Abstract
Decision-making on tick control practices is linked to the level of knowledge about livestock farming and to the social context in which individuals practice them. Tick infestation is one of the main problems in tropical livestock production. The objective of this study was to characterize tick-control related practices in two tropical livestock areas and their potential association with the level of tick infestation. A total of 139 farms were included in this survey. To determine this association, a multivariate logistic regression model was used. A stepwise model selection procedure was used and model validation was tested. Cattle husbandry as a main activity, the use of external paddocks, the use of amitraz, and the lack of mechanization on the farm were related with high tick infestation. On the other hand, owner involvement in the preparation of acaricide solution was identified as a protective factor against high tick infestation. At animal level, age (old), body condition status (thin), and lactation were also associated with high tick infestations, while Bos primigenius indicus cattle and their crosses reduced the probability of high tick infestations. The factors studied, such as herd size, education level of the owners, and veterinary guidance, varied from farm to farm. Nonetheless, these differences did not generate changes in the level of tick infestation. According to the area under the receiver operating characteristic curve (AUC-ROC), the model at farm level predicts a high level of infestation, with an accuracy of 72.00% and high sensitivity. In addition, at animal level, crossbreeding with indicus cattle and breeding selection for host resistance will be useful against high tick infestation. Likewise, the implementation of programs of capacitation and research on tick control for farmers, cowboys, and vets in these areas is necessary.
Keywords:
acaricide; cattle; Ecuador; protective factor; risk factor; tick; tick-borne diseases; tropical 1. Introduction
Livestock is a major economic activity in Ecuador. It contributes substantially to local nutrition, providing milk, meat, and derivatives that are in high demand by the population [1]. The agriculture sector, including livestock farming, represents around 7.80% of the total of Ecuador’s gross domestic product [2]. Ecuadorian cattle population is about 4.3 million heads, from 280,000 cattle farms nationwide [3]. Due to Ecuador’s location, most of the country, except for certain parts of highlands (places above 2500 m), experiences a humid tropical climate [4,5], providing favorable environmental conditions for the development of ectoparasites such as ticks. In fact, more than 75.00% of cattle herds are found in areas either infested or potentially infested with ticks [6].
Tick infestation causes significant economic losses in the livestock industry. Ticks transmit a wide range of pathogens that can cause tick-borne diseases (TBDs). The most important TBDs of cattle in Ecuador are anaplasmosis caused by rickettsia of the genus Anaplasma [7] and babesiosis caused by protozoa of the genus Babesia [8]. In addition to spreading pathogenic microorganisms, ticks cause weight loss, reduced milk production, and cause skin injures that can lead to secondary bacterial and fungal infections and even myasis (Cochlomyia hominivorax) [9,10,11]. Additional losses include the cost of treatment for clinical cases and the expenses derived from the indiscriminate use of acaricides for tick control. Likewise, the indiscriminate use of chemical compounds has increased the problem of tick multiresistance to acaricides, which has already been reported in Ecuador [11].
The livestock production present in tropical areas is extensive, with a low level of mechanization used, and grazing is the main source of food for animals [12]. Consequently, to increase milk production, farmers tend to introduce exotic breeds (Bos primigenious taurus), which often are susceptible to TBDs. In addition, for improving farm profitability, natural ecosystems are incorporated into production, clearing forests to plant non-native pasture, in order to expand the agricultural frontier [13,14]. These environmental and host modifications have had a major impact on the ecology of these parasites, causing the encounter rate between tick and host to be higher, leading to an increase in tick infestation [15,16]. However, decisions on tick control practices are usually linked to the level of knowledge about livestock farming and to the social context in which individuals practice these strategies [17]. Understanding the reasons that lead farmers to use particular control measures will contribute to holding back the advance of the threat that acaricides pose to the environment and public health and also increase farm productivity.
The three objectives of this study were to (i) describe two tropical dairy production areas located on the eastern and western foothills of the Ecuadorian Andes; (ii) relate tick control practices at animal and farm levels on the level of tick infestation; and (iii) identify tick species infesting cattle in these areas.
2. Results
2.1. Tick Species
In total, 1905 adult ticks were collected from 133 farms, 1345 ticks (70.60%) were females and 560 (29.40%) were males. In six farms, we did not find ticks on the animals examined. Tick prevalence in farms was estimated to be 95.70% (95% confidence internal, CI: 90–98). Table 1 shows the number of farms with tick presence and the tick species reported. Four species of ticks were morphologically identified. Rhipicephalus microplus (female: 1328; male: 553) being the most common species in the Northwest of Pichincha and Quijos river valley.

Table 1.
Ticks identified in the study areas.
2.2. Characteristics of Farming and Tick Control
A total of 139 farms were visited, 72 in the Quijos river valley, and 67 in the Northwest of Pichincha province (Table 2). According to the number of cattle, most of the farms visited in the two areas were medium farms (21 to 70 cattle; 54.17% in Quijos river valley, and 64.18% in Northwest of Pichincha), followed by small farms (1 to 20 cattle) in the Quijos river valley (38.89%) and large farms (more than 70 cattle; 23.88%) in Northwest of Pichincha. The principal activity in those areas is cattle husbandry, with a focus on milk production. Agriculture occupies the second place, being practiced at 22.20% in Quijos river valley and 37.31% in Northwest of Pichincha. In 19.44% (Quijos river valley) and 32.84% (Northwest of Pichincha) of farms these two activities are practiced jointly.

Table 2.
Characteristics of farming and tick control in Quijos river valley and Northwest of Pichincha.
With respect to the education level, the percentage of farmers with university education was higher in Northwest of Pichincha (29.85%) than in Quijos river valley (9.72%). In the two areas, most of the farms are non-mechanized (65.28% and 56.72%, respectively). However, the number of mechanized farms is twice as high (35.82%) in Northwest of Pichincha compared to Quijos river valley (16.67%). Only 43.06% in Quijos river valley and 37.31% in Northwest of Pichincha have a storage space to keep veterinary drugs. All the farms use grazing as the feeding method. In addition, 31.94% in Quijos river valley and 43.28% in Northwest of Pichincha cut and carry pasture, and this is mainly used to feed the lactating dairy cows. Fifty percent (36 out of 72 farms) of the farms in Quijos river valley and 25.37% (17 out of 67 farms) of the farms in Northwest of Pichincha do not have sufficient feeding paddocks in the total area on the farm and have to use external farm paddocks to feed their cattle. Most of the external paddocks in Quijos river valley are owned (63.89%) by the farmer but, in Northwest of Pichincha most of these are rented (64.71%), and the farmers pay an annual fee for their use.
This survey revealed that most cattle farms in Quijos river valley (87.50%) have veterinary support. Of these farms, 81.43% are managed by public veterinarians. In Northwest of Pichincha, in the majority of farms (55.22%) do not have the accompaniment of a veterinarian. On farms with veterinary accompaniment (44.78%), this service is private in half of the cases (50.91%).
All farms (100.00%) in the Quijos river valley use chemical treatment for tick control, and in the Northwest of Pichincha 95.52% used it. Only a few farms (4.48%) did not use chemical control, because they prefer uncommon control methods such as bath spray with entomopathogenic fungus, medicinal plants (Azadirachta indica), sulfocalcic broth, or sulfur supplementation in the diet. In most cases, the frequency of application of an acaricide treatment is less than once per month. The main method of application of acaricides was spraying with a hand sprayer (96.32%). In spray solution, the most commonly used acaricides were amides and organophosphates. Amides (amitraz) were used in 80.56% and 67.16% of the farms in Quijos river valley and Northwest of Pichincha, respectively. Organophosphates were used by 50.75% of all farms in Quijos river valley and 72.22% of the farms in Northwest of Pichincha. Ivermectin (macrocyclic lactone) was also commonly used for tick control by 77.78% of farms in the Quijos river valley and 86.57% in the Northwest of Pichincha (Table 2). This principle was administered parenterally (subcutaneously); however, in 19.65% and 6.90% of the farms in the Quijos river valley and Northwest of Pichincha it was applied topically (spraying). Among the farmers using ivermectin, 53.76% (Quijos river valley) and 50.00% (Northwest of Pichincha) use it on milking cattle. Although the two zones have different herd sizes and different levels of mechanization, both zones had similar percentages of high tick infestation (Quijos river valley with 41.67%; Northwestern of Pichincha with 40.29%) (p-Value > 0.05).
2.3. Tick-Infestation Associated Factors at Farm Level
Forty-one percent (95% CI: 32.84–49.68) (57/139) of farms had a high level of tick infestation. The variables included in the model are shown in Table 3. The initial model included 26 variables with (AIC: 206.40). The final model included eight factors significantly associated with high infestation level of ticks at farm level, selected for having the smallest value of AIC (AIC: 181.15).

Table 3.
Risk and protective explanatory variables for a high level of tick infestation at the farm level using univariate analysis.
The final logistic regression model is presented in Table 4. The results of the model showed that cattle husbandry as the principal economic activity has a positive association with high levels of tick infestation. The OR when raising animals is the principal activity was 3.96 (95% CI: 0.97–16.10; p-Value 0.053, marginally significant) times higher than when cattle husbandry is not the principal activity. Absence of mechanization on the farm has a positive association (risk explanatory variable) with high tick infestation. Indeed, semi-mechanized (OR = 4.48 with 95% CI: 1.02–19.53) and non-mechanized farms (OR = 5.11 with 95% CI: 1.14–22.86) had higher odds ratio to have high tick infestation than the mechanized farming.

Table 4.
Risk and protective explanatory variables for a high level of tick infestation at the farm level using a multivariable binary logistic regression model.
When the acaricide spray was prepared by the owner, there was less chance (OR = 0.19 with 95% CI: 0.06–0.061) of having animals with high tick infestation in the farms, in comparison with situations where the solution was prepared by employees.
2.4. Overall Weighted Score at Farm Level and Area under the Receiver Operating Characteristic Curve
The factors associated with a p-Value ≤ 0.10 in the final model (see Table 4) were used to calculate the weighted score. This threshold was used because of the relatively low number of farms sampled. Six covariates were aggregated as a unique overall weighted score (OWS) by farm, using the following formula:
where a = cattle husbandry is the principal activity; b2 = semi-mechanized farm; b3 = mechanized farm; c = farm with external paddocks; d = farm with veterinary support; e = the person who prepares the acaricide solution is the owner; f = use of amitraz.
| OWS = [(Presence_a = 1) × (OR_a)] + [(Presence_b2 = 1) × (OR_b2)] + |
| [(Presence_b3 = 1) × (OR_b3)] + [(Presence_c = 1) × (OR_c)] + [(Presence_d = 1) × (OR_d)] |
| + [(Absence_e = 1) × (1/OR_e)] + [(Presence_f = 1) × (OR_f)] |
With this formula, the minimum and the maximum theoretical values of the OWS are 5.11 and 21.08. The probability that a farm has a low or high level of tick infestation as a function of the result of the OWS is represented in Table 5.

Table 5.
The probability that a farm has a low or high level of tick infestation as a function of the overall weighted score.
The diagnostic discriminatory power of OWS was assessed by calculating the AUC-ROC (Figure 1). The AUC-ROC was 0.72 (95% CI: 0.63–0.80), with standard error = 0.043. Using the Youden index (0.34), the best cut-off to discriminate the level of tick infestation (high and low level) was OWS = 11.65. Applying this cut-off, the sensitivity was 94.74 and the specificity was 41.46%.
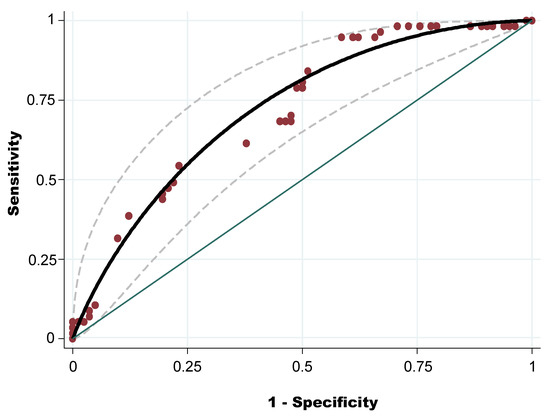
Figure 1.
Receiver operating characteristic curve of the overall weighted score of a high level of tick infestation at farm level.
2.5. Tick-Infestation Associated Factors at Animal Level
The covariates included in the analysis for tick infestation at animal level are shown in Table 6. In the univariate analysis six covariates were included. The sex of animals was discarded, given that only 27 out of 826 animals were males. For the final model, four covariates were associated with a high level of infestation at the animal level.

Table 6.
Risk explanatory factors for a high level of tick infestation at the animal level using a univariate analysis.
The final logistic regression model is presented in Table 7, and the results of the multivariable binary logistic regression model showed that cattle breed: Crossbreed: B. primigenious taurus × B. primigenious indicus (OR = 0.547 with 95% CI: 0.546–0.548) and B. p. indicus (OR = 0.539 with 95% CI: 0.538–0.540) were protective factors against a high level of infestation. In comparison to young animals, young adult and adults over 7 years old had an OR = 1.050 (95% CI: 1.048-1.051) and OR = 1.480 (95% CI: 1.478–1.482), respectively. Cows in lactating status had an OR = 2.287 (95% CI: 2.283–2.900) in comparison to other categories. Finally, for body condition status, good (OR = 1.21 with 95% CI: 1.21–1.21) and thin (OR = 1.992 with 95% CI: 1.990–1.995) conditions were risk explanatory variables for high level of infestation in comparison to fat animals.

Table 7.
Risk and protective factors for a high level of tick infestation at the animal level included in the final multivariable binary logistic regression model.
2.6. Overall Weighted Score (OWS) at Animal Level and Area under the Receiver Operating Characteristic Curve
The risk and protective explanatory variables with a p-Value ≤ 0.05 (see Table 6) were used to calculate the weighted score. Finally, four covariates were aggregated as a unique overall weighted score (OWS) by animal, using the following formula:
where g1= B. primigenious taurus g2= breed is a Crossbreed: B. primigenious taurus × B. primigenious indicus; g3 = breed is B. primigenious indicus; h2 = young adult animal; h3 = adult over 7 years old; i = lactating dairy cows; j2 = animal with good body condition status; and j3 = animal with thin body condition status.
| OWS = [(Absence_g1 = 1) × (1/OR_g2)] + [(Absence_g1 = 1) × (1/OR_g3)] + |
| [(Presence_h = 1) × (OR_h2)] + [(Presence_h3 = 1) × (OR_h3)] + [(Presence_i = 1) × (OR_i)] + |
| [(Presence_j2 = 1) × (OR_j2)] + [(Presence_j3 = 1) × (OR_j3)] |
With this formula, the minimum and the maximum theoretical values of the OWS were 0.00 and 7.59.
The diagnostic discriminatory power was assessed by calculating the AUC-ROC (Figure 2). The AUC-ROC was 0.56 (95% CI: 0.52–0.60) with standard error = 0.021. Using both the Youden index (0.087) the best cut-off to discriminate the level of tick infestation (high and low level) was OWS = 3.34. Applying this cut-off, the sensitivity was 89.14%, and the specificity was 19.54%.
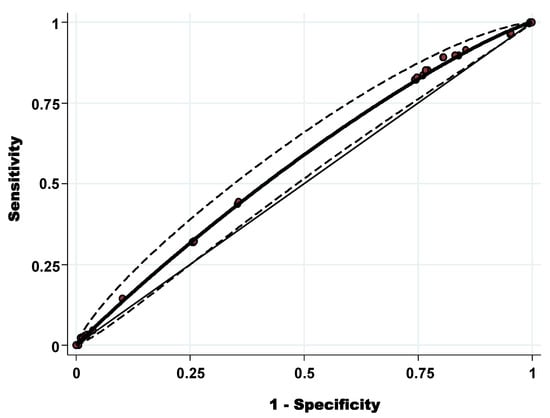
Figure 2.
Receiver operating characteristic curve of the overall weighted score of high level of tick infestation at animal level.
3. Discussion
R. microplus was the most collected and identified tick in both zones in this study, which confirms that it is the most common species on cattle. I. boliviensis, I. montoyanus, and A. mixtum were also identified on a few farms. Previous studies carried out in Ecuador have determined the presence of the R. microplus tick in Santo Domingo de los Colorados, Los Bancos, and Napo province [18,19,20,21,22,23,24]. Unpublished results obtained from the ‘Encuesta Nacional de Brucelosis, Tuberculosis y Garrapatas’, reported R. microplus as the most abundant species in tropical and subtropical areas of Ecuador. Nava et al. [25] and Aguilar-Domínguez et al. [26] reported the presence of A. mixtum larvae in the vegetation of coastal Ecuadorian localities. In addition, Guillén and Muñoz [20] identified Amblyomma spp. and Ixodes spp. on cattle at Santo Domingo de los Colorados. On the other hand, studies carried out in different parts of the Amazonia region have reported the presence of I. boliviensis [27] and I. montoyanus [28] on cattle.
In Ecuador, according to reports of several projects carried out by the Institute of Research in Zoonoses (unpublished data), Amblyomma maculatum, Amblyomma ovale, Haempahysalis juxtakochi, and Dermacentor nitens were also present on cattle. Species of the genus Amblyomma were reported by Enriquez et al. [29], Voltzit [30], and Maya et al. [22], who reported the presence of Amblyomma coelebs, Amblyomma triste, and Amblyomma cajennense, respectively, on cattle.
When looking at the results of the number of farmers with university studies, mechanized farms and herd size (farms with more than 70 animals), it is evident that cattle husbandry is a more developed activity in the Northwest of Pichincha compared to the Quijos river valley. All this can be associated to the fact that the two zones had a different historical trajectory. The Northwest of Pichincha began to practice cattle husbandry over 50 years ago. This activity has expanded over time in relation to its location in a transit zone between the Coast region and the Highlands region, which facilitates the entrance of animals from other areas and the exit of livestock products to their destinations; becoming an area that supplies livestock products to the capital of Ecuador located in the Highlands region, and providing more than 200,000 L of raw milk per day [31,32,33]. On the other hand, cattle husbandry in the Quijos river valley produces 55,000 L of milk per day [33,34] and is a family activity that grew thanks to the construction of roads to the Amazon region for oil exploitation between 1968 and 1972 [35]. In addition, with the incursion of the multinational company Nestlé, cattle husbandry displaced agricultural production, which was previously the main source of income in the area [34,36,37]. Although the two zones had different herd sizes, farm mechanization, and education levels, these two zones had similar percentages of high tick infestation (Quijos river valley with 41.67%; Northwestern of Pichincha with 40.29%). This finding shows that some factors may not have been considered. Although the climate is very similar in both areas, and the sampling was done in the rainy season, the dry season in Northwestern of Pichincha is longer.
Regarding tick control methods, both zones mainly use chemical control. The range of acaricide products available on the market is wide. However, the site of action does not have much variety, as it can be seen that most of the acaricides used in this study belong to one of these families amide, organophosphate, pyrethroid, macrocyclic lactone, and phenylpyrazolone, whose mode of action is at the level of the nervous system [38,39]. The only acaricide with a different mode of action is fluazuron (benzoylphenyl urea). This acaricide is relatively new on the market. It is applied as a pour on that affects the molting process. However, it is expensive and has a long residual life in meat and milk [40,41]. The limited use of this acaricide in the study areas is associated with its price. Ivermectin (macrocyclic lactone) also generates residues in milk and meat for several weeks after application [41], but it is used by 77.78% of farmers in the Quijos river valley and 86.57% in the Northwest of Pichincha. It is also used in lactating cows.
Acaricides were generally applied using hand sprayers, and the most usual acaricide applied in this survey was amitraz, an acaricide used extensively around the world, which entered in Ecuador in the 1960s [42]. Spraying consists of dissolving the correct dose of a wettable powder or flowable product in water [43]. However, this step is not followed by all farmers. Inadequate acaricide preparations (under-dosing or overdosing) and misapplications lead to the development of resistance [43,44], which has already been reported in Ecuador [11,22]. This fact would explain why farmers reduce the time between the treatments to less than one month in both zones (Quijos river valley, 80.56% and in Northwest of Pichincha, 67.16%). Bianchi et al., in 2003, [45] and Rodriguez-Vivas et al. in 2018 [39] reported that farmers used to apply control methods for ticks every month or whenever they observe a significant infestation level. Decreasing the interval between treatments is the first reaction when farmers observe that acaricide does not have the expected effect. Other common acaricides used are organophosphates, which despite being chemicals with a variable toxicity that can range from highly toxic to slightly toxic, and which have already been documented to cause neurological damage, are being used by farmers in the Quijos river valley in 77.78% and Northwest of Pichincha with 50.75% of farms. This is associated with the lack of knowledge that farmers have about the dangers of these acaricides. In addition to the fact that they are products freely sold in Ecuador, they do not need veterinary prescription [46], despite being banned in 32 countries (dichlorvos and trichlorfon) due to their harmful properties for the health and the environment [47].
At the animal level, the presence of crossbreed (B. primigenious taurus × B. primigenious indicus) and B. primigenious indicus breeds were protective to high levels of infestation in comparison with B. primigenious taurus cattle. This is because B. primigenious indicus cattle and their crossbreeds are genetically more resistant to ticks, which makes them adaptable to tropical climates [48,49,50]. The tolerance of B. primigenious indicus cattle is due to the coexistence and co-evolution of zebu cattle originating from Asia with R. microplus species also originating from the Asian continent, while European breeds (B. p. taurus) are more susceptible because they were less exposed in this evolution process, in addition to the fact that this type of cattle has thinner skin [14,51,52,53]. This is one of the main reasons why cattle breeds such as Jersey and Holstein have a higher level of tick infestation in tropical zones.
Cattle in the studied areas with ‘good’ and ‘thin’ body conditions have 1.21 and 2.00 higher odds ratio of high level of tick infestation than cattle with ‘fat’ body condition. This is due to the fact that low nutrition causes metabolic, endocrine, and immunological consequences that increase parasitism [54]. These results are consistent with those reported by Sutherst et al. [55], Tolleson et al. [56], and Abbas et al. [38], who associated poor nutrition in cattle with increased tick burdens. In addition, a study in sheep found that lean sheep had 50.00% more ticks than fat sheep [55]. However, this is a factor to be taken with caution, as the worse body condition is also a consequence of high tick infestation. An average of 40 ticks per day per animal may cause losses around of 20 kg of weight per year [57,58].
Another factor associated with a high level of tick infestation was lactation, these animals had almost three times higher risk (OR = 2.29) of a high-level infestation compared to an animal that was not in production. This could be related to the fact that cows in this period have a high level of prolactin and progesterone, altering their immune system and making them more susceptible to infection. Indeed, infestation added to production stresses, such as pregnancy or lactation, decrease resistance to infection [59,60,61,62]. Additionally, dry cows can be treated with ivermectin at higher doses, given the lifting of restrictions for ivermectin usage during the milking period.
Animal age also constitutes a risk explanatory variable; young adults (OR = 1.05) and adults over 7 years (OR = 1.48) animals had a higher risk of having high tick infestation than young animals. This result is consistent with the work of Swai et al. in 2005 [63] and Rehman et al. in 2017 [61], who found that mature animals had a higher chance of carrying ticks compared to calves. The low infestation in young cattle is related to maternal immunity transmitted during suckling, maternal grooming, management, and also because in young cattle, farmers avoid free grazing, while the reduced size of calves reduces the area of contact with ticks [64,65].
Farm mechanization was an important preventive factor for high level of tick infestation. The odds ratio of high tick infestation on cattle from semi-mechanized (OR = 4.48) and non-mechanized (OR = 5.11) farms was higher, compared to mechanized farming. This result is consistent with studies in which farms furnished with cattle handling systems were at less risk of having a higher tick infestation than unequipped farms; this fact has also been observed in farms with poor facilities, where incorrect use of acaricides led to acaricide resistance [66,67].
The lack of paddocks for livestock feeding causes farmers to mobilize their animals to external paddocks. Although this practice helps to maintain the production of cattle, the use of external paddocks increased the risk for high tick infestation by 2.08 times, with regard to the farms that did not move animals outside the farm. Even though these paddocks can be owned or rented, in most cases they are rented for only few months of the year and not only to one farm, but to several farms during the year. Causing them to be used by different animals than can easily transport ticks between farms. Studies carried out by Heath in 2016 [68] and Zannou et al. in 2020 [69] found that herd movements around and between farms and pasture management can all also have a bearing on the presence of high level of tick infestation in the animals and in the occurrence or progress of tick-borne diseases (TBD).
The person who prepares the solution for the acaricide spray is a critical factor for the risk to present a high level of tick infestation. When the acaricide solution was prepared by the owner, there was a lower level of tick infestation (OR = 0.19) compared to farms where the spray solution was prepared by employees. This could be related to the differences in the level of education between owners and employees. Additionally, when there are training events, the person who attends is in most cases the owner. The insufficient knowledge and lack of training leads to under or over-dosing acaricides, which results in decreased acaricide efficacy and an increase in resistant tick populations [42,66,70].
Cattle husbandry is an important activity in the studied areas. However, the practice of cattle raising as the main activity was a significant explanatory variable for the presence of high level of tick infestation (OR = 3.96). This fact could be associated with several causes; among them, cattle raising is an activity that has been inherited from generation to generation [71]; therefore, correct and incorrect knowledge about the use of acaricides have also been transmitted between generation, fostering resistance to the commonly used acaricides [11,21,42]. Dairy farmers in Ecuador do not have access to research or tools to implement an integrated tick management control, which is based on the appropriate combination of at least two control tools as animal management practices, selection of cattle breeds resistant to ticks, use of plant extracts, pasture management, vaccination, or biological control [39]. The implementation of these tools requires economic resources, which are in part limited because the lack of clear government policies for the establishment of basic milk prices [72]. Likewise, innovative control strategies are needed, because the areas studied here are ecologically vulnerable, so that the impact of acaricides may decrease the biodiversity in the zone.
In addition, capacitation programs on livestock management systems and tick control are needed. Their implementation will help farmers to make decisions that will improve livestock production. Knowing the number of animals that can be fed (system carrying capacity) from the farm’s paddocks and when animals can enter the paddocks will help to improve meat and milk production, obtaining quality products using the farm’s own resources [36,73]. Avoiding that, the deficit of pasture causes farmers to look for other grazing sites. From renting outside paddocks that increases the presence of a high level of infestation, to using entire landscape areas that are part of a forest and cause serious problems to biodiversity and soil stability [74].
Receiver operating characteristic (ROC) graphs are used in signal detection theory to depict the tradeoff between hit rates and false alarm rates of classifiers. This technique visualizes, organizes, and selects classifiers based on their performance [75,76]. At farm level the AUC-ROC of the OWS of high level of tick infestation was 0.72. This model, according to the Swets [76] scale is useful and can help to predict the potential level of tick infestation with an accuracy of 72.00%. Similarly, the sensitivity of the model prediction was very high for detecting farms with high levels of tick infestation. Indeed, if a farm has an OWS under 11, it has a high probability of having a low level of infestation. However, the specificity of the model was relatively low (41.00%), but this problem can be solved by a visit to the farm by a veterinarian, who can give appropriate guidance for the control of infestation, taking into account the specific context of the farm.
4. Materials and Methods
4.1. Study Area and Sampling Design
This cross-sectional survey was part of the project entitled ‘Socio-eco-epidemiology of ticks, tick-borne parasites, acaricide resistance and residual effects of acaricides in tropical Ecuadorian livestock: environmental, animal and public health impacts’. Sampling was conducted from November 2020 to March 2021 in two tropical regions of Ecuador. Area 1: Northwest of Pichincha Province in the Western Andean foothill, and Area 2: Quijos river valley in the Eastern Andean foothills.
Area 1 is located in the Northwest of Pichincha and is crossed by Chocó Andino of Pichincha Biosphere Reserve [77]. The Northwest of Pichincha is located on the western slopes of the Andes Mountains and has several altitudinal floors and microclimates [78,79,80]. Area 2 is located in the province of Napo and is located in the middle of two conservation areas, i.e., Cayambe Coca National Park and Sumaco Napo Galeras National Park [81]. Quijos river valley is located between the foothills of the eastern Andes Mountains and high jungle of the Amazon region [82].
The selection was made using snowball sampling techniques, where, with the help of community leaders and authorities, the farms were selected with an emphasis on small and medium herds.
4.2. Investigation of Risk Factors Socio-Eco-Epidemiological Survey
To identify risk factors associated with high levels of tick infestation, an epidemiological questionnaire called ‘Socio-eco-epidemiological survey of ticks and TBDs’ was administered in each farm (Supplementary File S1). The survey was validated by national and international experts in the field. It was also pilot-tested on three farms in each area (one small, one medium, and one large) to ensure that farmers understood all the questions. The questionnaire was divided into four parts: (A) farm general information and herd management; (B) tick and acaricides related information; (C) inputs, outputs, and labor force used in the farm; and (D) pharmacological inputs and farming practices. This survey consisted of a personal interview with the person who knows the most about farm management, irrespective of gender or age. The data were collected using the Epicollect-5 mobile application [83], except for part D which was collected in physical form.
4.3. Farms Selected
The average minimum distance between farms in Quijos river valley is 1.35 km (0.08–12.80 km), and in Northwest of Pichincha 4.34 km (0.34–24.69 km). The difference in distance between each zone is due to the fact that the farms in Quijos river valley are small and medium farms, and in Northwest of Pichincha there is a majority of medium and large farms.
According to the information collected in the survey, the farms were classified on the basis of the number of animals, as small (1 to 20 cattle), medium (21 to 70 cattle), and large (more than 70 cattle). The level of mechanization on the farm was classified using three criteria: infrastructures availability (corrals and cattle handling systems), the use of automatic or manual milking, and the usage of artificial insemination or natural services as reproduction method. A farm was considered mechanized if it met three criteria, semi-mechanized if meeting two, and non-mechanized if it only met one of the criteria.
The usage of external paddocks was considered if the paddocks used for cattle feeding were outside of the farm borders, regardless of whether the paddock was rented (paddocks of neighboring farms) or owned (paddocks of the same owner but in different locations). Paddock maintenance was defined according to two criteria: paddock topping (by scythe or machete) and paddock cleaning (feces removed). A farm performs paddock maintenance when it complies with one criterion.
Veterinary support was evaluated in four levels according to the presence of a veterinarian on the farm: permanent, sometimes, rarely, and never. The presence of a veterinarian permanently or sometimes was classified as the presence of veterinary support.
Knowledge on the presence of tick larvae in paddocks was a dichotomous answer ‘yes’ or ‘no’. With respect to the correct knowledge of tick location in the grass, this was classified as ‘yes’ if the farmer knows that tick larvae are located on flowers and the pasture canopy.
To identify the acaricide treatment used on the farm, acaricides were classified according to their active ingredient and the chemical group. The acaricide currently used was identified by inspection of the place where the drugs were stored and, with the help of a list of the main trade names, the acaricides used by the farmers in the 12 months prior to the visit were determined. Once the group to which the acaricide belongs was identified, the form and frequency of application, dosage, animals treated, and efficacy of the product, among others, were investigated.
4.4. Animals Sampled
The farms visited did not use acaricides for 10 days prior to the visit. In each farm, at least five animals were randomly selected, irrespective of breed and age. An individual health record form and general information about identification of animals (name or number), age, sex, and breed and clinical information, including a physical examination, behavior of the animal, and notations regarding observed physiological abnormalities (e.g., neoplasms and injuries), and level of tick infestation were filled out for each animal. A total of 883 individual health records were obtained.
The animals were classified according to their age into young, young adult, and adults over 7 years old. A young adult animal is between 24 and 83 months. The category adults over 7 years old, corresponds to cattle that are over the optimal culling age [84,85]. According to body condition, the animals were classified as thin (BCS 1-2), good (BCS 3), and fat (BCS 4-5), according to the body condition scale (BCS), which evaluates body condition from 1 to 5 points [86].
The areas where the study was carried out are dairy zones, and beef production is not significant, so this variable was not taken into account.
Color of the animals was categorized according to the most common colors present in the zones (black-white, black, brown, red, and white). Regarding breed, this was not used, because in the study areas there are no pure breeds, but rather farms use hybrids in the attempt to raise milk production. For this reason, macro groups were used: B. primigenious taurus, Crossbreed: B. primigenious taurus × B. primigenious indicus, and B. primigenious indicus.
4.5. Level of Infestation
The level of infestation of the animals was evaluated by a semi-quantitative visual inspection of the total bovine body (head, neck, back, loin, rump, arms, legs, ribs, chest, front flank, and udder) for approximately 8 min. The bovine body was divided into two parts (medial plane): right half and left half. Each half was subdivided into three zones: front third (from the head to the thoracic perimeter), the middle third (from the thoracic perimeter to the sacral bone), and the back third (from the sacral bone to the perineum). One-third was infested when it had 20 or more ingested females [87]. The presence of ticks in animals was rated in four levels: null, low, medium, and high. It was considered a low level of infestation if it was one-third infested, medium level of infestation two-thirds, and high level of infestation three-thirds infested.
4.6. Morphological Identification of Ticks
The tick samples were transported to the Entomology Unit of the Institute of Research in Zoonoses, located at Central University of Ecuador in Quito. For further morphological identification, the dichotomous keys and tick species descriptions of Guerrero [88] and Barros et al. [89] were used. The specimens were identified under stereomicroscope.
4.7. Statistical Analyses
All data from the questionnaire were exported to Microsoft Excel®, to be organized and cleaned. Inconsistencies across the data base were checked and verified by the interviewer and if necessary, the farmers were contacted by phone again. For the comparison of farming and tick control practices used in the survey areas, Fisher’s exact test was used; in this case the response variable was the survey area.
For the level of tick infestation, ordinal categorical levels (null, low, medium, and high) were assigned to a numerical scale from 0 to 3, and the average of the entire-rounded part of the five animal records with the infestation level was taken as a representation of the farm (level of tick infestation at the farm level). Farms with a score of 0 or 1 were referred to as having a low level of infestation, and with a score of 2 or 3 as having a high level of infestation. Tick infestation at the animal level was considered high when it was recorded as medium or high. Covariates with little or no variability were discarded from the analysis. Finally, a cleaned database was obtained with information of 826 animals (93.54% sampled), belonging to 139 farms (100.00% sampled).
At farm level, a univariate association test was applied, with 26 covariates. To identify the covariates for the final model, a stepwise procedure was practiced with a multiple logistic regression using the step AIC function from the MASS package [90] in the R environment [91]. For the analysis of explanatory factors at the animal level, six variables were included (age, sex, breed, color, body condition status, and lactating dairy cows). The glmer function from the lme4 package in R environment [91] was used to incorporate both fixed-effect parameters and random effects (farm to which they belong) in a linear predictor, through maximum likelihood [92]. The risk or protective explanatory variables with a p-Value ≤0.05 were associated with a high level of tick infestation.
A multivariate logistic model was used to combine and to evaluate the covariates. Variance inflation factor (VIF) was used to determine the degree of multicollinearity between the covariates. Function VIF from the REGCLASS package [93] in R [91] was used. If the explanatory variables were not redundant, then the VIF was equal to 1, but when the VIF value was greater than 5, they suggested the existence of multicollinearity [94,95].
A scoring system was developed using odds ratios (ORs) for each covariate (risk explanatory variable) in the final model. Each covariate was evaluated by its OR, and its presence/absence was coded as 1 or 0. When an OR was significantly less than one (protective explanatory variable), reverse coding of this variable was performed, its absence was recorded as 1, and the weight was 1/OR. All risk and protective explanatory variables were weighted as a single weighted overall score (OWS) by farm (farm-level risk explanatory variables) and by animal (animal-level risk explanatory variables). The area under the receiver operating characteristic curve (AUC-ROC) was used to measure performance for the OWS classification. The Youden index and ROC curve analysis were obtained by using Stata SE 14.2 [96]; likewise, this was used to estimate the best cut-off point. The Youden index was calculated to evaluate the performance of OWS in farms with high and low tick infestation levels. This Youden index was defined as sensitivity + specificity-1 [93]. The Swets [76] scale was used to qualify the usefulness of the model.
5. Conclusions
In conclusion, the two zones studied in the tropical part of Ecuador, had the same proportion of farms with high tick infestation, but different cattle management systems. Zone 1 of Noroccidente de Pichincha is a zone with a long history of cattle raising, better mechanization, and larger herd sizes than zone 2. Zone 2 (Quijos river valley) is a zone with a more recent establishment of livestock raising, where most farms are small and medium sized, and although it has a lower level of mechanization, they receive more support from public veterinarians. High level of infestation depends on management practices (use of amitraz with growing resistance, who prepares the acaricide solution, veterinary support, and cattle husbandry as the principal activity), infrastructure present on the farm (level of mechanization on the farm), and the usage of farm external paddocks. These factors can be considered as exploratory variables, which suggests that farmers try to generate income by practicing cattle raising as their main activity, even near natural areas. However, bad habits and practices, and lack of mechanization on the farm, can cause the level of tick infestation to be high, which makes them look for new forms of control that do not solve the problem, but cause extra expenses for production. In addition, the model found some associated factors helping to predict a high level of infestation with high sensitivity, which can contribute in a useful way to decision making on control of tick infestation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11040403/s1, File S1: Socio-Epidemiological Survey.
Author Contributions
Conceptualization, V.P., X.P.-O., R.R.-H., C.P., D.C.-B., J.G., S.E., S.A.-O., S.O.V., L.R.-G. and C.S.; V.P., X.P.-O., R.R.-H., S.O.V., L.R.-G. and C.S. contributed to the study design; V.P. and X.P.-O. collected field data; L.R.-G. and C.S. verified the underlying data; V.P. conducted the statistical analyses under the supervision of L.R.-G. and C.S.; V.P. drafted the manuscript; L.R.-G. and C.S. reviewed and edited the manuscript for clarity. All authors have read and agreed to the published version of the manuscript.
Funding
This survey was funded by the Academy of Research and Higher Education (ARES) through the Research for Development Project (PRD) entitled ‘Socio-eco-epidemiology of ticks, tick-borne parasites, acaricide resistance and residual effects of acaricides in tropical Ecuadorian livestock: environmental, animal and public health impacts’, which involves universities from Ecuador (CIZ, Universidad Central del Ecuador) and Belgium (UCLouvain and ULiège).
Institutional Review Board Statement
Due to the nature of the study and the low risk to participants, no formal Ethics Committee approval was required. All animals were treated with care, and the usual farm management of sample collection was followed, without mistreatment and ensuring animal welfare.
Informed Consent Statement
The farmers were properly informed and gave their written consent prior to sampling their animals.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.
Acknowledgments
Our thanks go to the Académie de Recherche et d’Enseignement Supérieur (ARES) for funding this research, the Universidad Central del Ecuador (UCE), UCLouvain and the University of Liège for hosting the project. In addition, our thanks go to all the farmers who participated, the community leaders, the field technicians, and the students of the Faculty of Veterinary Medicine and Agronomy of the Universidad Central del Ecuador, who participated in the field and laboratory work, thanks to whom this research was possible.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Franco Crespo, C.; Morales Carrasco, L.; Lascano Aimacaña, N.; Cuesta Chávez, A. Dinámica de los pequeños productores de leche en la Sierra centro de Ecuador. La. Granja. 2019, 30, 103–120. [Google Scholar] [CrossRef]
- Banco Central del Ecuador, B. Boletín Estadístico 4.3.2. Producto Interno Bruto Por Industria; Banco Central del Ecuador: Quito, Ecuador, 2018. [Google Scholar]
- Agrocalidad. Categorías de Población de Ganado Bovino de Ecuador; Ministerio de Agricultura y Ganadería: Quito, Ecuador, 2018; p. 1.
- Pourrut, P.; Róvere, O.; Romo, I.; Villacrés, H. Los Climas del Ecuador-Fundamentos Explicativos; Ministerio de Agricultura y Ganadería: Quito, Ecuador, 1983; p. 3.
- Torres, Y.; Rivas, J.; Pablos, C.; Perea, J.; Toro, P.; Angón, E.; García, A. Identificación e implementación de paquetes tecnológicos en ganadería vacuna de doble propósito. Caso Manabí-Ecuador. Rev. Mex. Cienc. Pecu. 2014, 5, 393–407. [Google Scholar] [CrossRef]
- Gioia, G.V.; Vinueza, R.L.; Marsot, M.; Devillers, E.; Cruz, M.; Petit, E.; Boulouis, H.J.; Moutailler, S.; Monroy, F.; Coello, M.A.; et al. Bovine anaplasmosis and tick-borne pathogens in cattle of the Galapagos Islands. Transbound. Emerg. Dis. 2018, 65, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Rymaszewska, A.; Grenda, S. Bacteria of the genus Anaplasma–characteristics of Anaplasma and their vectors: A review. Veterinární Med. 2008, 53, 573–584. [Google Scholar] [CrossRef]
- Greenberg, K.; Tahseen, M.; Davidson, A. Beware of Babesiosis: A rare and severe case causing death. Am. J. Emerg. Med. 2018, 36, 2337.e1–2337.e2. [Google Scholar] [CrossRef]
- Minjauw, B.; Mcleod, A. Tick-Borne Diseases and Poverty. The Impact of Ticks and Tick-Borne Diseases on the Livelihood of Small-Scale and Marginal Livestock Owners in India and Eastern and Southern Africa; DFID Animal Health Programme, Centre for Tropical Veterinary Medicine, University of Edinburgh: Edinburgh, UK, 2003; p. 116. [Google Scholar]
- Namgyal, J.; Tenzin, T.; Checkley, S.; Lysyk, T.J.; Rinchen, S.; Gurung, R.B.; Dorjee, S.; Couloigner, I.; Cork, S.C. A knowledge, attitudes, and practices study on ticks and tick-borne diseases in cattle among farmers in a selected area of eastern Bhutan. PLoS ONE 2021, 16, e0247302. [Google Scholar] [CrossRef]
- Rodríguez-Hidalgo, R.; Pérez-Otáñez, X.; Garcés-Carrera, S.; Vanwambeke, S.O.; Madder, M.; Benítez-Ortiz, W. The current status of resistance to alpha-cypermethrin, ivermectin, and amitraz of the cattle tick (Rhipicephalus microplus) in Ecuador. PLoS ONE 2017, 12, e0174652. [Google Scholar] [CrossRef]
- Torres, Y. Caracterización Socioeconómica de Pequeñas Explotaciones Ganaderas en la Provincia de Manabí; Universidad de Córdoba: Córdoba, Spain, 2012; p. 67. [Google Scholar]
- Ferrari, M. Garrapata, La resistencia del huédped como forma de control. Marca Líquida 2002, 12, 17–20. [Google Scholar]
- Bolaños, D. Distribución Geográfica y Caracterización Taxonómica de las Especies de Garrapatas Que Afectan al Ganado Bovino en la Provincia de Los Ríos; Universidad Central del Ecuador: Quito, Ecuador, 2016; p. 77. [Google Scholar]
- Canevari, J.T.; Mangold, A.J.; Guglielmone, A.A.; Nava, S. Population dynamics of the cattle tick Rhipicephalus (Boophilus) microplus in a subtropical subhumid region of Argentina for use in the design of control strategies. Med. Vet. Entomol. 2017, 31, 6–14. [Google Scholar] [CrossRef]
- Nava, S.; Mastropaolo, M.; Guglielmone, A.A.; Mangold, A.J. Effect of deforestation and introduction of exotic grasses as livestock forage on the population dynamics of the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) in northern Argentina. Res. Vet. Sci. 2013, 95, 1046–1054. [Google Scholar] [CrossRef]
- Broughan, J.M.; Judge, J.; Ely, E.; Delahay, R.J.; Wilson, G.; Clifton-Hadley, R.S.; Goodchild, A.V.; Bishop, H.; Parry, J.E.; Downs, S.H. A Review of risk factors for bovine tuberculosis infection in cattle in the UK and Ireland. Epidemiol. Infect. 2016, 144, 2899–2926. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; Cevallos, O.; Villarreal, P.; Carranza, M.; Carranza, H.; Pinargote, E. Prevalencia y detección por PCR anidada de Anaplasma marginale en bovinos y garrapatas en la zona central del Litoral Ecuatoriano. Cienc. Tecnol. 2015, 8, 11–17. [Google Scholar] [CrossRef]
- Vasco, K. Standardization of Melting Curve Analysis for the Detection of Babesia in Ticks using Nucleotide Polymorphisms; Universidad Central del Ecuador: Quito, Ecuador, 2014; p. 83. [Google Scholar]
- Guillén, N.; Muñoz, L. Estudio Taxonómico a Nivel de Género de Garrapatas en Ganado Bovino de la Parroquia Alluriquín-Santo Domingo de los Tsáchilas; Universidad de las Fuerzas Armadas de Ecuador: Sangolquí, Ecuador, 2013; p. 71. [Google Scholar]
- Pérez, X. Resistencia a Alfa-Cipermetrina, Ivermectina y Amitraz en Garrapatas Rhipicephalus Microplus (Canestrini, 1887) Colectadas en Cuatro Localidades; Universidad Central del Ecuador: Quito, Ecuador, 2016; p. 56. [Google Scholar]
- Maya-Delgado, A.; Madder, M.; Benítez-Ortíz, W.; Saegerman, C.; Berkvens, D.; Ron-Garrido, L. Molecular screening of cattle ticks, tick-borne pathogens and amitraz resistance in ticks of Santo Domingo de Los Tsáchilas province in Ecuador. Ticks Tick-Borne Dis. 2020, 11, 101492. [Google Scholar] [CrossRef] [PubMed]
- Jacho, M. Dinámica Poblacional de la Garrapata Rhipicephalus (Boophilus) Microplus en Ganado Bovino Lechero en el Cantón San Miguel de los Bancos; Universidad Central del Ecuador: Quito, Ecuador, 2015. [Google Scholar]
- Orozco, G. Distribución Espacial de Garrapatas que Afectan a las Ganaderías Ecuatorianas de las Tres Regiones, Usando Como Referencia la Línea Equinoccial; Universidad Central del Ecuador: Quito, Ecuador, 2018. [Google Scholar]
- Nava, S.; Beati, L.; Labruna, M.B.; Cáceres, A.G.; Mangold, A.J.; Guglielmone, A.A. Reassessment of the taxonomic status of Amblyomma cajennense (Fabricius, 1787) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of Amblyomma mixtum Koch, 1844, and Amblyomma sculptum Berlese, 1888 (Ixodida: Ixodidae). Ticks Tick-Borne Dis. 2014, 5, 252–276. [Google Scholar] [CrossRef]
- Aguilar-Domínguez, M.; Moo-Llanes, D.A.; Sánchez-Montes, S.; Becker, I.; Feria-Arroyo, T.P.; de León, A.P.; Romero-Salas, D. Potential distribution of Amblyomma Mixtum (Koch, 1844) in climate change scenarios in the Americas. Ticks Tick-Borne Dis. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Pesquera, C.; Portillo, A.; Palomar, A.M.; Oteo, J.A. Investigation of tick-borne bacteria (Rickettsia spp., Anaplasma spp., Ehrlichia spp. and Borrelia spp.) in ticks collected from Andean tapirs, cattle and vegetation from a protected area in Ecuador. Parasit. Vectors 2015, 8, 46. [Google Scholar] [CrossRef]
- Sistema de Información Aracnológica Registros de Ixodidae: Ixodes Montoyanus. Available online: http://entomologia.ec/arachnida/especie.php?especie=Ixodesmontoyanus&familia=Ixodidae (accessed on 13 January 2022).
- Enríquez, S.; Guerrero, R.; Arrivillaga-Henríquez, J.; Araujo, P.; Villacrés, E.; Enríquez, A.; Benítez-Ortíz, W. New Records of Ticks of Genus Amblyomma Koch, 1844 (Acari: Ixodidae) for Ecuador. Acta Parasitol. 2020, 65, 430–440. [Google Scholar] [CrossRef]
- Voltzit, O. A review of neotropical Amblyomma species (Acari: Ixodidae). Acarina 2007, 1, 3–134. [Google Scholar]
- Duchi, T.; Guevara, V. Análisis de Factores que Determinan la Sotenibilidad y Sustentabilidad de la Economía Socialy Solidaria Para la Industrialización y Comercialización de la Leche y sus Derivados en los Cantones Los Bancos, Pedro Vicente Maldonado y Puerto Quito; Universidad Politécnica Salesiana: Quito, Ecuador, 2013; p. 208. [Google Scholar]
- Jacome, E.; Morales, D. Diagnóstico Situacional de la Comercialización y Calidad del Ganado Bovino de Carne en Pacto, Gualea, Nanegal y Nanegalito Parroquias del Cantón Quito-Pichincha; Escuela Politécnica del Ejercito: Sangolquí, Ecuador, 2005; p. 98. [Google Scholar]
- Vizcarra, R. La Leche del Ecuador; Universidad Central del Ecuador: Quito, Ecuador, 2015; p. 183. [Google Scholar]
- Proaño, J.; Miño, R. Evaluación del Proyecto de Ganadería en el Cantón Quijos, Provincia de Napo (2007–2011), Ejecutado Por el Programa Regional Ecobona; Universidad Central del Ecuador: Quito, Ecuador, 2013; pp. 1–13. [Google Scholar]
- Grijalva, J.; Arévalo, V.; Wood, C. Expansión y trayectorias de la ganadería en la Amazonía: Estudio en el Valle de los Quijos y Piedemonte, en la selva alta del Ecuador. Publ. Miscelánea INIAP 2004, 125, 201. [Google Scholar]
- Leiva, T.; Barrionuevo, M. Relaciones de Género en los Sistemas Agropecuarios del Cantón Quijos; Escuela Politécnica del Ejercito: Sangolquí, Ecuador, 2010; pp. 1–63. [Google Scholar]
- Arévalo, V.; Andino, M.; Grijalva, J. Geopolítica y transformaciones agrarias: El valle de Los Quijos en la amazonia Ecuatoriana. Publ. Miscelánea INIAP 2008, 142, 102. [Google Scholar]
- Abbas, R.Z.; Zaman, M.A.; Colwell, D.D.; Gilleard, J.; Iqbal, Z. Acaricide resistance in cattle ticks and approaches to its management: The state of play. Vet. Parasitol. 2014, 203, 6–20. [Google Scholar] [CrossRef]
- Rodriguez-Vivas, R.I.; Jonsson, N.N.; Bhushan, C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol. Res. 2018, 117, 3–29. [Google Scholar] [CrossRef]
- Calligaris, I.; Oliveira, P.; Gislaine, C.; Gervasio, H.; Camargo, M. Action of the insect growth regulator fluazuron, the active ingredient of the acaricide acatak, in Rhipicephalus Sanguineus Nymphs (Latreille, 1806) (Acari: Ixodidae). Microsc. Res. Tech. 2013, 76, 1177–1185. [Google Scholar] [CrossRef]
- Frazzoli, C.; Mantovani, A. The environment-animal-human web: A “one health” view of toxicological risk. Frontiers in Public Health and Frontiers in Environmental Science. 2019, 5, 99–108. [Google Scholar] [CrossRef]
- Pérez, X. Distribución de la Resistencia a Los Acaricidas Amitraz, Ivermectina y Alfacipermetrina en Garrapatas Boophilus Microplus y Posibles Factores de Riesgo Asociados, en la Zona ±0.5 Grados de Latitud de la Línea Equinoccial de Ecuador; Universidad Central del Ecuador: Quito, Ecuador, 2019. [Google Scholar]
- Betancur, J.; Giraldo, C. Economic and health impact ofthe ticks in production animals. IntechOpen. 2019, 1, 1–19. [Google Scholar] [CrossRef]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef]
- Bianchi, M.W.; Barré, N.; Messad, S. Factors related to cattle infestation level and resistance to acaricides in Boophilus microplus tick populations in New Caledonia. Vet. Parasitol. 2003, 112, 75–89. [Google Scholar] [CrossRef]
- Agrocalidad. Manual Para el Registro de Empresas y Productos de Uso Veterinario; Ministerio de Agricultura y Ganadería: Quito, Ecuador, 2021; pp. 1–246.
- Bejarano, F. Los plaguicidas altamente peligrosos en México. Red Acción Sobre Plaguicidas Altern. En México C 2017, 17, 1–351. [Google Scholar]
- Maryam, J.; Babar, M.E.; Nadeem, A.; Hussain, T. Genetic variants in interferon gamma (IFN-γ) gene are associated with resistance against ticks in Bos taurus and Bos indicus. Mol. Biol. Rep. 2012, 39, 4565–4570. [Google Scholar] [CrossRef]
- Piper, E.K.; Jackson, L.A.; Bielefeldt-Ohmann, H.; Gondro, C.; Lew-Tabor, A.E.; Jonsson, N.N. Tick-susceptible Bos taurus cattle display an increased cellular response at the site of larval Rhipicephalus (Boophilus) microplus attachment, compared with tick-resistant Bos indicus cattle. Int. J. Parasitol. 2010, 40, 431–441. [Google Scholar] [CrossRef]
- de Castro, J.J. Sustainable tick and tickborne disease control in livestock improvement in developing countries. Vet. Parasitol. 1997, 71, 77–97. [Google Scholar] [CrossRef]
- Burrow, H.M.; Mans, B.J.; Cardoso, F.F.; Birkett, M.A.; Kotze, A.C.; Hayes, B.J.; Mapholi, N.; Dzama, K.; Marufu, M.C.; Githaka, N.W.; et al. Towards a new phenotype for tick resistance in beef and dairy cattle: A review. Anim. Prod. Sci. 2019, 59, 1401. [Google Scholar] [CrossRef]
- Condori, R.; Ibáñez, T.; Hernández, R.; Ochoa, R.; Loza-Murguia, M.G. Frecuencia relativa de Boophilus microplus (Canestrini 1888) & Amblyomma cajennense (Fabricius 1787) (Acari: Ixodida) en ganado bovino, en la zona de colonización de Yucumo, Provincia Gral. José Ballivián Departamento Del Beni, Bolivia. J. Selva Andina Res. Soc. 2010, 1, 13–22. [Google Scholar] [CrossRef]
- Shyma, K.P.; Gupta, J.P.; Singh, V. Breeding strategies for tick resistance in tropical cattle: A sustainable approach for tick control. J. Parasit. Dis. 2015, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Kelly, P. Interactions of malnutrition and immune impairment, with specific reference to immunity against parasites. Parasite Immunol. 2006, 28, 577–588. [Google Scholar] [CrossRef]
- Sutherst, R.W.; Kerr, J.D.; Maywald, G.F.; Stegeman, D.A.; Tolleson, D.R.; Carstens, G.E.; Welsh, T.H.; Teel, P.D.; Strey, O.F.; Longnecker, M.T.; et al. The effect of season and nutrition on the resistance of cattle to the tick Boophilus microplus. Aust. J. Agric. Res. 1983, 90, 3442–3450. [Google Scholar] [CrossRef]
- Tolleson, D.R.; Carstens, G.E.; Welsh, T.H.; Teel, P.D.; Strey, O.F.; Longnecker, M.T.; Prince, S.D.; Banik, K.K. Plane of nutrition by tick-burden interaction in cattle: Effect on growth and metabolism. J. Anim. Sci. 2012, 90, 3442–3450. [Google Scholar] [CrossRef]
- Frisch, J.E.; O’Neill, C.J.; Kelly, M.J. Using genetics to control cattle parasites—the Rockhampton experience. Int. J. Parasitol. 2000, 30, 253–264. [Google Scholar] [CrossRef]
- Manjunathachar, H.V.; Saravanan, B.C.; Kesavan, M.; Karthik, K.; Rathod, P.; Gopi, M.; Tamilmahan, P.; Balaraju, B.L. Economic importance of ticks and their effective control strategies. Asian Pac. J. Trop. Dis. 2014, 4, S770–S779. [Google Scholar] [CrossRef]
- Bilkis, M.; Mondal, M.; Rony, S.; Islam, M.; Begum, N. Host determinant based prevalence of ticks and lice in cattle (Bos indicus) at Bogra district of Bangladesh. Prog. Agric 2011, 22, 65–73. [Google Scholar] [CrossRef]
- Taye, D.R.; Assefa, K.; Hika, W. Prevalence of major ectoparasites of calves and associated risk factors in and around Bishoftu town. Afr. J. Agric. Res. 2015, 10, 1127–1135. [Google Scholar] [CrossRef]
- Rehman, A.; Nijhof, A.M.; Sauter-Louis, C.; Schauer, B.; Staubach, C.; Conraths, F.J. Distribution of ticks infesting ruminants and risk factors associated with high tick prevalence in livestock farms in the semi-arid and arid agro-ecological zones of Pakistan. Parasit. Vectors 2017, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.B.; Rangel, C.P.; De Azevedo Baêta, B.; Da Fonseca, A.H. Influence of the physiological state on infestation by Rhipicephalus microplus in dairy cows. Ticks Tick-Borne Dis. 2013, 4, 52–56. [Google Scholar] [CrossRef]
- Swai, E.S.; Mbise, A.N.; Kessy, V.; Kaaya, E.; Sanka, P.; Loomu, P.M. Farm constraints, cattle disease perception and tick management practices in Pastoral Maasai community-Ngorongoro, Tanzania. Livest. Res. Rural Dev. 2005, 7, 599–610. [Google Scholar]
- González, F.; Becerril, C.; Torres, G.; Díaz, P.; Santellano, E.; Rosendo, A. Infestación natural por Amblyomma cajennense y Boophilus microplus en bovinos criollo lechero tropical durante la epoca de lluvias. Agrociencia 2009, 43, 577–584. [Google Scholar]
- Utech, K.B.W.; Wharton, R.H. Breeding for resistance to Boophilus microplus in Australian Illawarra Shorthorn and Brahman X Australian Illawarra Shorthorn cattle. Aust. Vet. J. 1982, 58, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, N.N.; Mayer, D.G.; Green, P.E. Possible risk factors on queensland dairy farms for acaricide resistance in cattle tick (Boophilus microplus). Vet. Parasitol. 2000, 88, 79–92. [Google Scholar] [CrossRef]
- Miyama, T.; Byaruhanga, J.; Okamura, I.; Uchida, L.; Muramatsu, Y.; Mwebembezi, W.; Vudriko, P.; Makita, K. Effect of chemical tick control effect of chemical tick control practices on tick infestation and Theileria parva infection in an intensive dairy production region of Uganda. Ticks Tick-Borne Dis. 2020, 11, 101438. [Google Scholar] [CrossRef]
- Heath, A.C.G. Biology, Ecology and distribution of the tick, Haemaphysalis longicornis Neumann (Acari: Ixodidae) in New Zealand. N. Z. Vet. J. ISSN 2016, 64, 10–20. [Google Scholar] [CrossRef]
- Zannou, O.M.; Ouedraogo, A.S.; Biguezoton, A.S.; Lempereur, L.; Patrick, K.; Emmanuel, Y.; Sébastien, A.; Lenaert, M.; Toe, P.; Farougou, S.; et al. First digital characterization of the transhumance corridors through benin used by cattle herds from Burkina Faso and associated risk scoring regarding the invasion of Rhipicephalus (Boophilus) microplus. Transbound. Emerg. Dis. 2021, 68, 2079–2093. [Google Scholar] [CrossRef]
- Kamran, K.; Ali, A.; Villagra, C.A.; Bazai, Z.A.; Iqbal, A.; Sajid, M.S. Hyalomma Anatolicum resistance against ivermectin and fipronil is associated with indiscriminate use of acaricides in southwestern Balochistan, Pakistan. Parasitol. Res. 2021, 120, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Vayas, T.; Mayorga, F.; Freire, C. Sector Ganadero: Análisis 2014–2019; Universidad Técnica de Ambato: Ambato, Ecuador, 2019; p. 5. [Google Scholar]
- Banco Central del Ecuador, Reporte de coyuntura sector agropecuario. Dir. Nac. Sínt. Macroeconómica Gest. Coyunt. Previs. Económicas Diseño 2020, 93, 12.
- Ruiz, F.Á.; Gz-Janica, H.L. Efectos Ambientales y Socio-Económicos del Sistema de Producción Ganadero con Enfoque Ambientalmente Sostenible y El Sistema Tradicional, Implementados en las Fincas Escocia y Alejandría, Respectivamente en el Municipio de Montería, Departamento de Córdob; Pontificia Universidad Javeriana: Bogotá, Colombia, 2012; p. 107. [Google Scholar]
- Cingolani, A.M.; Noy-Meir, I.; Renison, D.D.; Cabido, M. La ganadería extensiva, ¿es compatible con la conservación de la biodiversidad y de los suelos? Ecol. Austral 2008, 18, 253–271. [Google Scholar]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Swets, J. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Reserva de Biósfera Chocó Andino de Pichincha. Available online: https://www.chocoandinopichincha.com (accessed on 23 September 2021).
- Consejo Municial PVM. Caracterización Cantonal y Parroquial: Cantón Pedro Vicente Maldonado; Gobierno Provincial de Pichincha: Quito, Ecuador, 2011; pp. 165–182.
- Echeverría, J. Conociendo Quito: El Noroccidente del DMQ, un territorio de alta biodiversidad, cultura y empredimientos sostenibles. Inst. Ciudad 2017, 1, 1–8. [Google Scholar]
- HCPP. Proyecto de Desarrollo Rural Integral del Occidente de Pichincha-Fase II; Unidad de Desarrollo Integral: León, Mexico, 2000.
- Cárdenas, J. Informe de educación ambiental EcoFondo-Napo. EcoFondo 2010, 7, 1–7. [Google Scholar]
- Guamán, S.; Gonzáles, R.; Carrasco, R.; Guamán, F. Caracterización de los sistemas ganaderos de aptitud lechera en El Valle del Quijos, provincia del Napo, Ecuador. Eur. Sci. J. 2019, 15, 279–292. [Google Scholar] [CrossRef][Green Version]
- Epicollect, Epicollect 5. Imp. Coll. 2021. Available online: https://five.epicollect.net (accessed on 24 September 2021).
- Wondatir Workie, Z.; Gibson, J.P.; van der Werf, J.H.J. Analysis of culling reasons and age at culling in australian dairy cattle. Anim. Prod. Sci. 2021, 61, 680. [Google Scholar] [CrossRef]
- Zijlstra, J.; Jiayang, M.; Zhijun, C.; Van der Fels, B. Longevity and culling rate: How to improve? Available online: https://www.wur.nl/en/Publication-details.htm?publicationId=publication-way-353135313130 (accessed on 19 March 2022).
- Ferguson, J.D.; Galligan, D.T.; Thomsen, N. Principal descriptors of body condition score in holstein cows. J. Dairy Sci. 1994, 77, 2695–2703. [Google Scholar] [CrossRef]
- Hüe, T.; Fontfreyde, C. Development of a new approach of pasture management to control infestation. Trop. Anim. Health Prod. 2019, 51, 1989–1995. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R. Las garrapatas de Venezuela (Acarina: Ixodoidea): Listado de especies y claves para su identificación. Bol Dir Malariol Saneam Ambient 1996, 36, 1–24. [Google Scholar]
- Barros-Battesti, D.; Arzua, M.; Bechara, H. Carrapatos de Importância Médico-Veterinária da Região Neotropical: Um Guia Ilustrado Para Identificação de Espécies; University of São Paulo: São Paulo, Brazil, 2006; p. 223. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org/ (accessed on 17 June 2021).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Petrie, A. Regclass: Tools for an Introductory Class in Regression and Modeling; Wiley-Black Well: Hoboken, NJ, USA, 2020. [Google Scholar]
- Daoud, J.I. Multicollinearity and regression analysis. J. Phys. Conf. Ser. 2018, 949, 012009. [Google Scholar] [CrossRef]
- Mandeville, P.B.; Concato, J. ¿Por qué se deben centrar las covariables en regresión lineal? Cienc. UANL 2008, XI, 300–305. [Google Scholar]
- StataCorp. Stata statistical software: Release 14.2. College Station. 2015; Available online: https://www.stata.com/ (accessed on 8 February 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).