A.P.; Wassenaar, J.W.; Hughes, S.G.; Soslow, J.H. Cardiovascular magnetic resonance evaluation of soldiers after recovery from symptomatic SARS-CoV-2 infection: A case-control study of cardiovascular post-acute sequelae of SARS-CoV-2 infection (CV PASC). J. Cardiovasc. Magn. Reson. 2021, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.; Berg, S.; Berntson, L.; Berthold, E.; Brodin, P.; Backstrom, F.; Compagno, M.; Fasth, A.; Lingman Framme, J.; Horne, A.; et al. Population-based study of multisystem inflammatory syndrome associated with COVID-19 found that 36% of children had persistent symptoms. Acta Paediatr. 2022, 111, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Deitchman, A.N.; Torres, L.; Iyer, N.S.; Munter, S.E.; Nixon, C.C.; Donatelli, J.; Thanh, C.; Takahashi, S.; Hakim, J.; et al. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell. Rep. 2021, 36, 109518. [Google Scholar] [CrossRef]
- Arthur, J.M.; Forrest, J.C.; Boehme, K.W.; Kennedy, J.L.; Owens, S.; Herzog, C.; Liu, J.; Harville, T.O. Development of ACE2 autoantibodies after SARS-CoV-2 infection. PLoS ONE 2021, 16, e0257016. [Google Scholar] [CrossRef]
- Patterson, B.K.; Francisco, E.B.; Yogendra, R.; Long, E.; Pise, A.; Rodrigues, H.; Hall, E.; Herrera, M.; Parikh, P.; Guevara-Coto, J.; et al. Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection. Front. Immunol. 2021, 12, 746021. [Google Scholar] [CrossRef]
- Ryan, F.J.; Hope, C.M.; Masavuli, M.G.; Lynn, M.A.; Mekonnen, Z.A.; Yeow, A.E.L.; Garcia-Valtanen, P.; Al-Delfi, Z.; Gummow, J.; Ferguson, C.; et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. 2022, 20, 26. [Google Scholar] [CrossRef]
- Twohig, K.A.; Nyberg, T.; Zaidi, A.; Thelwall, S.; Sinnathamby, M.A.; Aliabadi, S.; Seaman, S.R.; Harris, R.J.; Hope, R.; Lopez-Bernal, J.; et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: A cohort study. Lancet Infect. Dis. 2022, 22, 35–42. [Google Scholar] [CrossRef]
- Hart, W.S.; Miller, E.; Andrew, N.J.; Waight, P.; Maini, P.K.; Funk, S.; Thompson, R.N. Generation time of the alpha and delta SARS-CoV-2 variants: An epidemiological analysis. Lancet Infect. Dis. 2022, 22, 603–610. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, L.; Misasi, J.; Pegu, A.; Zhang, Y.; Harris, D.R.; Olia, A.S.; Talana, C.A.; Yang, E.S.; Chen, M.; et al. Structural basis for potent antibody neutralization of SARS-CoV-2 variants including B.1.1.529. Science 2022, 376, eabn8897. [Google Scholar] [CrossRef]
- Menni, C.; Valdes, A.M.; Polidori, L.; Antonelli, M.; Penamakuri, S.; Nogal, A.; Louca, P.; May, A.; Figueiredo, J.C.; Hu, C.; et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: A prospective observational study from the ZOE COVID Study. Lancet 2022, 399, 1618–1624. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post COVID-19 Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, jiac136. [Google Scholar] [CrossRef]
- Kenny, G.; McCann, K.; O’Brien, C.; Savinelli, S.; Tinago, W.; Yousif, O.; Lambert, J.S.; O’Broin, C.; Feeney, E.R.; De Barra, E.; et al. Identification of Distinct Long COVID Clinical Phenotypes Through Cluster Analysis of Self-Reported Symptoms. Open Forum Infect. Dis. 2022, 9, ofac060. [Google Scholar] [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef]
- Liu, Y.H.; Chen, Y.; Wang, Q.H.; Wang, L.R.; Jiang, L.; Yang, Y.; Chen, X.; Li, Y.; Cen, Y.; Xu, C.; et al. One-Year Trajectory of Cognitive Changes in Older Survivors of COVID-19 in Wuhan, China: A Longitudinal Cohort Study. JAMA Neurol. 2022, e220461. [Google Scholar] [CrossRef]
- Hernandez-Romieu, A.C.; Carton, T.W.; Saydah, S.; Azziz-Baumgartner, E.; Boehmer, T.K.; Garret, N.Y.; Bailey, L.C.; Cowell, L.G.; Draper, C.; Mayer, K.H.; et al. Prevalence of Select New Symptoms and Conditions Among Persons Aged Younger Than 20 Years and 20 Years or Older at 31 to 150 Days After Testing Positive or Negative for SARS-CoV-2. JAMA Netw. Open 2022, 5, e2147053. [Google Scholar] [CrossRef]
- Anderson, E.M.; Diorio, C.; Goodwin, E.C.; McNerney, K.O.; Weirick, M.E.; Gouma, S.; Bolton, M.J.; Arevalo, C.P.; Chase, J.; Hicks, P.; et al. Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) Antibody Responses in Children With Multisystem Inflammatory Syndrome in Children (MIS-C) and Mild and Severe Coronavirus Disease 2019 (COVID-19). J. Pediatric. Infect. Dis. Soc. 2021, 10, 669–673. [Google Scholar] [CrossRef]
- Janssen, N.A.F.; Grondman, I.; de Nooijer, A.H.; Boahen, C.K.; Koeken, V.; Matzaraki, V.; Kumar, V.; He, X.; Kox, M.; Koenen, H.; et al. Dysregulated Innate and Adaptive Immune Responses Discriminate Disease Severity in COVID-19. J. Infect. Dis. 2021, 223, 1322–1333. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A.; Solomatina, L.; Chereshnev, V. SARS-CoV-2-Specific Immune Response and the Pathogenesis of COVID-19. Int. J. Mol. Sci. 2022, 23, 1716. [Google Scholar] [CrossRef]
- Junqueira, C.; Crespo, A.; Ranjbar, S.; de Lacerda, L.B.; Lewandrowski, M.; Ingber, J.; Parry, B.; Ravid, S.; Clark, S.; Schrimpf, M.R.; et al. FcgammaR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature 2022. [Google Scholar] [CrossRef]
- Afrin, L.B.; Weinstock, L.B.; Molderings, G.J. COVID-19 hyperinflammation and post-Covid-19 illness may be rooted in mast cell activation syndrome. Int. J. Infect. Dis. 2020, 100, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Caraffa, A.; Tete, G.; Gallenga, C.E.; Ross, R.; Kritas, S.K.; Frydas, I.; Younes, A.; Di Emidio, P.; Ronconi, G. Mast cells activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19. J. Biol. Regul. Homeost. Agents 2020, 34, 1629–1632. [Google Scholar] [CrossRef]
- Weinstock, L.B.; Brook, J.B.; Walters, A.S.; Goris, A.; Afrin, L.B.; Molderings, G.J. Mast cell activation symptoms are prevalent in Long-COVID. Int. J. Infect. Dis. 2021, 112, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Ricke, D.O. Two Different Antibody-Dependent Enhancement (ADE) Risks for SARS-CoV-2 Antibodies. Front. Immunol. 2021, 12, 640093. [Google Scholar] [CrossRef] [PubMed]
- McClary-Gutierrez, J.S.; Mattioli, M.C.; Marcenac, P.; Silverman, A.I.; Boehm, A.B.; Bibby, K.; Balliet, M.; de Los Reyes, F.L., 3rd; Gerrity, D.; Griffith, J.F.; et al. SARS-CoV-2 Wastewater Surveillance for Public Health Action. Emerg. Infect. Dis. 2021, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Gómez, H.R.; Morfín-Otero, R.; González-Díaz, E.; Esparza-Ahumada, S.; León-Garnica, G.; Rodríguez-Noriega, E. The Multifaceted Manifestations of Multisystem Inflammatory Syndrome during the SARS-CoV-2 Pandemic. Pathogens 2022, 11, 556. https://doi.org/10.3390/pathogens11050556
Pérez-Gómez HR, Morfín-Otero R, González-Díaz E, Esparza-Ahumada S, León-Garnica G, Rodríguez-Noriega E. The Multifaceted Manifestations of Multisystem Inflammatory Syndrome during the SARS-CoV-2 Pandemic. Pathogens. 2022; 11(5):556. https://doi.org/10.3390/pathogens11050556
Chicago/Turabian StylePérez-Gómez, Héctor Raúl, Rayo Morfín-Otero, Esteban González-Díaz, Sergio Esparza-Ahumada, Gerardo León-Garnica, and Eduardo Rodríguez-Noriega. 2022. "The Multifaceted Manifestations of Multisystem Inflammatory Syndrome during the SARS-CoV-2 Pandemic" Pathogens 11, no. 5: 556. https://doi.org/10.3390/pathogens11050556
APA StylePérez-Gómez, H. R., Morfín-Otero, R., González-Díaz, E., Esparza-Ahumada, S., León-Garnica, G., & Rodríguez-Noriega, E. (2022). The Multifaceted Manifestations of Multisystem Inflammatory Syndrome during the SARS-CoV-2 Pandemic. Pathogens, 11(5), 556. https://doi.org/10.3390/pathogens11050556





