Welder’s Anthrax: A Review of an Occupational Disease
Abstract
1. Introduction
2. Review of Cases of Welder’s Anthrax
3. Welding Processes and Exposures
4. Possible Mechanisms of Infection and Disease
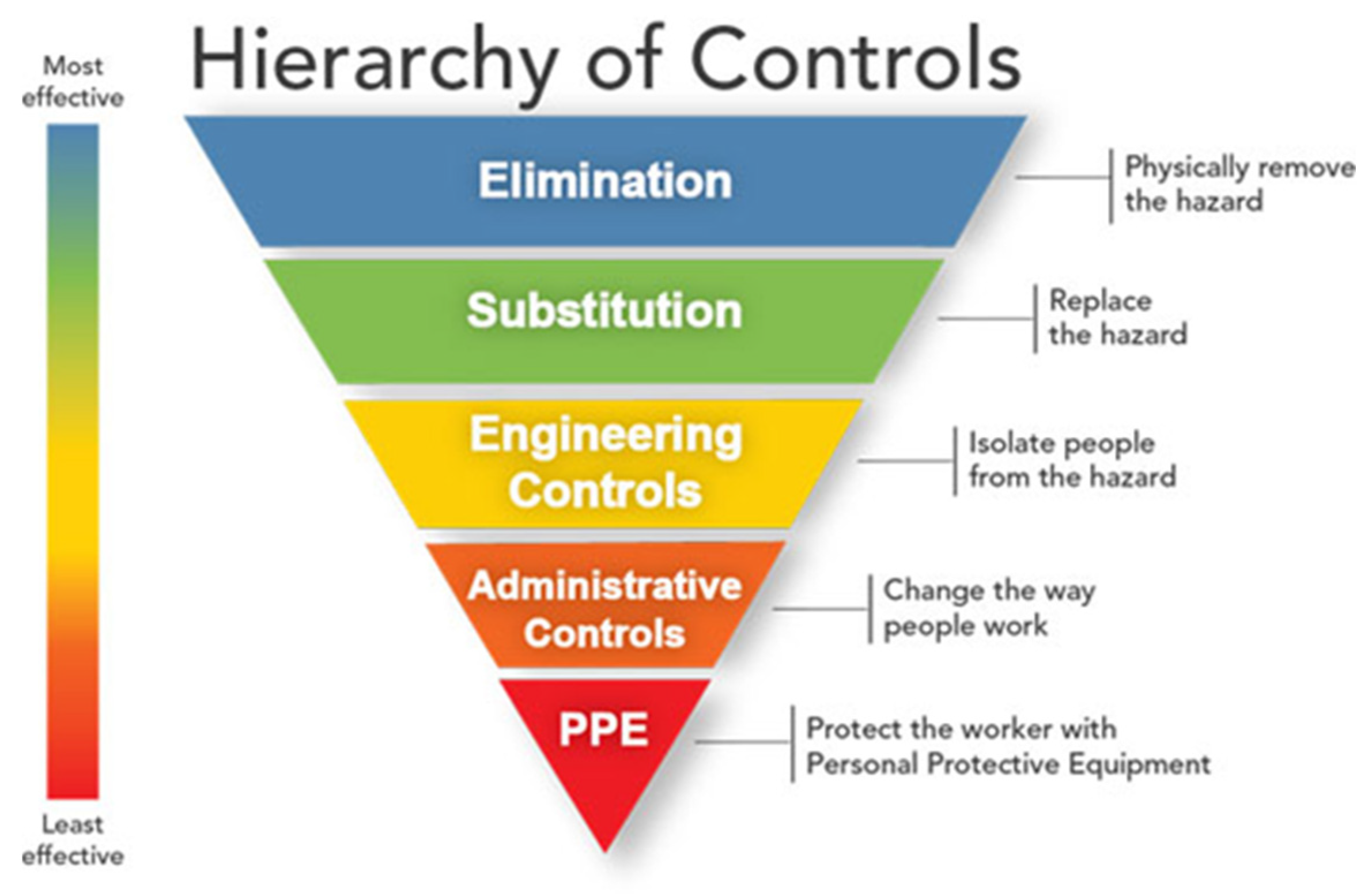
5. Occupational/Public Health Prevention Measures
6. Clinical Considerations and Medical Countermeasures
7. Public Health Implications
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Du, J.; Lai, Q.; Zeng, R.; Ye, D.; Xu, J.; Shao, Z. Proposal of Nine Novel Species of the Bacillus cereus Group. Int. J. Syst. Evol. Micribiol. 2017, 67, 2499–2508. [Google Scholar] [CrossRef] [PubMed]
- Hoffmaster, A.R.; Ravel, J.; Rasko, D.A.; Chapman, G.D.; Chute, M.D.; Marston, C.K.; De, B.K.; Sacchi, C.T.; Fitzgerald, C.; Mayer, L.W.; et al. Identification of Anthrax Toxin Genes in a Bacillus cereus Associated with an Illness Resembling Inhalation Anthrax. Proc. Natl. Acad. Sci. USA 2004, 101, 8449–8454. [Google Scholar] [CrossRef]
- Okinaka, R.T.; Cloud, K.; Hampton, O.; Hoffmaster, A.R.; Hill, K.K.; Keim, P.; Koehler, T.M.; Lamke, G.; Kumano, S.; Mahillon, J.; et al. Sequence and Organization of pXO1, the Large Bacillus anthracis Plasmid Harboring the Anthrax Toxin Genes. J. Bacteriol. 1999, 181, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
- Okinaka, R.; Cloud, K.; Hampton, O.; Hoffmaster, A.; Hill, K.; Keim, P.; Koehler, T.; Lamke, G.; Kumano, S.; Manter, D.; et al. Sequence, Assembly and Analysis of pX01 and pX02. J. Appl. Microbiol. 1999, 87, 261–262. [Google Scholar] [CrossRef]
- Green, B.D.; Battisti, L.; Koehler, T.M.; Thorne, C.B.; Ivins, B.E. Demonstration of a Capsule Plasmid in Bacillus anthracis. Infect. Immun. 1985, 49, 291–297. [Google Scholar] [CrossRef]
- Bottone, E.J. Bacillus cereus, a Volatile Human Pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef] [PubMed]
- Frankard, J.; Li, R.; Taccone, F.; Struelens, M.J.; Jacobs, F.; Kentos, A. Bacillus cereus Pneumonia in a Patient with Acute Lymphoblastic Leukemia. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 725–728. [Google Scholar] [CrossRef]
- Katsuya, H.; Takata, T.; Ishikawa, T.; Sasaki, H.; Ishitsuka, K.; Takamatsu, Y.; Tamura, K. A Patient with Acute Myeloid Leukemia who Developed Fatal Pneumonia Caused by Carbapenem-Resistant Bacillus cereus. J. Infect. Chemother. 2009, 15, 39–41. [Google Scholar] [CrossRef]
- Miyata, J.; Tasaka, S.; Miyazaki, M.; Yoshida, S.; Naoki, K.; Sayama, K.; Asano, K.; Fujiwara, H.; Ohkusu, K.; Hasegawa, N.; et al. Bacillus cereus Necrotizing Pneumonia in a Patient with Nephrotic Syndrome. Intern. Med. 2013, 52, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.M.; Hair, J.G.; Hebert, M.; Hebert, L.; Roberts, F.J., Jr.; Weyant, R.S. Fulminating Bacteremia and Pneumonia Due to Bacillus cereus. J. Clin. Microbiol. 1997, 35, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Hoffmaster, A.R.; Hill, K.K.; Gee, J.E.; Marston, C.K.; De, B.K.; Popovic, T.; Sue, D.; Wilkins, P.P.; Avashia, S.B.; Drumgoole, R.; et al. Characterization of Bacillus cereus Isolates Associated with Fatal Pneumonias: Strains are Closely Related to Bacillus anthracis and Harbor B. anthracis Virulence Genes. J. Clin. Microbiol. 2006, 44, 3352–3360. [Google Scholar] [CrossRef] [PubMed]
- Avashia, S.B.; Riggins, W.S.; Lindley, C.; Hoffmaster, A.; Drumgoole, R.; Nekomoto, T.; Jackson, P.J.; Hill, K.K.; Williams, K.; Lehman, L.; et al. Fatal Pneumonia among Metalworkers Due to Inhalation Exposure to Bacillus cereus Containing Bacillus anthracis Toxin Genes. Clin. Infect. Dis. 2007, 44, 414–416. [Google Scholar] [CrossRef]
- Wright, A.M.; Beres, S.B.; Consamus, E.N.; Long, S.W.; Flores, A.R.; Barrios, R.; Richter, G.S.; Oh, S.Y.; Garufi, G.; Maier, H.; et al. Rapidly Progressive, Fatal, Inhalation Anthrax-Like Infection in a Human: Case Report, Pathogen Genome Sequencing, Pathology, and Coordinated Response. Arch. Pathol. Lab. Med. 2011, 135, 1447–1459. [Google Scholar] [CrossRef]
- Pena-Gonzalez, A.; Rodriguez-R, L.M.; Marston, C.K.; Gee, J.E.; Gulvik, C.A.; Kolton, C.B.; Saile, E.; Frace, M.; Hoffmaster, A.R.; Konstantinidis, K.T. Genomic Characterization and Copy Number Variation of Bacillus anthracis Plasmids pXO1 and pXO2 in a Historical Collection of 412 Strains. mSystems 2018, 3, e00065-18. [Google Scholar] [CrossRef]
- Marston, C. (National Center for Emerging and Zoonotic Infectious Diseases, Atlanta, GA, USA). Personal Communication, 2021.
- Dawson, P.; Schrodt, C.A.; Feldmann, K.; Traxler, R.M.; Gee, J.E.; Kolton, C.B.; Marston, C.K.; Gulvik, C.A.; Antonini, J.M.; Negrón, M.E.; et al. Notes from the Field: Fatal Anthrax Pneumonia in Welders and Other Metalworkers Caused by Bacillus cereus Group Bacteria Containing Anthrax Toxin Genes—U.S. Gulf Coast States, 1994–2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1453–1454. [Google Scholar] [CrossRef]
- Meselson, M.; Guillemin, J.; Hugh-Jones, M.; Langmuir, A.; Popova, I.; Shelokov, A.; Yampolskaya, O. The Sverdlovsk Anthrax Outbreak of 1979. Science 1994, 266, 1202–1208. [Google Scholar] [CrossRef]
- Walker, D. Sverdlovsk Revisited: Pulmonary Pathology of Inhalational Anthrax Versus Anthraxlike Bacillus cereus Pneumonia. Arch. Path. Lab. Med. 2012, 136, 235. [Google Scholar] [CrossRef]
- U.S. Bureau of Labor Statistics, Occupational Employment and Wage Statistics. Occupational Employment and Wages, May 2020. 51-4121: Welders, Cutters, Solderers, and Brazers. Available online: https://www.bls.gov/oes/current/oes514121.htm (accessed on 10 August 2021).
- Minnesota Population Center. Integrated Public Use Microdata Series, International, Version 6.4. [dataset]; University of Minnesota: Minneapolis, MN, USA, 2015. [Google Scholar] [CrossRef]
- Villaume, J.E.; Wasti, K.; Liss-Suter, D.; Hsiao, S. Effects of Welding on Health; American Welding Society: Miami, FL, USA, 1979; Volume 1. [Google Scholar]
- Howden, D.G.; Desmeules, M.J.A.; Saracci, R.; Sprince, N.L.; Herber, P.I. Respiratory Hazards of Welding: Occupational Exposure Characterization. Am. Rev. Respir. Dis. 1988, 138, 1047–1048. [Google Scholar]
- The James F. Lincoln Arc Welding Foundation (Ed.) Arc-welding fundamentals. In The Procedure Handbook of Arc Welding, 15th ed.; The James F. Lincoln Arc Welding Foundation: Cleveland, OH, USA, 2000; pp. 1.3–1.11. [Google Scholar]
- Zimmer, A.T.; Biswas, P. Characterization of the Aerosols Resulting from Arc Welding Processes. J. Aerosol. Sci. 2001, 32, 993–1008. [Google Scholar] [CrossRef]
- Antonini, J.M.; Afshari, A.A.; Stone, S.; Chen, B.; Scwegler-Berry, D.; Fletcher, W.G.; Goldsmith, W.T.; Vandestouwe, K.H.; McKinney, W.; Castranova, V.; et al. Design, Construction, and Characterization of a Novel Robotic Welding Fume Generator and Inhalation Exposure System for Laboratory Animals. J. Occup. Environ. Hyg. 2006, 3, 194–203. [Google Scholar] [CrossRef]
- NIOSH. Criteria for a Recommended Standard: Welding, Brazing and Thermal C NIOSH. In Criteria for a Recommended Standard: Welding, Brazing and Thermal Cutting; U.S. Department of Health and Human Services, Centers for Disease Control, National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 1988; pp. 88–110. Available online: https://www.cdc.gov/niosh/docs/88-110/pdfs/88-110.pdf?id=10.26616/NIOSHPUB88110 (accessed on 25 March 2022).
- Bowler, R.M.; Gysens, S.; Diamond, E.; Nakagawa, S.; Drezgic, M.; Roles, H.A. Manganese Exposure: Neuropsychological and Neurological Symptoms and Effects in Welders. Neurotoxicology 2006, 27, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Lundin, J.I.; Checkoway, H.; Criswell, S.R.; Hobson, A.J.; Harris, R.C.; Swisher, L.M.; Evanoff, B.A.; Racette, B.A. Screening for early detection of parkinsonism using a self-administered questionnaire: A cross-sectional epidemiologic study. Neurotoxicology 2014, 45, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Tharr, D.; Wallace, M.E.; Lentz, T.J.; Fajen, J.M.; Palassis, J.; Case Studies. Assessment of Students’ Exposure to Welding Fumes in a Vocational School Welding Shop. Appl. Occup. Environ. Hyg. 1997, 12, 712–715. [Google Scholar] [CrossRef]
- Susi, P.; Goldberg, M.; Barnes, P.; Stafford, E. Use of Task-based Exposure Assessment Model (TBEAM) for Assessment of Metal Fume Exposures during Welding and Thermal Cutting. Appl. Occup. Environ. Hyg. 2000, 15, 26–38. [Google Scholar] [CrossRef]
- Korczynski, R.E. Occupational Health Concerns in the Welding Industry. Appl. Occup. Environ. Hyg. 2000, 15, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Susi, P.; Flynn, M.R. Manganese and Welding Fume Exposure and Control in Construction. J. Occup. Environ. Hyg. 2007, 4, 943–951. [Google Scholar] [CrossRef]
- Torén, K.; Blanc, P.D.; Naidoo, R.N.; Murgia, N.; Qvarfordt, I.; Aspevall, O.; Dahlman-Hoglund, A.; Schioler, L. Occupational Exposure to Dust and to Fumes, Work as a Welder and Invasive Pneumococcal Disease Risk. Occup. Environ. Med. 2019, 77, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.H.; Moon, K.T.; Kim, J.Y.; Choe, S.W. The Risk of Hospitalisation for Infectious Pneumonia in Mineral Dust Exposed Industries. Occup. Environ. Med. 2011, 68, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Torén, K.; Qvarfordt, I.; Bergdahl, I.A.; Järvholm, B. Increased Mortality from Infectious Pneumonia after Occupational Exposure to Inorganic Dust, Metal Fumes and Chemicals. Thorax 2011, 66, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Marrie, T.J.; Garg, S.; Kellner, J.D.; Tyrrell, G.J.; SPAT Group. Welders Are at Increased Risk for Invasive Pneumococcal Disease. Int. J. Infect. Dis. 2010, 14, e796–e799. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.T.; Cullinan, P.; Rice, S.; Brown, T.; Coggon, D. Mortality from Infectious Pneumonia in Metal Workers: A Comparison with Deaths from Asthma in Occupations Exposed to Respiratory Sensitisers. Thorax 2009, 64, 983–986. [Google Scholar] [CrossRef]
- Marongiu, A.; Hasan, O.; Ali, A.; Bakhsh, S.; George, B.; Irfan, N.; Minelli, C.; Canova, C.; Schofield, S.; De Matteis, S.; et al. Are Welders More at Risk of Respiratory Infections? Findings from a Cross-sectional Survey and Analysis of Medical Records in Shipyard Workers: The WELSHIP Project. Thorax 2016, 71, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Blanc, P.D.; Annesi-Maesano, I.; Balmes, J.R.; Cummings, K.J.; Fishwick, D.; Miedinger, D.; Murgia, N.; Naidoo, R.N.; Reynolds, C.J.; Sigsgaard, T.; et al. The Occupational Burden of Nonmalignant Respiratory Diseases. An Official American Thoracic Society and European Respiratory Society Statement. Am. J. Respir. Crit. Care Med. 2019, 199, 1312–1334. [Google Scholar] [CrossRef]
- Lockey, J.E.; Schenker, M.B.; Howden, D.G.; Desmeules, M.J.; Saracci, R.; Sprince, N.L.; Harber, P.I. Current Issues in Occupational Lung Disease. Am. Rev. Respir. Dis. 1988, 138, 1047–1050. [Google Scholar]
- Wergeland, E.; Iversen, B.G. Deaths from Pneumonia after Welding. Scand. J. Work Environ. Health 2001, 27, 353. [Google Scholar] [CrossRef] [PubMed]
- Coggon, D.; Inskip, H.; Winter, P.; Pannett, B. Lobar Pneumonia: An Occupational disease in Welders. Lancet 1994, 344, 41–43. [Google Scholar] [CrossRef]
- Palmer, K.T.; Poole, J.; Ayres, J.G.; Mann, J.; Burge, P.S.; Coggon, D. Exposure to Metal Fume and Infectious Pneumonia. Am. J. Epidemiol. 2003, 157, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Antonini, J.M.; Stone, S.; Roberts, J.R.; Chen, B.; Schwegler-Berry, D.; Afshari, A.A.; Frazer, D.G. Effect of Short-Term Stainless Steel Welding Fume Inhalation Exposure on Lung Inflammation, Injury, and Defense Responses in Rats. Toxicol. Appl. Pharmacol. 2007, 223, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Antonini, J.M.; Roberts, J.R.; Stone, S.; Chen, B.T.; Schwegler-Berry, D.; Frazer, D.G. Short-term Inhalation Exposure to Mild Steel Welding Fume Had No Effect on Lung Inflammation and Injury but Did Alter Defense Responses to Bacteria in Rats. Inhal. Toxicol. 2009, 21, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Antonini, J.M.; Taylor, M.D.; Millecchia, L.; Bebout, A.R.; Roberts, J.R. Suppression in Lung Defense Responses after Bacterial Infection in Rats Pretreated with Different Welding Fumes. Toxicol. Appl. Pharmacol. 2004, 200, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Antonini, J.M.; Roberts, J.R. Chromium in Stainless Steel Welding Fume Suppresses Lung Defense Responses against Bacterial Infection in Rats. J. Immunotoxicol. 2007, 4, 117–127. [Google Scholar] [CrossRef]
- Daou, N.; Buisson, C.; Gohar, M.; Vidic, J.; Bierne, H.; Kallassy, M.; Lereclus, D.; Nielsen-LeRoux, C. IlsA, a Unique Surface Protein of Bacillus cereus Required for Iron Acquisition from Heme, Hemoglobin and Ferritin. PLoS Pathog. 2009, 5, e1000675. [Google Scholar] [CrossRef] [PubMed]
- Honsa, E.S.; Maresso, A.W. Mechanisms of iron import in anthrax. Biometals 2011, 24, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Doig, A.T.; McLaughlin, A.G. X-ray Appearances of the Lungs of Electric Arc Welders. Lancet 1936, 1, 771–775. [Google Scholar] [CrossRef]
- Enzer, N.; Sander, O.A. Chronic Lung Changes in Electric Arc Welders. J. Ind. Hyg. 1938, 20, 333–350. [Google Scholar]
- Kleinfeld, M.; Messite, J.; Kooyman, O.; Spiro, J. Welders’ Siderosis: A Clinical Roentgenographic and Physiological Study. Arch. Environ. Health 1969, 19, 70–73. [Google Scholar] [CrossRef]
- Morgan, W.K.C. On Welding, Wheezing, and Whimsy. Am. Ind. Hyg. Assoc. J. 1989, 50, 59–69. [Google Scholar] [CrossRef]
- Antonini, J.M.; Roberts, J.R.; Stone, S.; Chen, B.T.; Schwegler-Berry, D.; Chapman, R.; Zeidler-Erdely, P.C.; Andrews, R.N.; Frazer, D.G. Persistence of Deposited Metals in the Lungs after Stainless Steel and Mild Steel Welding Fume Inhalation in Rats. Arch. Toxicol. 2011, 85, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Kalliomaki, P.-L.; Kalliomaki, K.; Rahkonen, E.; Aittoniemi, K. Lung Retention of Welding Fumes and Ventilatory Lung Functions. A Follow-Up Study among Shipyard Welders. Ann. Occup. Hyg. 1983, 27, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Li, G.j.; Zhang, L.; Lu, L.; Wu, P.; Zheng, W. Occupational Exposure to Welding Fume among Welders: Alterations of Manganese, Iron, Zinc, Copper, and Lead in Body Fluids and the Oxidative Stress Status. J. Occup. Environ. Med. 2004, 46, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Pesch, B.; Weiss, T.; Kendzia, B.; Henry, J.; Lehner, M.; Lotz, A.; Heinze, E.; Käfferlein, H.U.; Van Gelder, R.; Berges, M.; et al. Levels and Predictors of Airborne and Internal Exposure to Manganese and Iron among Welders. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 291–298. [Google Scholar] [CrossRef]
- Friede, E.; Rachow, D.O. Symptomatic Pulmonary Disease in Arc Welders. Ann. Intern. Med. 1961, 54, 121–127. [Google Scholar] [PubMed]
- Ioannou, G.N.; Dominitz, J.A.; Weiss, N.S.; Heagerty, P.J.; Kowdley, K.V. The Effect of Alcohol Consumption on the Prevalence of Iron Overload, Iron Deficiency, and Iron Deficiency Anemia. Gastroenterology 2004, 126, 1293–1301. [Google Scholar] [CrossRef]
- Armand, P.; Kim, H.T.; Rhodes, J.; Sainvil, M.-M.; Cutler, C.; Ho, V.T.; Koreth, J.; Alyea, E.P.; Hearsey, D.; Neufeld, E.J.; et al. Iron Overload in Patients with Acute Leukemia or MDS Undergoing Myeloablative Stem Cell Transplantation. Biol. Blood Marrow Transpl. 2011, 17, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.B.; Myers, G.; Wang, H. Association between Neonatal Iron Overload and Early Human Brain Development in Premature Infants. Early Hum. Dev. 2012, 88, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Dheeba, B.; Sampathkumar, P. Evaluation of Heavy Metal Contamination in Surface Soil around Industrial Area, Tamil Nadu, India. Int. J. Chem. Tech. Res. 2012, 4, 1229–1240. [Google Scholar]
- Adekeye, E.A.; Ojo, M.A.; Ajayi, O.O. Contributions of Metal Welding Workshops to Environmental Pollution in Akure Metropolis, Ondo State, Nigeria. J. Environ. Iss. Agric. Dev. Ctry. 2011, 3, 1–7. [Google Scholar]
- Su, C.P.; de Perio, M.A.; Cummings, K.J.; McCague, A.B.; Luckhaupt, S.E.; Sweeney, M.H. Case Investigations of Infectious Diseases Occurring in Workplaces, United States, 2006-2015. Emerg. Infect. Dis. 2019, 25, 397–405. [Google Scholar] [CrossRef]
- OSHA. Recommended Practices for Safety and Health Programs. 2016. Available online: https://www.osha.gov/sites/default/files/OSHA3885.pdf (accessed on 9 December 2021).
- OSHA. Controlling Hazardous Fume and Gases during Welding. 2013. Available online: https://www.osha.gov/Publications/OSHA_FS-3647_Welding.pdf (accessed on 9 December 2021).
- NIOSH. Dust Control. Handbook for Industrial Minerals Mining and Processing, 2nd ed.; Cecala, A.B., O’Brien, A.D., Schall, J., Colinet, J.F., Franta, R.J., Schultz, M.J., Haas, E.J., Robinson, J., Patts, J., Holen, B.M., et al., Eds.; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health: Pittsburgh, PA, USA, 2019; DHHS (NIOSH) Publication No. 2019–124, RI 9701. [CrossRef][Green Version]
- OSHA. OSHA Hazard Communication Standard-29 CFR 1910.1200. 2021. Available online: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1200 (accessed on 9 December 2021).
- OSHA. OSHA Respiratory Protection Program Standard-29 CFR 1910.134. Available online: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134 (accessed on 9 December 2021).
- Donoghue, A.M.; Wesdock, J.C. Pneumococcal Vaccination for Welders: Global Deployment within a Multi-national Corporation. Am. J. Ind. Med. 2019, 62, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.T.; Cosgrove, M. Vaccinating Welders against Pneumonia. Occup. Environ. Med. 2012, 69, 932. [Google Scholar] [CrossRef]
- Brézillon, C.; Haustant, M.; Dupke, S.; Corre, J.P.; Lander, A.; Franz, T.; Monot, M.; Couture-Tosi, E.; Jouvion, G.; Leendertz, F.H.; et al. Capsules, Toxins and AtxA as Virulence Factors of Emerging Bacillus cereus biovar anthracis. PLoS Negl. Trop. Dis. 2015, 9, e0003455. [Google Scholar] [CrossRef]
- Bower, W.A.; Schiffer, J.; Atmar, R.L.; Keitel, W.A.; Friedlander, A.M.; Liu, L.; Yu, Y.; Stephens, D.S.; Quinn, C.P.; Hendricks, K.; et al. Use of Anthrax Vaccine in the United States: Recommendations of the Advisory Committee on Immunization Practices, 2019. MMWR Recomm. Rep. 2019, 68, 1–14. [Google Scholar] [CrossRef]
- Wright, J.G.; Quinn, C.P.; Shadomy, S.; Messonnier, N. Use of Anthrax Vaccine in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm. Rep. 2010, 59, 1–30. [Google Scholar]
- Hendricks, K.A.; Wright, M.E.; Shadomy, S.V.; Bradley, J.S.; Morrow, M.G.; Pavia, A.T.; Rubinstein, E.; Holty, J.E.; Messonnier, N.E.; Smith, T.L.; et al. Centers for Disease Control and Prevention Expert Panel Meetings on Prevention and Treatment of Anthrax in Adults. Emerg. Infect. Dis. 2014, 20, e130687. [Google Scholar] [CrossRef] [PubMed]
- de Perio, M.A.; Materna, B.L.; Sondermeyer Cooksey, G.L.; Vugia, D.J.; Su, C.P.; Luckhaupt, S.E.; McNary, J.; Wilken, J.A. Occupational Coccidioidomycosis Surveillance and Recent Outbreaks in California. Med. Mycol. 2019, 57, S41–S45. [Google Scholar] [CrossRef]
- National Institute for Occupational Safety and Health. Collecting and Using Industry and Occupation Data. Available online: https://www.cdc.gov/niosh/topics/coding/default.html (accessed on 27 May 2021).
| Patient | Year of Diagnosis | Age Race/Ethnicity Sex | Occupation | Worksite State | Other Work Information | Co-Morbidities | Anthrax Toxin Genes | Strain | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 1994 | 42 male * | Welder | LA | None mentioned | None | Yes | B. cereus G9241 # | Recovered | [2] |
| B | 2003 | 39 white male | Welder | TX | Welder for 19 years | Mild asthma, hypertension, hyperlipidemia | Yes | B. cereus 03BB87 # | Died | [11,12] |
| C | 2003 | 56 black male | Metalworker | TX | Worked in foundry, grinding metal for polishing and operating machine | 40 pack per year smoker | Yes | B. cereus 03BB102 | Died | [11,12] |
| D | 2007 | 47 female * | Welder | LA | Shipyard-related | None | Yes | B. cereus LA2007 # | Died | [14,15] |
| E | 2011 | 39 Hispanic male | Welder | TX | None mentioned | None | Yes | B. cereus Elc2 | Died | [13] |
| F | 2020 | 39 white male | Welder | LA | Welded on oil tank on new A36 mild carbon steel using a shielded metal arc welding (or stick) process | Hypertension, gastroesophageal reflux, 25 pack per year smoker, alcohol use disorder | Yes | B. cereus LA2020 # | Recovered, received antitoxin | [16] |
| G | 2020 | 34 Hispanic male | Welder | TX | Worked as a welder for 10 years. Worked in a fabrication shop on low-carbon mild steel using Metal Inert Gas (MIG) | Childhood epilepsy, alcohol use disorder | Yes | B. cereus TX2020 | Died | [16] |
| Patient | Type of Environmental Sample Collected | No. of Environmental Samples Collected | Analytic Method | No. Positive Environmental Samples | Isolate Notes |
|---|---|---|---|---|---|
| B [12] | Settled dust and dirt | Not published | Culture | 1 B.cereus isolate from dust samples from cart | Positive for pXO2 cap genes but not anthrax toxin genes; isolate distinct from clinical isolates |
| C [12] | Settled dust and dirt | Not published | Culture | 0 | NA |
| F [16] | Soil, gravel, settled dust and dirt swabs from oil tank * | 132 | RT-PCR and culture | 10 total PCR-positive 4 soil samples from oil tank 4 gravel samples from around worksite 2 swabs from grinder tools and cabinets | One isolate was grown and was a genetic match to the patient’s clinical isolate |
| G [16] | Soil, settled dust and dirt (sponge and swabs) from surfaces, broom bristles | 108 | RT-PCR | 0 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Perio, M.A.; Hendricks, K.A.; Dowell, C.H.; Bower, W.A.; Burton, N.C.; Dawson, P.; Schrodt, C.A.; Salzer, J.S.; Marston, C.K.; Feldmann, K.; et al. Welder’s Anthrax: A Review of an Occupational Disease. Pathogens 2022, 11, 402. https://doi.org/10.3390/pathogens11040402
de Perio MA, Hendricks KA, Dowell CH, Bower WA, Burton NC, Dawson P, Schrodt CA, Salzer JS, Marston CK, Feldmann K, et al. Welder’s Anthrax: A Review of an Occupational Disease. Pathogens. 2022; 11(4):402. https://doi.org/10.3390/pathogens11040402
Chicago/Turabian Stylede Perio, Marie A., Katherine A. Hendricks, Chad H. Dowell, William A. Bower, Nancy C. Burton, Patrick Dawson, Caroline A. Schrodt, Johanna S. Salzer, Chung K. Marston, Karl Feldmann, and et al. 2022. "Welder’s Anthrax: A Review of an Occupational Disease" Pathogens 11, no. 4: 402. https://doi.org/10.3390/pathogens11040402
APA Stylede Perio, M. A., Hendricks, K. A., Dowell, C. H., Bower, W. A., Burton, N. C., Dawson, P., Schrodt, C. A., Salzer, J. S., Marston, C. K., Feldmann, K., Hoffmaster, A. R., & Antonini, J. M. (2022). Welder’s Anthrax: A Review of an Occupational Disease. Pathogens, 11(4), 402. https://doi.org/10.3390/pathogens11040402







