Diagnosis of Coxiella burnetii Cattle Abortion: A One-Year Observational Study
Abstract
1. Introduction
2. Results
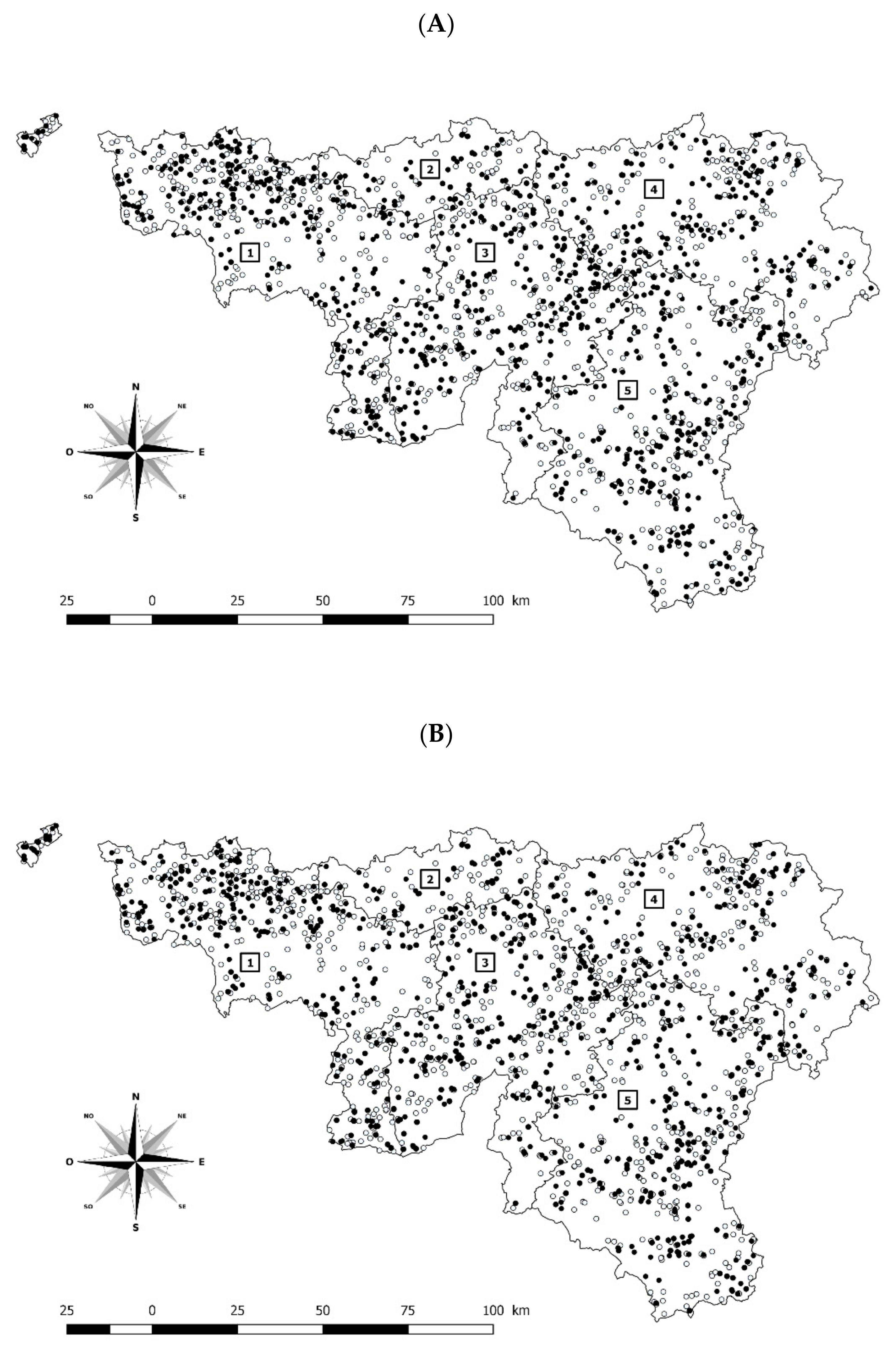
2.1. Representativeness of Samples
2.1.1. Representativeness of Samples for ELISA Performed All over the Study Period (Sera from the Mothers)
2.1.2. Representativeness of Samples for PCR Performed Only since 2020 (Tissues from Fetuses)
2.2. Prevalence of Coxiella burnetii Abortion Using Polymerase Chain Reaction
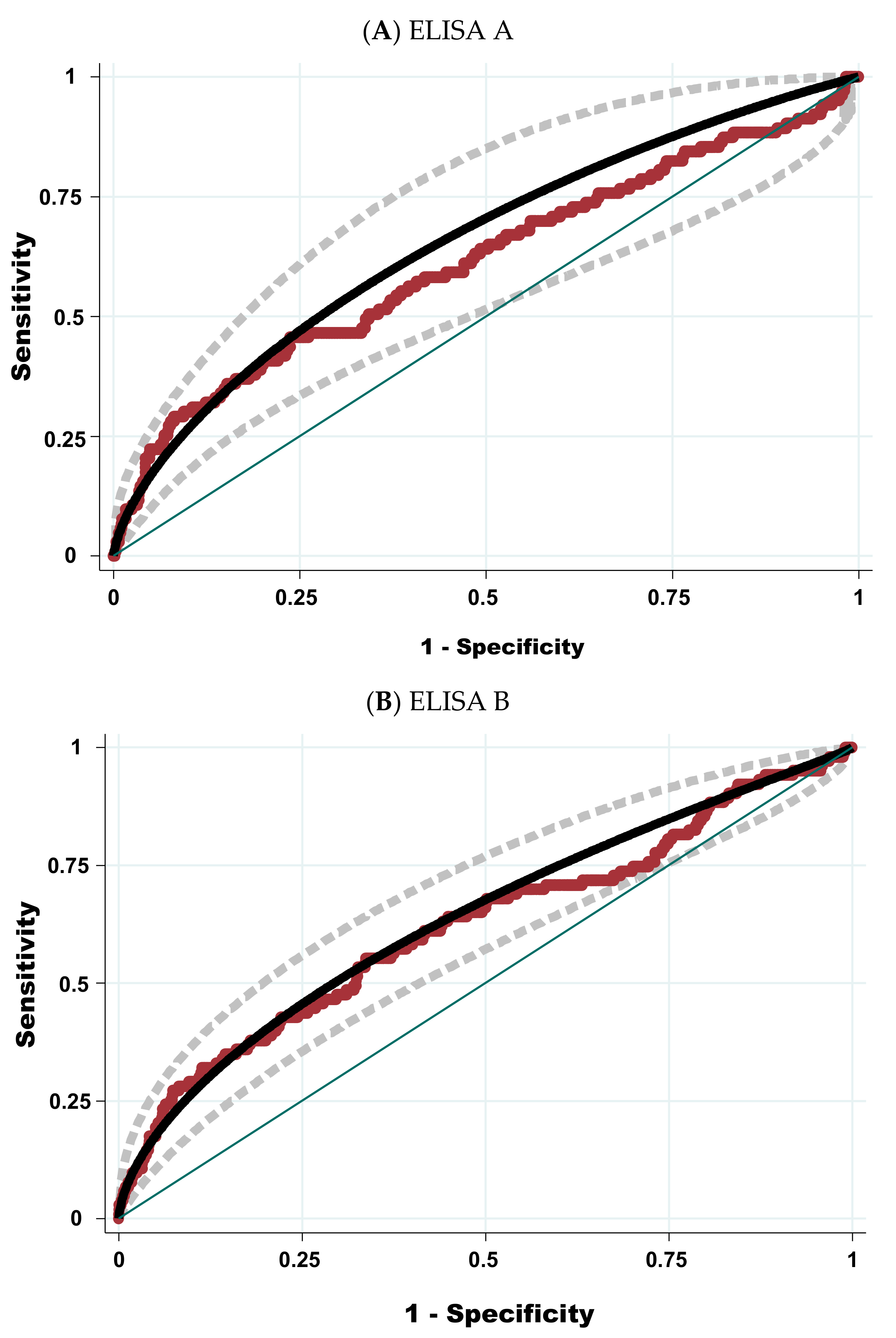
2.3. Estimation of the Sensitivity, Specificity, Positive and Negative Predictive Values of the Two ELISA Tests Relative to the Polymerase Chain Reaction
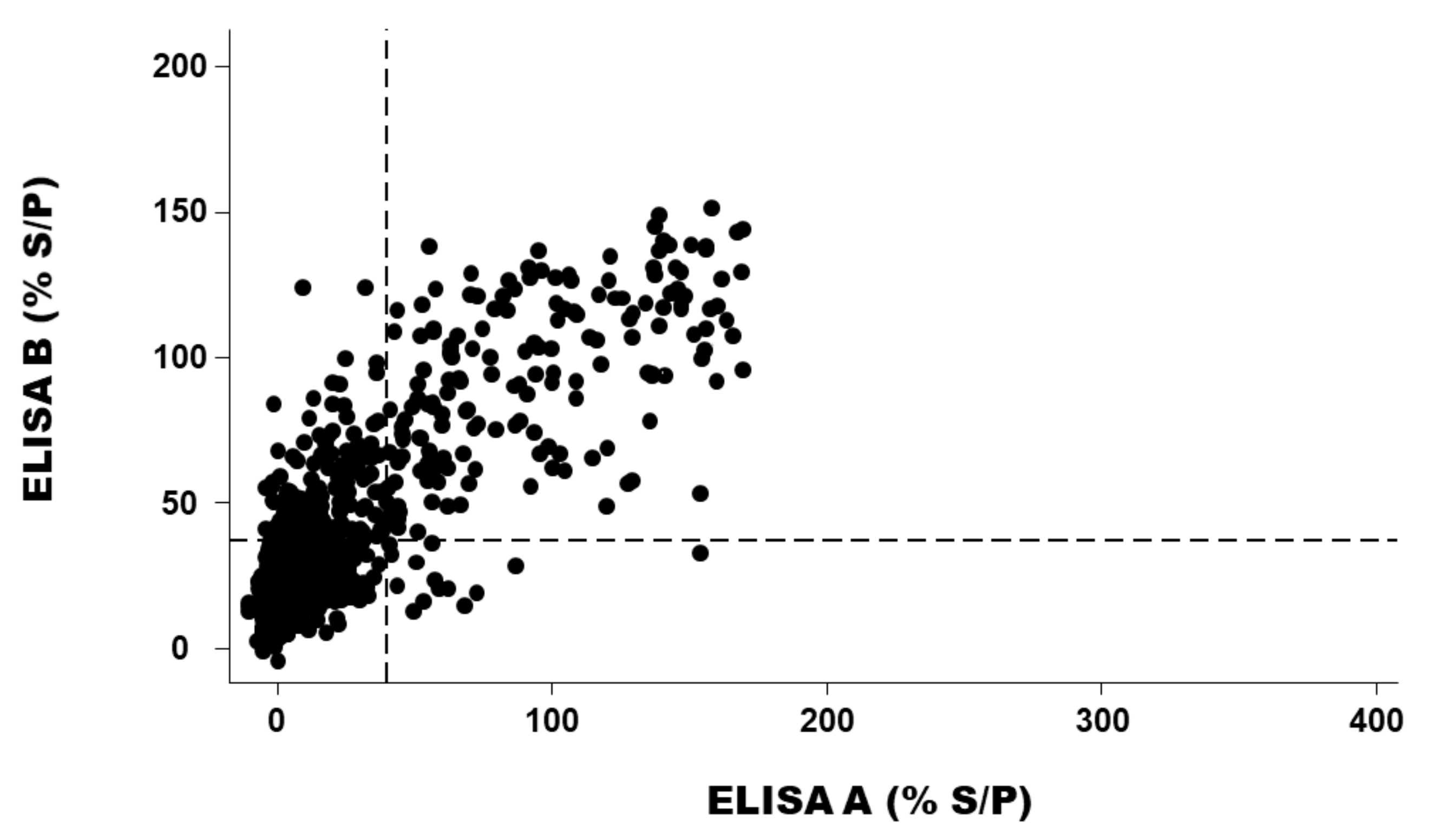
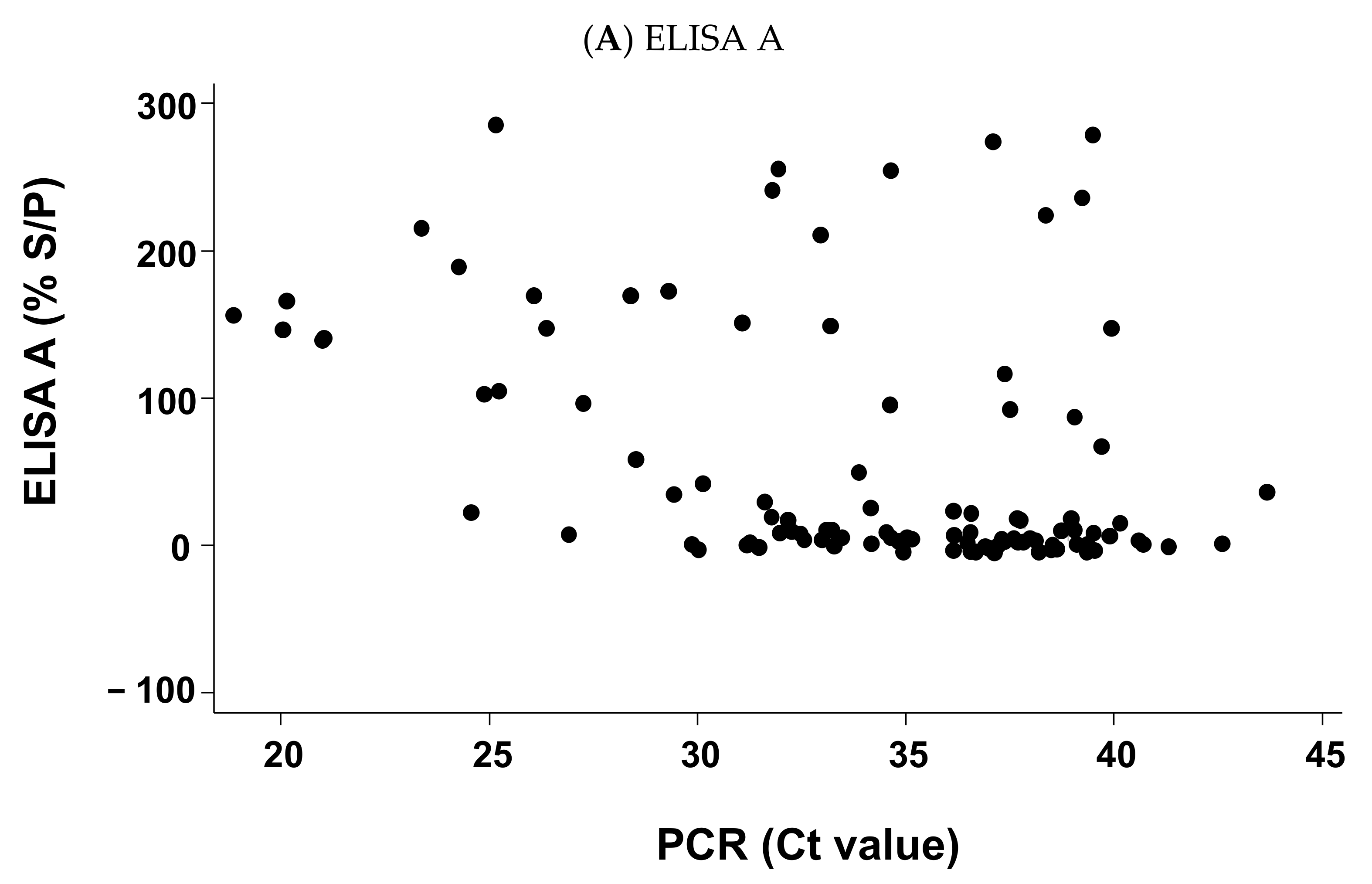
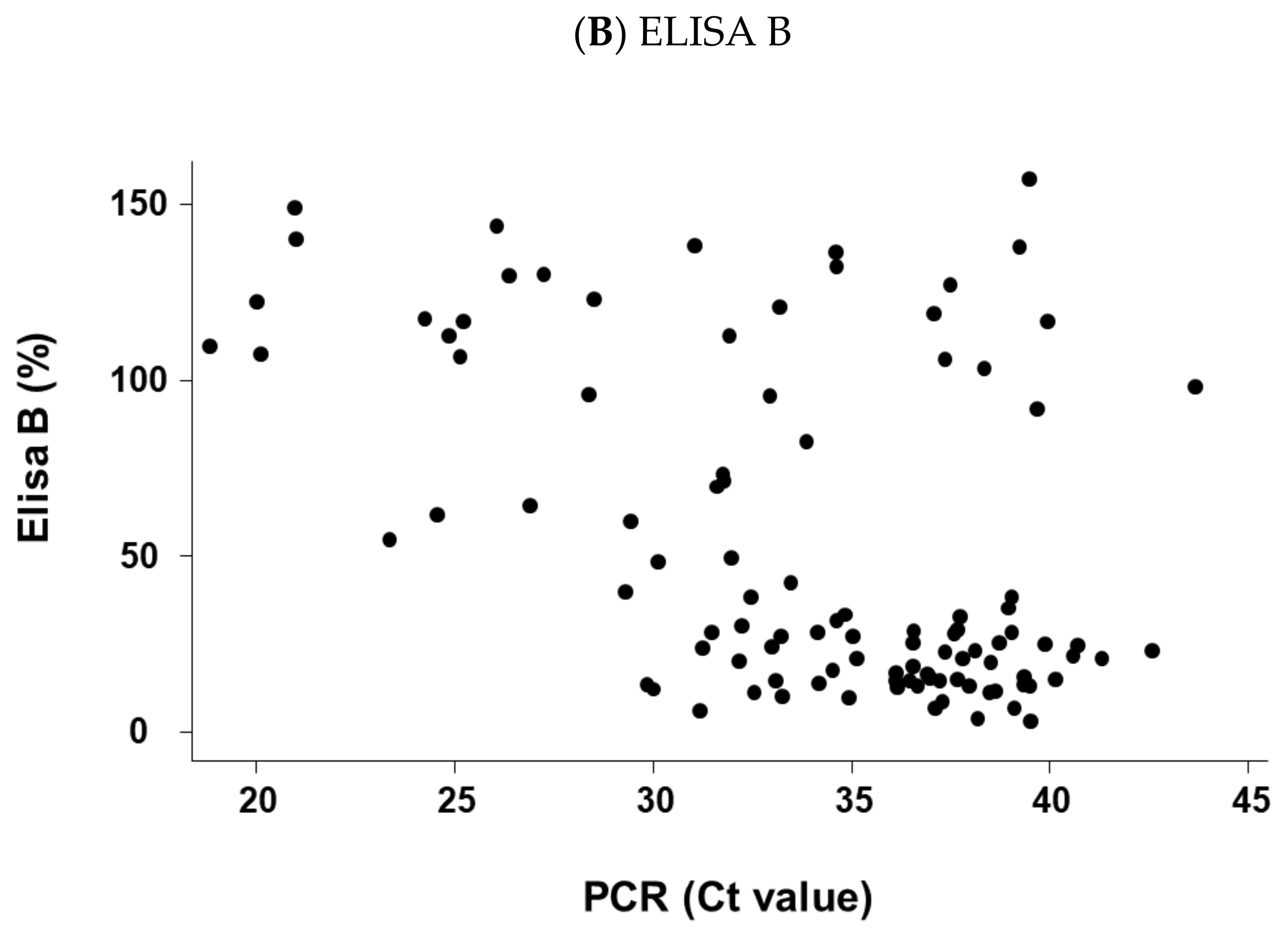
2.4. For Positive PCR Results Only, Relation between the Cycle Threshold (Ct) Values of the Sample Result of ELISA A or ELISA B
2.5. Explanatory Variables Related to Q Fever
2.6. Optimisation of the Receiver Operating Characteristic Curve (AUC-ROC) Using Additional Anamnestic Information
2.6.1. Use of Results of ELISA Test Only
2.6.2. Mixing Result of ELISA Test and Anamnestic Information to Increase the Area under the Receiver Operating Characteristic Curve
+ (si(C6 = “ Free-stall on slatted floors”; 1*3.45; si(C6 = “ Free-stall on
straw”; 1 × 2.55; 0))) + (C7 × 2) + (C8 × 4.85) + (C9 × 2.22) + (C10 × 1.83)
3. Discussion
4. Materials and Methods
4.1. Study Area
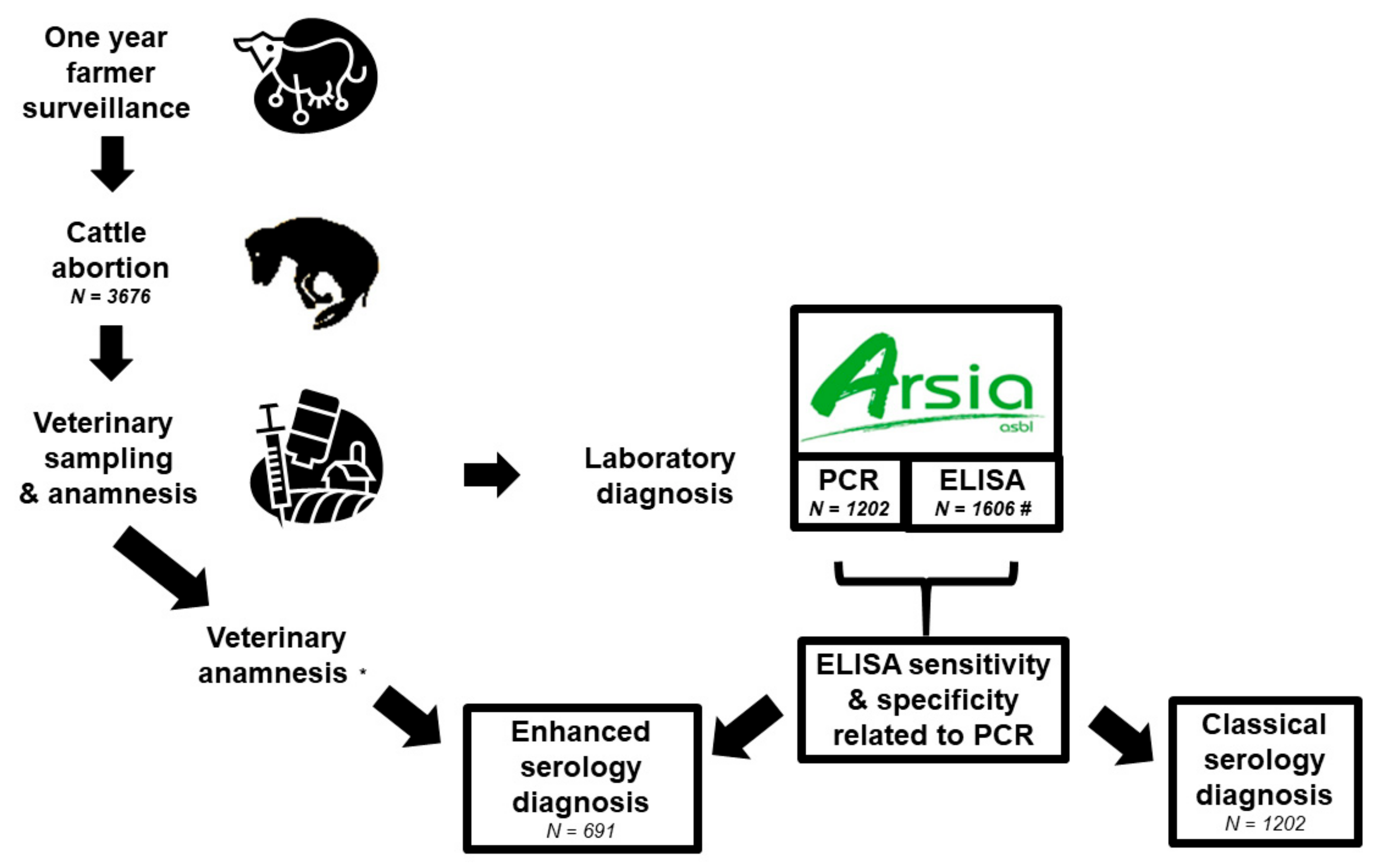
4.2. Sampling Design
4.3. Detection of Coxiella burnetii Using Polymerase Chain Reaction (PCR)
4.4. Enzyme-Linked Immunosorbent Assay (ELISA) Tests
4.4.1. First Indirect ELISA Test [A]
4.4.2. Second Indirect ELISA Test [B]
4.5. Statistical Analysis
4.5.1. Basis Statistics
4.5.2. Receiver Operating Characteristic Curve
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porter, S.R.; Czaplicki, G.; Mainil, J.; Guattéo, R.; Saegerman, C. Q Fever: Current state of knowledge and perspectives of research of a neglected zoonosis. Int. J. Microbiol. 2011, 2011, 248418. [Google Scholar] [CrossRef] [PubMed]
- Guatteo, R.; Seegers, H.; Taurel, A.-F.; Joly, A.; Beaudeau, F. Prevalence of Coxiella burnetii infection in domestic ruminants: A critical review. Vet. Microbiol. 2011, 149, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dal Pozzo, F.; Martinelle, L.; Léonard, P.; Renaville, B.; Renaville, R.; Thys, C.; Smeets, F.; Czaplicki, G.; Van Esbroeck, M.; Saegerman, C. Q fever serological survey and associated risk factors in veterinarians, southern Belgium, 2013. Transbound. Emerg. Dis. 2017, 64, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Tomaiuolo, S.; Boarbi, S.; Fancello, T.; Michel, P.; Desqueper, D.; Grégoire, F.; Callens, J.; Fretin, D.; Devriendt, B.; Cox, E.; et al. Phylogeography of human and animal Coxiella burnetii strains: Genetic fingerprinting of Q Fever in Belgium. Front. Cell Infect. Microbiol. 2021, 10, 625576. [Google Scholar] [CrossRef] [PubMed]
- Huijsmans, C.J.J.; Schellekens, J.J.A.; Wever, P.C.; Toman, R.; Savelkoul, P.H.M.; Janse, I.; Hermans, M.H. Single-nucleotide-polymorphism genotyping of Coxiella burnetii during a Q fever outbreak in The Netherlands. Appl. Environ. Microbiol. 2011, 77, 2051–2057. [Google Scholar] [CrossRef]
- Rabaza, A.; Giannitti, F.; Fraga, M.; Macías-Rioseco, M.; Corbellini, L.G.; Riet-Correa, F.; Hirigoyen, D.; Turner, K.M.E.; Eisler, M.C. Serological Evidence of Human Infection with Coxiella burnetii after Occupational Exposure to Aborting Cattle. Vet. Sci. 2021, 8, 196. [Google Scholar] [CrossRef]
- Pouquet, M.; Bareille, N.; Guatteo, R.; Moret, L.; Beaudeau, F. Coxiella burnetii infection in humans: To what extent do cattle in infected areas free from small ruminants play a role? Epidemiol. Infect. 2020, 148, e232. [Google Scholar] [CrossRef] [PubMed]
- Vanderburg, S.; Rubach, M.P.; Halliday, J.E.B.; Cleaveland, S.; Reddy, E.A.; Crump, J.A. Epidemiology of Coxiella burnetii infection in Africa: A OneHealth systematic review. PLoS Negl. Trop. Dis. 2014, 8, e2787. [Google Scholar] [CrossRef] [PubMed]
- Czaplicki, G.; Houtain, J.Y.; Mullender, C.; Porter, S.R.; Humblet, M.F.; Manteca, C.; Saegerman, C. Apparent prevalence of antibodies to Coxiella burnetii (Q fever) in bulk tank milk from dairy herds in southern Belgium. Vet. J. 2012, 192, 529–531. [Google Scholar] [CrossRef]
- Saegerman, C.; Speybroeck, N.; Dal Pozzo, F.; Czaplicki, G. Clinical indicators of exposure to Coxiella burnetii in dairy herds. Transbound. Emerg. Dis. 2015, 62, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Taurel, A.F.; Guatteo, R.; Lehebel, A.; Joly, A.; Beaudeau, F. Vaccination using phase I vaccine is effective to control Coxiella burnetii shedding in infected dairy cattle herds. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 1–9. [Google Scholar] [CrossRef]
- van Engelen, E.; Schotten, N.; Schimmer, B.; Hautvast, J.L.A.; van Schaik, G.; van Duijnhoven, Y.T.H.P. Prevalence and risk factors for Coxiella burnetii (Q fever) in Dutch dairy cattle herds based on bulk tank milk testing. Prev. Vet. Med. 2014, 117, 103–109. [Google Scholar] [CrossRef]
- Renault, V.; Damiaans, B.; Sarrazin, S.; Humblet, M.F.; Lomba, M.; Ribbens, S.; Riocreux, F.; Koenen, F.; Cassart, D.; Dewulf, J.; et al. Classification of adult cattle infectious diseases: A first step towards prioritization of biosecurity measures. Transbound. Emerg. Dis. 2018, 65, 1991–2005. [Google Scholar] [CrossRef] [PubMed]
- Hanzen, C.H. Propédeutique et Pathologies de la Reproduction Mâle et Femelle, 4th ed.; Biotechnologies de la Reproduction; Pathologies de la Glande Mammaire; Université de Liège, Faculté de Médecine Vétérinaire, Service d’Obstétrique et de Pathologie de la Reproduction des Ruminants, équidés et Porcs: Liege, Belgium, 2000. [Google Scholar]
- Bronner, A.; Hénaux, V.; Vergne, T.; Vinard, J.-L.; Morignat, E.; Hendrikx, P.; Calavas, D.; Gay, E. Assessing the mandatory bovine abortion notification system in France using unilist capture-recapture approach. PLoS ONE 2013, 8, e63246. [Google Scholar] [CrossRef]
- Parisi, A.; Fraccalvieri, R.; Cafiero, M.; Miccolupo, A.; Padalino, I.; Montagna, C.; Capuano, F.; Sottili, R. Diagnosis of Coxiella burnetii-related abortion in Italian domestic ruminants using single-tube nested PCR. Vet. Microbiol. 2006, 118, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Mioni, M.S.R.; Henker, L.C.; Teixeira, W.S.R.; Lorenzett, M.P.; Labruna, M.B.; Pavarini, S.P.; Driemeier, D.; Rousset, É.; Sidi-Boumedine, K.; Thiéry, R.; et al. Molecular detection of Coxiella burnetii in aborted bovine fetuses in Brazil. Acta Trop. 2021, 2021, 106258. [Google Scholar] [CrossRef] [PubMed]
- Fox-Lewis, A.; Isteed, K.; Austin, P.; Thompson-Faiva, H.; Wolfgang, J.; Ussher, J.E. A Case of Imported Q Fever in New Zealand. NZMJ 2019, 132, 92–94. Available online: https://journal.nzma.org.nz/journal-articles/a-case-of-imported-q-fever-in-new-zealand (accessed on 30 November 2021).
- Arricau-Bouvery, N.; Rodolakis, A. Is Q fever an emerging or reemerging zoonoses? Vet. Res. 2005, 36, 327–350. [Google Scholar] [CrossRef] [PubMed]
- Niemczuk, K.; Szymańska-Czerwińska, M.; Śmietanka, K.; Bocian, L. Comparison of diagnostic potential of serological, molecular and cell culture methods for detection of Q fever in ruminants. Vet. Microbiol. 2014, 171, 147–152. [Google Scholar] [CrossRef]
- Sidi-Boumedine, K.; Rousset, E.; Henning, K.; Ziller, M.; Niemczuk, K.; Roest, H.I.J.; Thiéry, R. Development of Harmonised Schemes for the Monitoring and Reporting of Q-Fever in Animals in the European Union; EFSA Scientific Report on Question No EFSA-Q-2009-00511; EFSA Supporting Publications: Parma, Italy, 2010; 48p. [Google Scholar]
- Guatteo, R.; Joly, A.; Beaudeau, F. Shedding and serological patterns of dairy cows following abortions associated with Coxiella burnetii DNA detection. Vet. Microbiol. 2012, 155, 430–433. [Google Scholar] [CrossRef]
- Vaidya, V.M.; Malik, S.V.S.; Kaur, S.; Kumar, S.; Barbuddhe, S.B. Comparison of PCR, immunofluorescence assay, and pathogen isolation for diagnosis of Q Fever in humans with spontaneous abortions. J. Clin. Microbiol. 2008, 46, 2038–2044. [Google Scholar] [CrossRef]
- Böttcher, J.; Vossen, A.; Janowetz, B.; Alex, M.; Gangl, A.; Randt, A.; Meier, N. Insights into the dynamics of endemic Coxiella burnetii infection in cattle by application of phase-specific ELISAs in an infected dairy herd. Vet. Microbiol. 2011, 151, 291–300. [Google Scholar] [CrossRef]
- Djellata, n.; Yahimi, A.; Hanzen, C.; Saegerman, C.; Kaidi, R. Prevalence and factors associated with a higher or lower risk of exposure to Coxiella burnetii, Chlamydia abortus and Toxoplasma gondii in dairy cows that have aborted in Algeria. Rev. Sci. Tech. 2019, 38, 761–786. [Google Scholar] [CrossRef] [PubMed]
- Agger, J.F.; Paul, P.; Christoffersen, A.-B.; Agerholm, J.S. Risk factors for Coxiella burnetii antibodies in bulk tank milk from Danish dairy herds. Acta Vet. Scand. 2013, 55, 80. [Google Scholar] [CrossRef] [PubMed]
- Simensen, E.; Østerås, O.; Bøe, K.E.; Kielland, K.; Ruud, L.E.; Næss, G. Housing system and herd size interactions in Norwegian dairy herds; associations with performance and disease incidence. Acta Vet. Scand. 2010, 52, 14. [Google Scholar] [CrossRef] [PubMed]
- Boroduske, A.; Trofimova, J.; Kibilds, J.; Papule, U.; Sergejeva, M.; Rodze, I.; Grantina-Ievina, L. Coxiella burnetii (Q fever) infection in dairy cattle and associated risk factors in Latvia. Epidemiol. Infect. 2017, 145, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Halos, L.; Bord, S.; Cotté, V.; Gasqui, P.; Abrial, D.; Barnouin, J.; Boulouis, H.J.; Vayssier-Taussat, M.; Vourch, G. Ecological Factors Characterizing the Prevalence of Bacterial Tick-Borne Pathogens in Ixodes ricinus Ticks in Pastures and Woodlands. Appl. Environ. Microbiol. 2010, 76, 4413–4420. [Google Scholar] [CrossRef]
- Eldin, C.; Mélenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.-L.; Maurin, M.; Raoult, D. From Q fever to Coxiella burnetii infection: A paradigm change. Clin. Microbiol. Rev. 2017, 30, 115–190. [Google Scholar] [CrossRef] [PubMed]
- Gardon, J.; Héraud, J.M.; Laventure, S.; Ladam, A.; Capot, P.; Fouquet, E.; Favre, J.; Weber, S.; Hommel, D.; Hulin, A.; et al. Suburban transmission of Q fever in French Guiana: Evidence of a wild reservoir. J. Infect. Dis. 2001, 184, 278–284. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taurel, A.-F.; Guatteo, R.; Joly, A.; Seegers, H.; Beaudeau, F. Seroprevalence of Q fever in naturally infected dairy cattle herds. Prev. Vet. Med. 2011, 101, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Piñero, A.; Ruiz-Fons, F.; Hurtado, A.; Barandika, J.; Atxaerandio, R.; García-Pérez, A. Changes in the dynamics of Coxiella burnetii infection in dairy cattle: An approach to match field data with the epidemiological cycle of C. burnetii in endemic herds. J. Dairy Sci. 2014, 97, 2718–2730. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Agger, J.F.; Markussen, B.; Christoffersen, A.B.; Agerholm, J.S. Factors associated with Coxiella burnetii antibody positivity in Danish dairy cows. Prev. Vet. Med. 2012, 107, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Nicas, M.; Hubbard, A.E.; Reingold, A.L. The infectious dose of Coxiella burnetii (Q Fever). Appl. Biosaf. 2006, 11, 32–41. [Google Scholar] [CrossRef]
- Delooz, L.; Czaplicki, G.; Gregoire, F.; Dal Pozzo, F.; Pez, F.; Kodjo, A.; Saegerman, C. Serogroups and genotypes of Leptospira spp. strains from bovine aborted foetuses. Transbound. Emerg. Dis. 2018, 65, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Arsia. Rapport Annuel 2020; ARSIA asbl, Ed.; Jean Detiffe: Ciney, Belgium, 2021; 81p, Available online: www.arsia.be (accessed on 28 December 2021).
- Saegerman, C.; Diep, A.N.; Renault, V.; Donneau, A.-F.; Stamatakis, L.; Coppieters, W.; Michel, F.; Breuer, C.; Dandoy, M.; Ek, O.; et al. A two-month field cohort study of SARS-CoV-2 in saliva of BNT162b2 vaccinated nursing home workers. Commun. Med. 2022, 2, 1. [Google Scholar] [CrossRef]
- Petrie, A.; Watson, P. Statistics for Veterinary and Animal Science; John Wiley & Sons, Ltd.: West Sussex, UK, 2013; 391p. [Google Scholar]
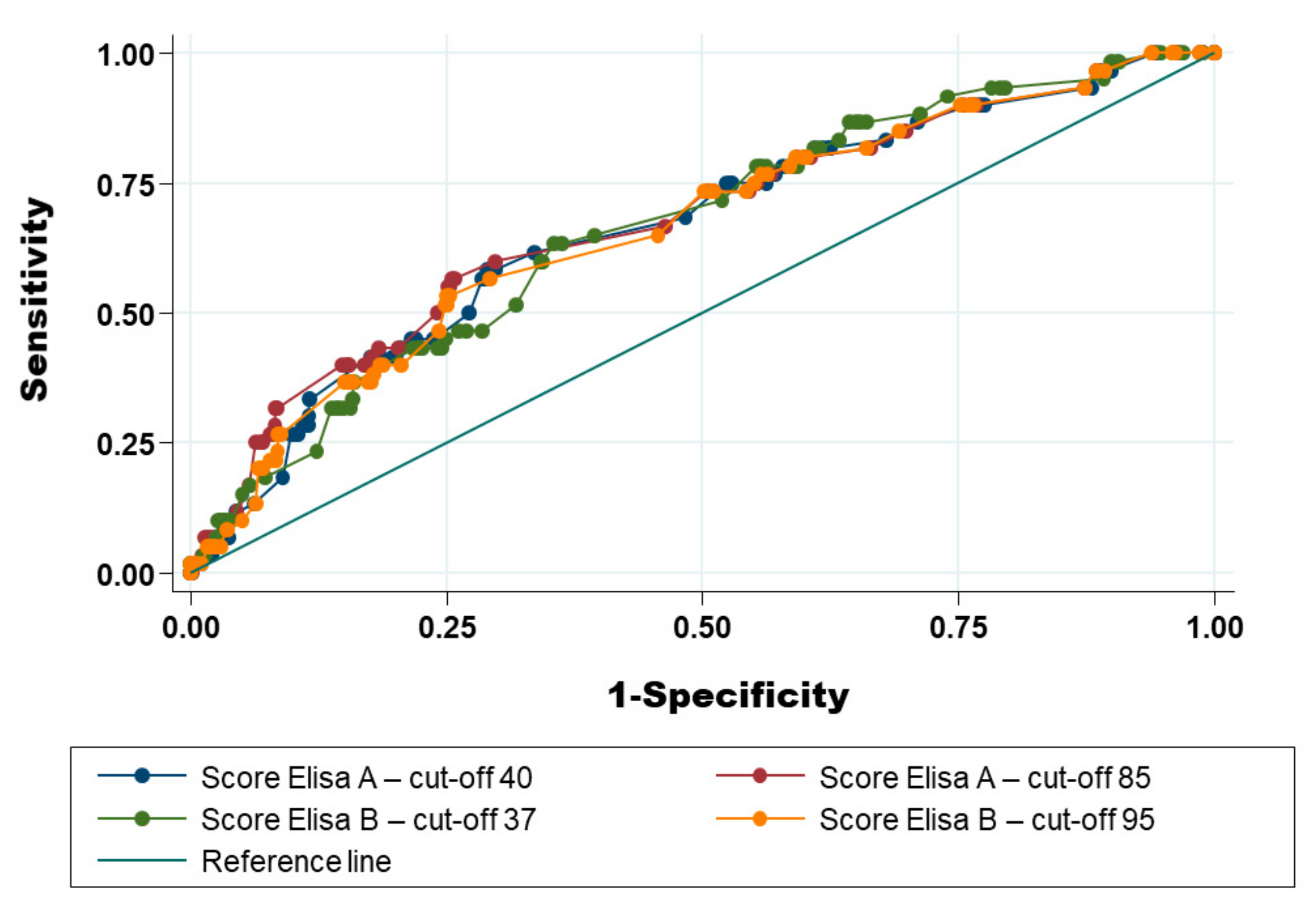
| Scenario and Cut-Off Used | Sensitivity in % (95% CI) | Specificity in % (95% CI) | Positive Predictive Value in % (95% CI) | Negative Predictive Value in % (95% CI) | Coefficient Kappa (95% CI) |
|---|---|---|---|---|---|
| ELISA [A] Cut-off: % S/P > 40 | 33.01 (24.06–42.97) | 86.02 (83.84–88.01) | 17.99 (12.79–24.22) | 93.26 (91.54–94.71) | 0.14 (0.08–0.19) |
| ELISA [A] Cut-off: % S/P > 85 | 28.16 (19.73–37.87) | 91.88 (90.12–93.42) | 24.37 (16.97–33.09) | 93.23 (91.57–94.65) | 0.19 (0.13–0.24) |
| ELISA [B] Cut-off: % S/P > 37 | 42.72 (33.02–52.85) | 77.19 (74.60–79.63) | 14.81 (10.98–19.37) | 93.55 (91.18–95.06) | 0.11 (0.06–0.15) |
| ELISA [B] Cut-off: % S/P > 95 | 27.18 (18.88–36.84) | 92.60 (90.90–94.08) | 24.45 (17.63–34.65) | 93.19 (91.54–94.61) | 0.11 (0.06–0.15) |
| Variable | Code | Modality | OR | (95% CI) | p-Value | At Risk or Protective |
|---|---|---|---|---|---|---|
| ELISA [A] using the cut-off of the producer (% S/P > 40) | C1 | Negative | Ref | - | - | |
| Positive | 3.03 | (1.94–4.73) | <0.001 | At risk | ||
| ELISA [A] using the cut-off determined by the ROC curve (% S/P > 85) | C2 | Negative | Ref | - | - | |
| Positive | 4.65 | (2.89–7.49) | <0.001 | At risk | ||
| ELISA [B] using the cut-off of the producer (% S/P > 37) | C3 | Negative | Ref | - | - | |
| Positive | 2.52 | (1.67–3.82) | <0.001 | At risk | ||
| ELISA [B] using the cut-off determined by the ROC curve (% S/P > 95) | C4 | Negative | Ref | - | - | |
| Positive | 4.68 | (2.87–7.62) | <0.001 | At risk | ||
| AI or natural mating | C5 | 1 or 2 AI | Ref | - | - | |
| >2 AI | 1.32 | (0.45–3.88) | 0.75 | |||
| Natural mating | 0.45 | (0.26–0.78) | 0.005 | Protective | ||
| Winter stabling of animals | C6 | Tethered housing | Ref | - | - | |
| Free-stall on slatted floors (A) | 3.45 | (1.31–9.11) | 0.012 | At risk | ||
| Free-stall on straw (B) | 2.55 | (1.09–5.95) | 0.03 | At risk | ||
| (A) and (B) | 7.97 | (0.30–215.21) | 0.22 | |||
| Pasture surrounded by trees or hedges | C7 | No | Ref | - | - | |
| Yes | 2.00 | (1.02–3.93) | 0.044 | At risk | ||
| Contact with goats | C8 | No | Ref | - | - | |
| Yes | 4.85 | (1.22–19.26) | 0.025 | At risk | ||
| Proportion of dairy in the herd | C9 | <20% | Ref | - | - | |
| ≥20% | 2.22 | (1.30–3.78) | 0.004 | Risk | ||
| Use of well water in winter | C10 | No | Ref | - | - | |
| Yes | 1.83 | (1.02–3.28) | 0.041 | Risk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saegerman, C.; Grégoire, F.; Delooz, L. Diagnosis of Coxiella burnetii Cattle Abortion: A One-Year Observational Study. Pathogens 2022, 11, 429. https://doi.org/10.3390/pathogens11040429
Saegerman C, Grégoire F, Delooz L. Diagnosis of Coxiella burnetii Cattle Abortion: A One-Year Observational Study. Pathogens. 2022; 11(4):429. https://doi.org/10.3390/pathogens11040429
Chicago/Turabian StyleSaegerman, Claude, Fabien Grégoire, and Laurent Delooz. 2022. "Diagnosis of Coxiella burnetii Cattle Abortion: A One-Year Observational Study" Pathogens 11, no. 4: 429. https://doi.org/10.3390/pathogens11040429
APA StyleSaegerman, C., Grégoire, F., & Delooz, L. (2022). Diagnosis of Coxiella burnetii Cattle Abortion: A One-Year Observational Study. Pathogens, 11(4), 429. https://doi.org/10.3390/pathogens11040429







