Abstract
Pseudomonas protegens (strain DSMZ 13134) is a biocontrol agent with promising antagonistic activity hinging on antibiosis against the fungal forest pathogens Heterobasidion spp. Here, by using High-Performance Liquid Chromatography coupled to Mass Spectrometry (HPLC-MS), we assessed whether monocultures of P. protegens (strain DSMZ 13134) produce the three major determinants of biocontrol activity known for the genus Pseudomonas: 2,4-diacetylphloroglucinol (2,4-DAPG), pyoluteorin (PLT), and pyrrolnitrin (PRN). At the tested culture conditions, we observed the production of PLT at concentrations ranging from 0.01 to 10.21 mg/L and 2,4-DAPG at a concentration not exceeding 0.5 mg/L. Variations of culture conditions involving culture medium, incubation temperature, and incubation period had no consistent influence on PLT production by the bacterium. Assays using culture medium amended with PLT at the same concentration of that present in cell-free filtrate of the bacterium, i.e., 3.77 mg/L, previously documented as effective against Heterobasidion spp., showed a remarkable activity of PLT against genotypes of all the four Heterobasidion species present in Europe, including the non-native invasive H. irregulare. However, such antifungal activity decreased over time, and this may be a constraint for using this molecule as a pesticide against Heterobasidion spp. When the bacterium was co-cultured in liquid medium with genotypes of the different Heterobasidion species, an increased production of PLT was observed at 4 °C, suggesting the bacterium may perform better as a PLT producer in field applications under similar environmental conditions, i.e., at low temperatures. Our results demonstrated the role of PLT in the inhibition of Heterobasidion spp., although all lines of evidence suggest that antibiosis does not rely on a single constitutively produced metabolite, but rather on a plethora of secondary metabolites. Findings presented in this study will help to optimize treatments based on Pseudomonas protegens (strain DSMZ 13134) against Heterobasidion spp.
1. Introduction
A variety of Plant Growth-Promoting Rhizobacteria (PGPR) exhibit antagonistic effects against plant pathogens via nutrient competition, induced resistance, priming, and antibiosis [1,2,3]. Pseudomonas spp. have been widely studied for their biocontrol potential [1,2,4]. They show antagonistic effects against a wide range of fungal plant pathogens, including ascomycetes, basidiomycetes, and mitosporic fungi [1,2,3]. The genus Pseudomonas is reported to inhibit pathogens by antibiosis through the secretion of secondary metabolites [1,2,4,5,6]. Different Pseudomonas species secrete a plethora of secondary metabolites with antibiotic activity, including pyoluteorin (PLT), pyrrolnitrin (PRN), 2,4-diacetylphloroglucinol (2,4-DAPG), phenazines, cyclic lipopeptides, and volatile compounds [1,2,3,6]. Based on genomic information, other undetected secondary metabolites could also be secreted [4].
Diseases caused by the fungal pathogens Heterobasidion spp. include destructive root and butt rots in coniferous forests of the Northern Hemisphere [7]. Members of the species complex H. annosum (Fr.) Bref. sensu lato (s.l.) are native to Europe., including H. abietinum Niemelä & Korhonen, H. annosum sensu stricto (s.s.) (Fr.) Bref., hereafter referred to as H. annosum, and H. parviporum Niemelä & Korhonen which are mainly associated with Abies alba Mill., Pinus spp., and Picea abies (L.) Karst., respectively [7]. In central Italy, stone pine (P. pinea L.) stands along the west coastline are threatened by the invasive North American H. irregulare Garbel. & Otrosina [8]. Following a pest risk analysis [9], this non-native Heterobasidion species is currently recommended for regulation under the European and Mediterranean Plant Protection Organization (EPPO) A2 list. Disease management implies treating freshly cut stumps with either chemical or biological products to prevent stump infection by spores and subsequent spreading of the fungi to neighboring healthy trees through root contacts [7].
Our study focuses on the PGPR Pseudomonas protegens (strain DSMZ 13134), which is the active component of the bio fungicide Proradix® (SP Sourcon Padena GmbH, Tübingen, Germany) currently commercialized against black scurf caused by Rhizoctonia solani J.G. Kühn and silver scab caused by Helmintosporium solani Durieu & Mont. on potatoes and other tubers. Previous laboratory and field studies had shown the ability of this PGPR to display significant antagonistic effects towards the fungal forest pathogens Heterobasidion spp., making it a candidate biocontrol agent against the Heterobasidion species [10,11,12,13]. Moreover, the cell-free filtrate (CFF) of the same bacterium displayed the ability to inhibit both mycelial growth (100% inhibition) and conidial germination (99% inhibition) of the pathogen in vitro [12] and performed even better than the bio fungicide Proradix® in experiments conducted in controlled conditions and in the forest on stumps of several host tree species [12,13]. It has been shown that antibiosis is likely the main mechanism of action of P. protegens (strain DSMZ 13134) against Heterobasidion spp. [12,13], as well as towards other fungal pathogens [14]. The secondary metabolites with antifungal activity present in the CFF are the putative determinants of both Proradix® and CFF efficacy, but those compounds and their mechanisms of action remain completely unknown. Knowing the mechanisms of action of a biocontrol agent can help with the optimization of the disease control, but it is also required for registration where risks for humans and the environment, including risks for resistance development, have also to be indicated [4,15]. Regulation in the EU makes a distinction between biocontrol agents able to produce secondary metabolites with antimicrobial activity in situ and such compounds in product without living cells of the biocontrol agent [4].
This study investigated the formation and the concentration of three of the major known determinants of biocontrol activity of Pseudomonas spp., namely 2,4-DAPG, PLT and PRN [1,2,3,6,16] in the CFF of P. protegens (strain DSMZ 13134) used as both an amendment in medium inhibiting Heterobasidion spp. in vitro and a stump treatment in a previous field study [12,13]. The antifungal activity of the selected secondary metabolites present in the CFF, and its contribution to the antifungal activity of the raw CFF, were evaluated in vitro at different times against the four Heterobasidion spp. currently occurring in Europe, including the non-native invasive H. irregulare. In addition, we assessed whether the variety and yield of such metabolites could be enhanced by acting on culture conditions (culture medium, incubation temperature, and incubation period). Our ultimate aim was to explore whether the production of these compounds by P. protegens (strain DSMZ 13134) is temperature-controlled during the interaction with Heterobasidion species. This could help to predict the production of metabolites when the bacterium (i.e., Proradix®) is applied on stump surfaces in infested sites under different environmental conditions.
2. Results
2.1. Presence and Concentration of Selected Secondary Metabolites in the CFF of P. protegens (Strain DSMZ 13134)
To determine whether the selected secondary metabolites are produced by P. protegens (strain DSMZ 13134), we generated a monoculture of the bacterium in Luria–Bertani (LB) broth for 24 h at 25 °C. The CFF of the monoculture was obtained by filtering the bacterial culture aseptically through a 0.22 μm filter membrane. Chemical analysis of CFF through High-Performance Liquid Chromatography coupled to Mass Spectrometry (HPLC-MS) allowed us to determine the presence of PLT at the concentration of 3.77 mg/L (Table 1). Neither 2,4-DAPG nor PRN were detected in the CFF of P. protegens (strain DSMZ 13134) (Table 1).

Table 1.
Concentration of 2,4-diacetylphloroglucinol (2,4-DAPG), pyoluteorin (PLT), and pyrrolnitrin (PRN) in monocultures of P. protegens (strain DSMZ 13134). Monocultures were grown in Luria–Bertani, King B, and a modified King B broth at 4 °C, 25 °C, and 30 °C and incubating them for 24 h and 7 days.
2.2. Effects of PLT on Mycelial Growth of Heterobasidion spp.
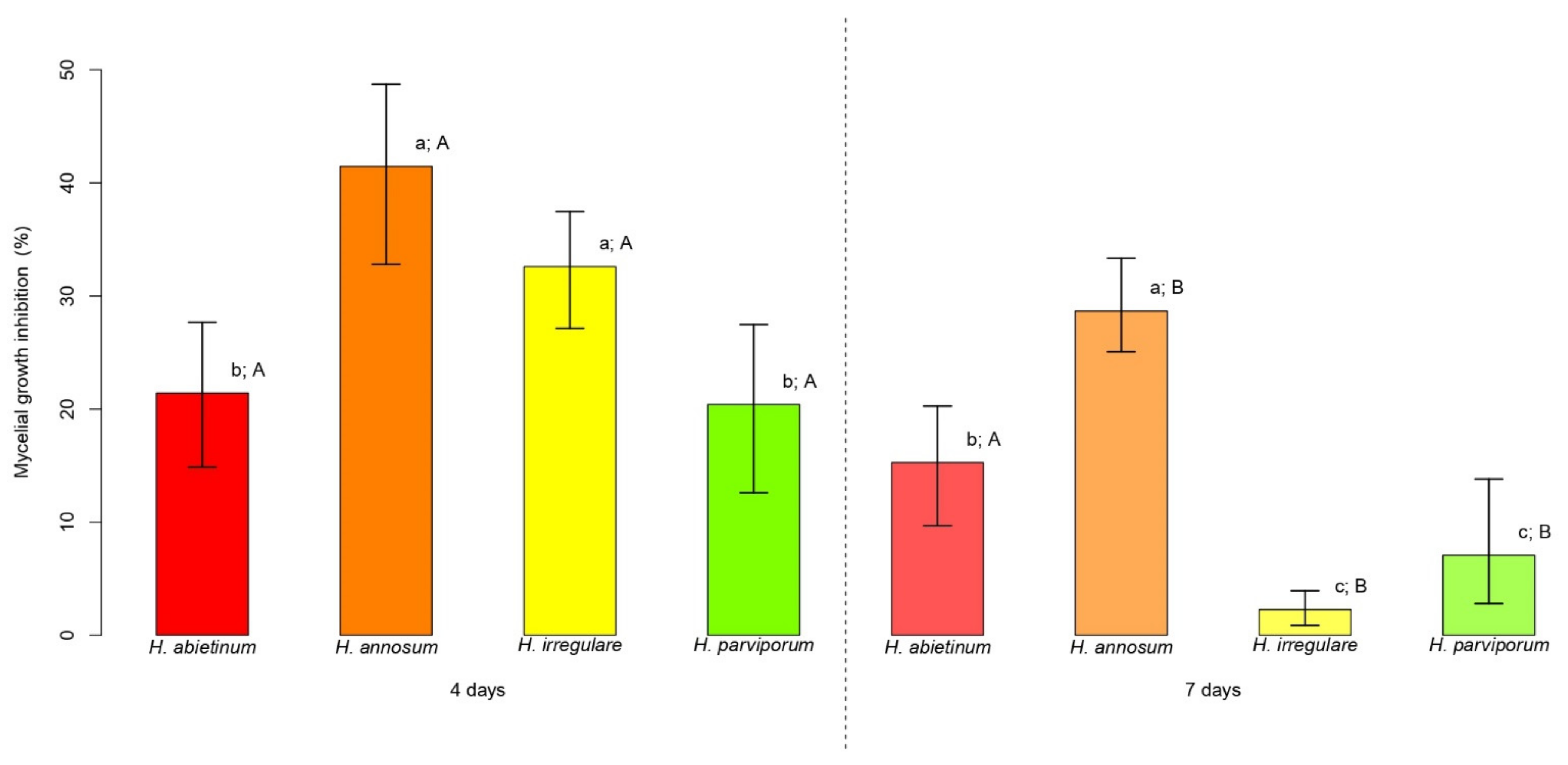
PLT at the concentration of 3.77 mg/L (consistent with that present in CFF) was assayed for mycelial growth inhibition (MGI in %) of Heterobasidion genotypes. The average MGI of Heterobasidion spp. ranged from 20.4% (12.6–27.5% 95% CI) to 41.5% (32.8–48.7% 95% CI) at 4 days, and from 2.3% (0.9–3.9% 95% CI) to 28.7% (25.1–33.3% 95% CI) at 7 days (Figure 1). At 4 days, H. annosum and H. irregulare attained average values of MGI over 40% and 30%, respectively, which were significantly higher than those observed for H. abietinum and H. parviporum, both at around 20% (p < 0.05) (Figure 1). H. annosum was the most inhibited species at 7 days with an average MGI around 30%, a value twofold higher than that attained by H. abietinum (p < 0.05) (Figure 1). At the same time point, H. irregulare and H. parviporum were significantly less inhibited than the other Heterobasidion species (p < 0.05), attaining average values of MGI around 2 and 7%, respectively (Figure 1). From the first to the second time point, a reduction in the average MGI was observed for all Heterobasidion species achieving approximately −6% for H. abietinum (p > 0.05), −13% for H. annosum and H. parviporum (p < 0.05), and −30% for H. irregulare (p < 0.05) (Figure 1).
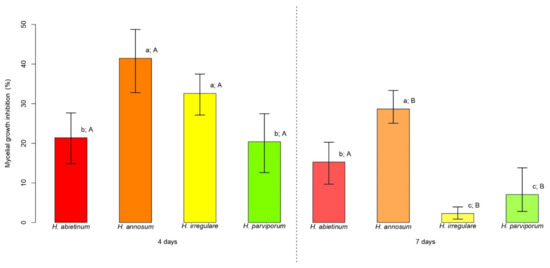
Figure 1.
Mycelial growth inhibition (MGI) comparisons. For each time point (4 and 7 days) bars indicate the average MGI displayed by the four species of Heterobasidion. Error bars refer to the lower and upper bounds of the 95% confidence interval. Above each bar, lowercase letters refer to the comparisons of average MGI values among Heterobasidion species for each time point, while upper case letters refer to the comparison of the two time points for the same species. Different letters indicate significant differences (p < 0.05).
2.3. Effects of Culture Conditions on Secondary Metabolites Production by Monocultures of P. protegens (Strain DSMZ 13134)
To evaluate how cultural parameters may affect secondary metabolites production in P. protegens (strain DSMZ 13134), we tested a panel of monocultures of the bacterium growing at different conditions. Pseudomonas protegens (strain DSMZ 13134) produced PLT at concentrations ranging from 0.01 to 10.21 mg/L depending on cultural conditions (Table 1). Quantification of 2,4-DAPG was possible only in monocultures grown in LB for 24 h at 4 and 30 °C, whereas in the other culture conditions, 2,4-DAPG was under the limit of quantification (LOQ, 0.5 mg/L) or it was not detected (Table 1). Concentrations of PRN were always under the detection limit (LOD) (Table 1).
Statistical analysis carried out with unbiased recursive partitioning tree model showed that the concentration of PLT produced by monocultures of P. protegens (strain DSMZ 13134) was not significantly associated with either culture medium, incubation temperature, or incubation period (p > 0.05) (Table 2). The average concentration of PLT was 2.39 mg/L (1.68–3.32 mg/L 95% CI) (Figure 2).

Table 2.
Association between concentration of pyoluteorin (PLT) and culture conditions of P. protegens (strain DSMZ 13134). The c statistics and its p-value are reported for each of the factors tested for their association with the response variable. Multiple values of c marked by a numeric index in subscript refer to subsequent splits of the tree model. Significant c values (p < 0.05) are indicated by the symbol *.
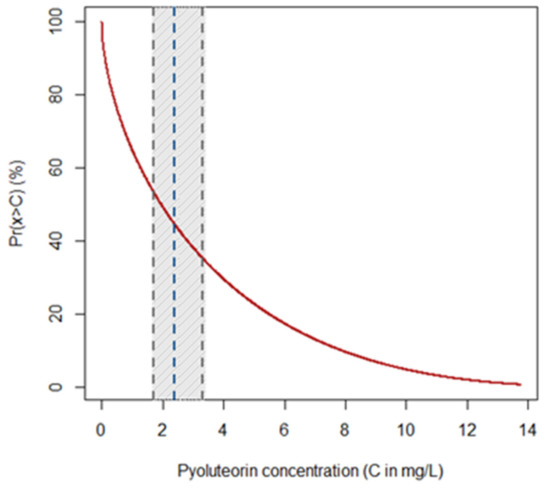
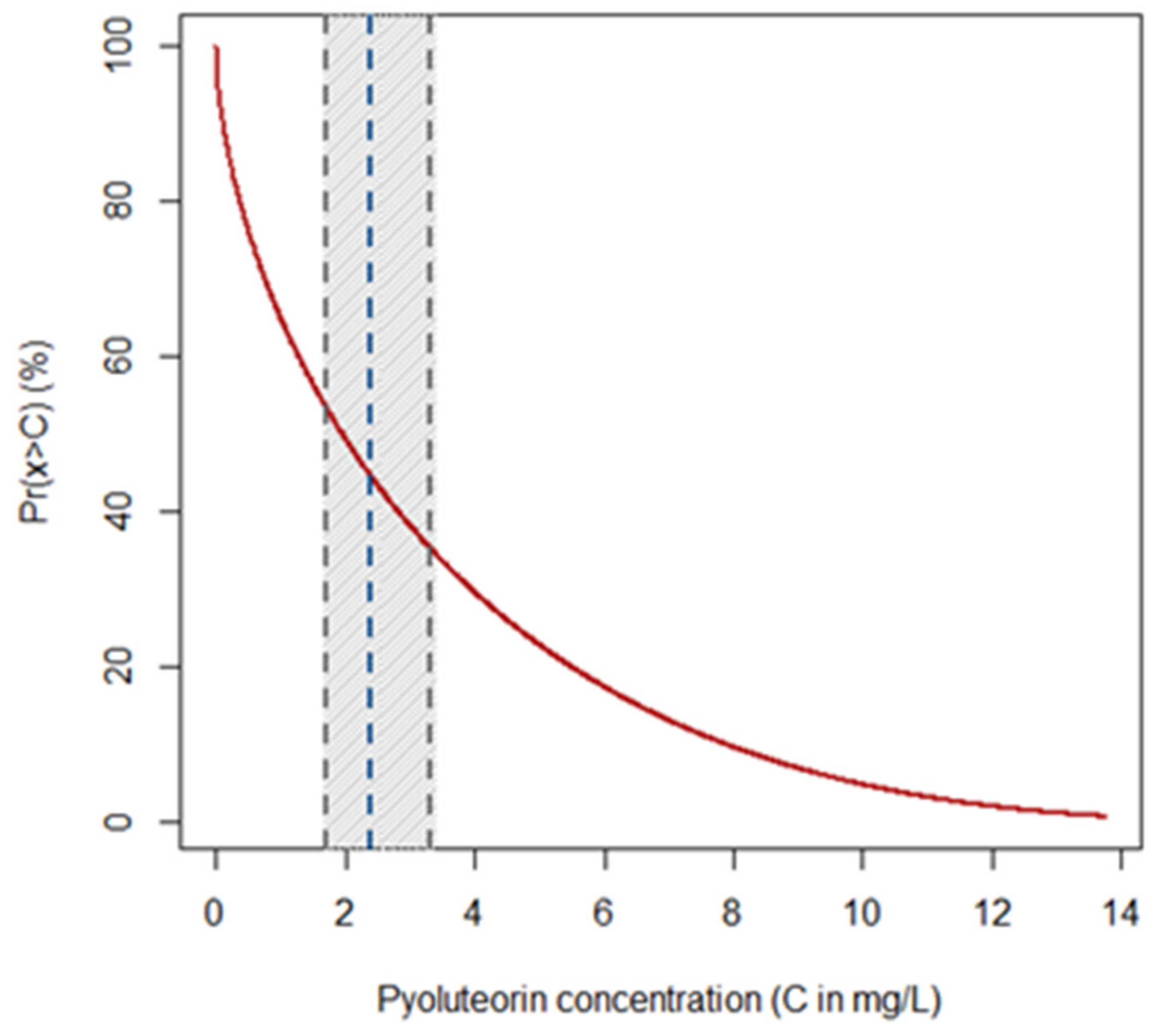
Figure 2.
Probability of retrieving pyoluteorin (PLT) from monocultures of P. protegens (strain DSMZ 13134) with concentrations over a given threshold. The graph shows the thresholds C of PLT concentration (in mg/L) on the x-axis, while the y-axis reports the probability ( in %) that P. protegens (strain DSMZ 13134) may release higher concentrations of the compound. The red curve quantifies the probability, while the blue dashed lines represent the average expected concentration of PLT along with the lower and upper bounds (gray dashed lines) of its 95% confidence interval (gray strip).
Among the distribution types included in the Pearson system of generalized frequency curves, type I attained the lowest AIC (68.0), while all others displayed AIC values over 200. The equation of the corresponding upper tail probability distribution function obtained was:
where the probability that monocultures of P. protegens (strain DSMZ 13134) release PLT with a concentration x higher than a given threshold C is provided by the integral of the Pearson curve of type I (Figure 2). In the above equation, is the Euler Gamma function, while the curve parameters obtained through maximum likelihood estimation were , and . The curve shows that PLT is produced by monocultures of P. protegens (strain DSMZ 13134) (i.e., the concentration of the metabolite is higher than 0) with a probability approaching 100% (Figure 2). The same curve indicates that the probability of retrieving PLT with a concentration of up to 3.77 mg/L (i.e., mean concentration measured in the CFF) is approximately 70% (Figure 2).
2.4. Effects of Co-Cultures of P. protegens (Strain DSMZ 13134) with Heterobasidion spp. on Secondary Metabolites Production by the Bacterium
We explored whether the production of secondary metabolites by P. protegens (strain DSMZ 13134) was enhanced or reduced during the interaction with Heterobasidion species at different temperatures. The ability to produce PLT was confirmed in co-culture experiments (Table 3; Figure 3). Pseudomonas protegens (strain DSMZ 13134) produced PLT at concentrations ranging from 0.07 to 9.90 mg/L in extracts of all co-cultures except one with H. abietinum at 30 °C (Table 3). At all tested temperatures, P. protegens (strain DSMZ 13134) in co-culture produced 2,4-DAPG under the LOQ (Table 3). Again, despite some variations in culture conditions, P. protegens (strain DSMZ 13134) failed to produce a detectable concentration of PRN (Table 3).

Table 3.
Concentration of 2,4-diacetylphloroglucinol (2,4-DAPG), pyoluteorin (PLT), and pyrrolnitrin (PRN) produced in co-cultures of P. protegens (strain DSMZ 13134) and one genotype of different Heterobasidion species. Co-cultures were grown in King B broth at 4 °C, 25 °C, and 30 °C, and incubating them for 7 days.
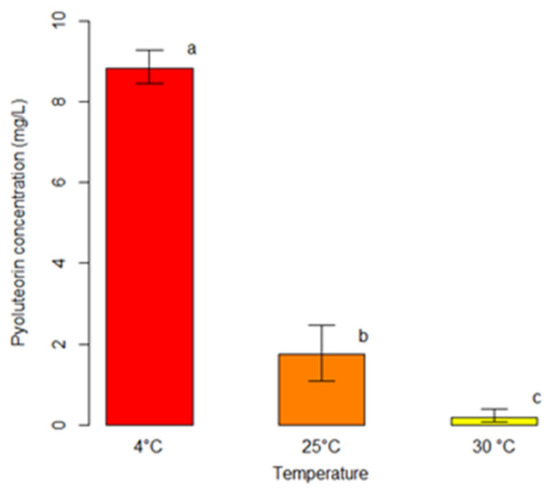
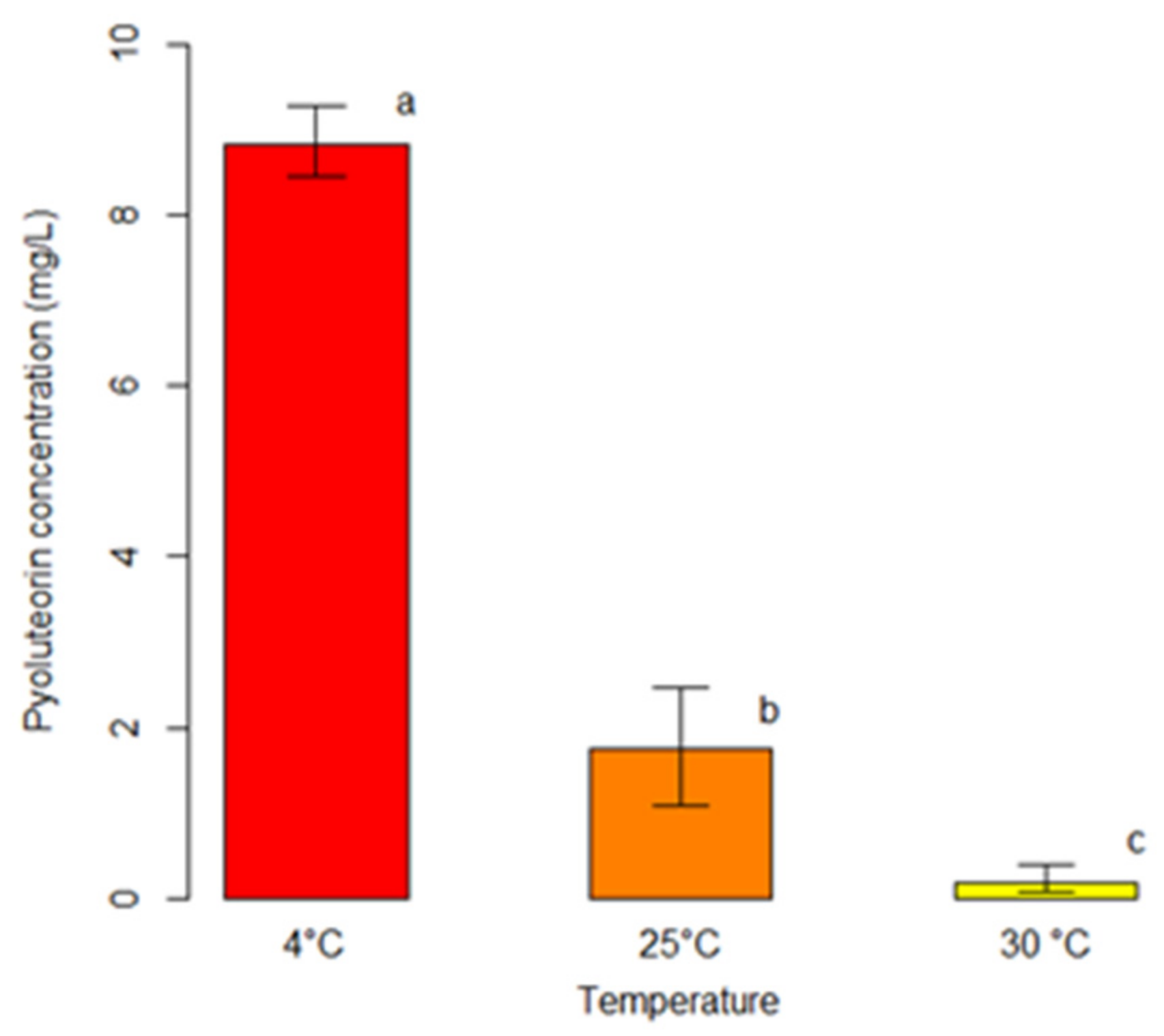
Figure 3.
Comparison among average concentrations of pyoluteorin (PLT) produced by P. protegens (strain DSMZ 13134) in co-culture with Heterobasidion spp. at different temperatures. For each temperature, bars indicate the average concentration of PLT (mg/L). Error bars refer to the lower and upper bounds of the 95% confidence interval. Different letters above the bars indicate significant differences (p < 0.05).
The outcomes of the unbiased recursive partitioning tree model pointed out that only the incubation temperature exerted a significant effect on the production of PLT from P. protegens (strain DSMZ 13134) in co-culture with Heterobasidion spp. (p < 0.05) (Table 2). Conversely, the co-culture type did not display any significant association with the response variable (p > 0.05) (Table 2). Decreasing incubation temperatures were associated with raising concentrations of PLT ranging from an average of 0.18 mg/L at 30 °C, to 1.74 mg/L at 25 °C, up to 8.84 mg/L at 4 °C (p < 0.05) (Figure 3).
3. Discussion
Plant Growth-Promoting Rhizobacteria are ubiquitous in nature, and they may have a range of agricultural, environmental, and industrial applications. The genus Pseudomonas is highly appreciated for its plant growth promoting traits and because of its ability to produce a range of secondary metabolites with antibiotic activity [2,3,5,6]. Among the investigated secondary metabolites, only PLT was present in the CFF of P. protegens (strain DSMZ 13134). Pyoluteorin is a polyketide metabolite produced by fluorescent Pseudomonas known for its fungicidal, bactericidal, and herbicidal activities [2,17]. The concentration of PLT in the CFF was measured as high as 3.77 mg/L. This concentration is consistent with those found in the well-studied rhizosphere bacterium P. protegens Pf-5 [18,19].
PLT at the same concentration present in the CFF, i.e., 3.77 mg/L, was assayed against the mycelial growth of Heterobasidion spp. to determine the individual contribution of PLT to the overall efficacy of the raw CFF, which was previously shown to be 100% [12]. The probability distribution function obtained from the Pearson system showed that the concentration of PLT present in the CFF is slightly over the average, but there is a large probability (70%) that P. protegens (strain DSMZ 13134) can release up to this concentration. Conversely, testing higher concentrations might be not sound because only in 30% of cases the bacterium is expected to produce PLT over the threshold of 3.77 mg/L. In this study, PLT proved to have antifungal activity against all the tested Heterobasidion species. Yet, its efficacy decreased significantly after 7 days of incubation against all Heterobasidion species except H. abietinum. The decline in the effectiveness may be due to PLT stability, as suggested by a previous experimental study on the degradation of PLT showing that temperature, solution pH and UV irradiation had a strong influence on its degradation [20]. Interestingly, PLT was found to be unstable in acid and in alkaline solutions, and under UV irradiation, making its use as a pesticide difficult without any modification to improve the stability [20]. In vitro culture conditions might have increased PLT instability, thus reducing its efficacy against the fungal pathogens over time. Significant reductions of the efficacy of PLT in a few days may be a constraint for using this molecule as a pesticide against Heterobasidion spp. Because stumps remain susceptible to infection by these pathogens for a few weeks [7].
In a previous work, the same Heterobasidion genotypes used in this study were completely inhibited when grown on media containing the CFF, without differences in sensitivity among different species and over time [12]. Our results clearly document that PLT is not the sole compound responsible for Heterobasidion inhibition, as PLT alone can explain at best 41.5% of the total inhibition caused by the CFF. Consequently, all lines of evidence suggest that antibiosis does not rely on a single constitutively produced metabolite, but rather on a plethora of secondary metabolites.
The optimization of culture conditions to enhance secondary metabolites production has gained attention for its potential to implement the success of biocontrol-based products and to maximize disease control [3,4,15]. Alterations of culture conditions may have a pronounced influence on yield enhancement and de novo induction of secondary metabolites [21,22]. In the present study, P. protegens (strain DSMZ 13134) was efficient in producing PLT, but this production was not affected by any of the tested cultural parameters (culture medium, incubation temperature and incubation period). Although it has been reported that P. protegens strains can be considered as a new bacterial group able to produce 2,4-DAPG, PLT and PRN [23], it appears that our P. protegens strain is not capable of producing high levels of 2,4-DAPG or PRN.
Since these metabolites are usually produced and released by microorganisms in small quantities [4,16,24], although their production is strongly dependent on nutrient availability [4,15,16,25], even substantial variation of culture conditions may not be able to increase their production. The modified King B (KBM) broth, containing a larger amount of glycerol, should have indeed enhanced PLT production over 2,4-DAPG production [26,27,28]. However, this was not observed in our experiment: in fact, the substrate did not affect PLT production in our bacterial strain. Pyoluteorin and 2,4-DAPG production is interrelated in P. protegens and other Pseudomonas species, despite the independent biochemical and genetic determinants for their biosynthesis [29,30,31]. Brodhagen et al. [29] have demonstrated that PLT production is induced by positive autoregulation in P. fluorescens Pf-5. In addition to its autoregulatory role, PLT repressed 2,4-DAPG production [29]. Such complex interactions may have occurred in our experiments as well.
The probability distribution curve obtained from the Pearson system can be regarded as the PLT production fingerprint of P. protegens (strain DSMZ 13134) growing in monocultures. The curve is more informative than the simple average of the PLT concentration since it allows one to quantify the probability of retrieving up to, or more than, a given amount of the compound. More importantly, the curve clearly shows that the probability of obtaining PLT from monocultures of P. protegens (strain DSMZ 13134) is almost 100%, despite the fact that the concentrations can be variable and with a high probability density centered on low values. This may have a practical significance, for instance, to compare production curves of PLT or other secondary metabolites under different conditions. In fact, from an applied perspective, optimization for the production of the preferred secondary metabolites during the fermentation process of the target biocontrol agent appears as a valuable strategy toward the enhancement of the biocontrol agent itself [4,15]. This strategy is focusing on the development of a formulation that contains secondary metabolites together with living cells of the producing biocontrol agent so that the performance in field is expected to be the result of the combined effects of secondary metabolites and the potential production of additional secondary metabolites in situ [4]. This appears as a valuable alternative to the commercialization of secondary metabolites without the biocontrol agent. In fact, strictly speaking, in the absence of living cells such secondary metabolites have to be considered chemicals in the EU [4], implying an extensive characterization for risk assessment, thus increasing substantially the costs for registration [4].
In the attempt to improve our understanding of the strategies adopted by P. protegens (strain DSMZ 13134) in response to Heterobasidion spp., we assessed the effects of the interaction between the bacterium and the four Heterobasidion species currently occurring in Europe on the production of 2,4-DAPG, PLT and PRN at different temperatures (4 °C, 25 °C and 30 °C). Such temperatures were chosen to simulate the widest range of environmental conditions at which stump treatments are performed and hence antagonist–pathogen interaction may occur. In fact, co-culture experiments simulate natural scenarios where bacteria and fungi co-inhabit and interact in a same confined environment, in this model system a stump surface, thereby exerting intense microbial competition and interspecies crosstalk [32,33,34]. The outcomes of our co-culture experiment confirmed that P. protegens (strain DSMZ 13134) is capable of producing PLT. The concentration of 2,4-DAPG was again found under 0.5 mg/L, whereas no PRN production was observed. The incubation temperature of 4 °C is conducive to the production of PLT in co-culture, indicating that the antagonist–pathogen interaction at low temperatures might lead to an increased production of this antifungal compound. Among abiotic factors, temperature has been reported to affect antibiotic production by bacterial biocontrol agents [35,36,37,38,39]. However, there is not a general defined temperature at which the production of secondary metabolites by Pseudomonas strains is optimized because different strains have their own requirements [35,36,37,39]. Since we did not observe a similar trend in monocultures of P. protegens (strain DSMZ 13134), the increased production of PLT at low temperatures might be due to the interaction between the bacterium and the pathogens. The microbial co-culture is used as an experimental tool to increase the yield and variety of secondary metabolites; thus, microbial competition is deliberately provoked to activate silent metabolic pathways and/or to up-regulate gene expression [32,33,34]. Hence, we can only speculate that Proradix® may perform better under similar environmental conditions, i.e., at low temperatures, thanks to its higher production of the antifungal compound PLT. An increased production of PLT on the stump surface may also be expected if the product is kept at low temperatures before use. It would be interesting to verify if a remarkably increased production of PLT, or other antifungal compounds, occurs when the bacterium grows in co-culture with saprobic, non-pathogenic fungi. It should be noted that among the possible approaches to improve biocontrol, two different scenarios based on assembled consortia of microorganisms are predicted [4]. On one side, the selection of helper strains applied to support the biocontrol agent in its establishment, survival and antagonistic activity, e.g., the production of antibiotic compounds [4]. On the other side, the application of biocontrol products consisting of different biocontrol strains combining different modes of action [4].
4. Materials and Methods
4.1. Microorganisms and Culture Conditions
Pseudomonas protegens (strain DSMZ 13134) was provided by SP Sourcon Padena GmbH (Tübeningen, Germany) and stored in Luria–Bertani (LB) broth containing 30% glycerol at −80 °C. Fresh cultures were started from frozen stocks for each experiment by inoculating 100 µL into LB broth (10 g of tryptone, 5 g of yeast extract, 10 g of NaCl, 1 L of H2O [pH 7.2]) and incubating at 25 °C for 24 h while shaking.
Fungal genotypes of each species of H. annosum s.l. occurring in Europe, i.e., H. abietinum, H. annosum, H. irregulare, and H. parviporum, were selected among those tested against P. protegens (strain DSMZ 13134) in a previous study [12] (Table 4). All genotypes of H. annosum s.l. were preserved in the culture collection of the University of Turin and maintained at 4 °C in pure culture on malt extract agar (MEA) (30 g of malt extract, 3 g of enzymatic digest of soybean meal, 15 g of agar-agar, 1 L of H2O [pH 5.6]). Inoculum for the experiments was grown by transferring a MEA plug from these maintenance cultures to fresh MEA and incubating plates at 25 °C for 7 days.

Table 4.
Heterobasidion genotypes used in this study. Asterisks after the accession numbers indicate genotypes selected for co-culture experiments of P. protegens (strain DSMZ 13134) and Heterobasidion spp.
Other culture media used in this study were King B (KB) broth (20 g of proteose peptone, 1.5 g of K2HPO4, 1.5 g of MgSO4 ∙7H2O, 10 mL of glycerol, 1 L of H2O [pH 7.6]) [40] and a KBM broth (20 g of proteose peptone, 2.5 g of K2HPO4, 6 g of MgSO4· 7H2O, 15 mL of glycerol, 1 L of H2O [pH 7.6]).
4.2. Production of Monocultures of P. protegens (Strain DSMZ 13134) for the Quantification of 2,4-DAPG, PLT and PRN
A set of monocultures of P. protegens (strain DSMZ 13134) was subjected to different culture conditions to determine whether cultural parameters may affect secondary metabolite production. Monocultures were grown in the dark with constant shaking (100 rpm) in conical flasks (250 mL) containing 100 mL of LB, KB, or KBM broth. Previous studies had shown that LB broth was conducive to the production of an array of secondary metabolites able to inhibit Heterobasidion mycelial growth and conidial germination [12], leading to a reduction in both the colonized area and incidence of Heterobasidion on stumps [13]. King B broth-based media are routinely used in laboratory to enhance production of secondary metabolites in fluorescent Pseudomonas strains [6,29,41,42].
An aliquot (1 mL) of the fresh culture of P. protegens (strain DSMZ 13134) was inoculated in each conical flask. Monocultures were incubated at 4, 25, and 30 °C and harvested after 24 h and 7 days of incubation. Incubation temperatures of 4 and 30 °C were chosen to test the influence of low and high temperature stresses on secondary metabolites production by P. protegens (strain DSMZ 13134). An incubation temperature of 25 °C and harvesting at 24 h of growth were the conditions used in the previous studies, where the CFF was obtained by culturing P. protegens (strain DSMZ 13134) in LB broth with constant shaking at 25 °C for 24 h (OD600 of 1.1) [12,13]. The 7-day harvesting period was chosen because secondary metabolism usually occurs at the late growth phase of the producing microorganisms [43]. For each culture medium (LB, KB, and KBM broth) and temperature (4, 25, and 30 °C), triplicate samples were established.
Monocultures were filtered to obtain a sample free from bacterial cells for the analysis by HPLC-MS. Cells were pelleted by centrifugation at 4000 rpm for 10 min, and the supernatant was filtered aseptically through a 0.22 μm filter membrane.
4.3. Production of Co-Cultures of P. protegens (Strain DSMZ 13134) and Heterobasidion spp. for the Quantification of 2,4-DAPG, PLT and PRN
A set of co-cultures of P. protegens (strain DSMZ 13134) and Heterobasidion spp. was subjected to different temperatures to explore how the production of 2,4-DAPG, PLT and PRN by P. protegens (strain DSMZ 13134) was modulated during the interaction between the bacterium and the fungal pathogens. To prepare co-cultures of P. protegens (strain DSMZ 13134) and Heterobasidion spp., 250 mL conical flasks were used, containing 100 mL of KB broth.
For each species of H. annosum s.l., the genotype displaying the highest growth rates in antagonism assays conducted previously [12] was selected for this experiment (Table 4). Three mycelial plugs (7 mm in diameter) from an actively growing colony of each fungal genotype were inoculated in each flask and incubated in the dark with constant shaking (100 rpm) at 25 °C for 7 days. An aliquot (1 mL) of P. protegens (strain DSMZ 13134) fresh culture was subsequently transferred into the culture broths of Heterobasidion spp. Co-cultures were then incubated in the dark at 4, 25, and 30 °C and harvested after 7 days. Incubation temperatures were chosen to simulate the range of temperatures at which stump treatments may be performed and hence the interaction between the bacterium and the fungal pathogens may occur. For each combination of culture medium (KB) and temperature (4, 25, and 30 °C), three conical flasks were established. Co-cultures were filtered to obtain a sample free from bacterial cells for analysis by HPLC-MS.
4.4. Quantification of 2,4-DAPG, PLT, PRN Produced by P. protegens (Strain DSMZ 13134)
All reagents used for the quantification of secondary metabolites were analytical or LC-MS grade and were provided by Sigma-Aldrich (Milan, Italy). Pure 2,4-DAPG and PLT were provided by D.B.A. ITALIA (Milan, Italy), while pure PRN by Merck Life Science (Milan, Italy). The CFF resulting from cultures were purified using solid phase extraction (SPE) cartridges (Strata C18-E, 500 mg, 6 mL Phenomenex, Torrance, CA, USA). The SPE were previously activated with 3 mL of acetonitrile, washed with 2 mL of acidified water at pH 2, and the samples were then eluted to a final volume of 3 mL with a water-acetonitrile mix (1/1 volume).
The HPLC-MS system was a Varian MS-310 triple quadrupole mass spectrometer equipped with an electrospray ionization (ESI) source and 212 LC pump (Agilent, Milan, Italy). Separation was performed on a Kinetex C18 column (5 μm, 50 × 2.0 mm, Phenomenex, Torrance, CA, USA). The mobile phase solvents were water (A) and acetonitrile (B), both containing 0.1% (v/v) formic acid. The mobile phase gradient was from 90% to 10% A in 10 min (0.2 mL/min flow rate), then from 10% to 90% A in 2 min and the conditions were maintained for 3 min.
4.5. Inhibition of Mycelial Growth of Heterobasidion spp. by PLT
The poisoned food technique was used to assess the in vitro inhibitory activity against Heterobasidion spp. of secondary metabolites present in the CFF of monocultures of P. protegens (strain DSMZ 13134) cultured in LB broth for 24 h at 25 °C. This CFF corresponds to the CFF of P. protegens (strain DSMZ 13134) used in previous studies [12,13]. The only secondary metabolite detected was PLT at the concentration of 3.77 mg/L (see results); therefore, the antifungal activity of PLT was assessed on MEA amended with PLT at 3.77 mg/L concentration poured into 6 cm Petri plates. Pyoluteorin (D.B.A. ITALIA, Milan, Italy) was suspended in acetonitrile (100 mg/L) and added to the medium after autoclaving for 20 min at 121 °C, when the medium had cooled to approximately 50 °C, to yield the final concentration of 3.77 mg/L. Five genotypes for each species of H. annosum s.l. (Table 4) were used as target pathogens for the examination of antifungal activity of PLT. MEA plates not amended with PLT inoculated with fungal genotypes acted as controls. A MEA plug (5 mm in diameter) from an actively growing colony of each genotype was inoculated in the center of each Petri plate. Three Petri plates were prepared for each Heterobasidion genotype. Petri plates were incubated in the dark at 25 °C allowing a comparison with the results of the inhibition assays carried out with the raw CFF of P. protegens (strain DSMZ 13134) conducted under the same incubation temperature and with the same Heterobasidion genotypes [12].
Colony radii of Heterobasidion genotypes were measured (in mm) in treated (rT) plates (i.e., amended with PLT) and control (rC) plates (not amended with PLT) along two perpendicular axes after 4 and 7 days of incubation, and the two measurements for each day were averaged. As described previously [12], the MGI of Heterobasidion spp. was determined by calculating (in %) the radial reduction observed in treated plates in relation to the corresponding control plates with the following equation:
4.6. Statistical Analyses
The average values of MGI were calculated and compared among Heterobasidion species for each time point (i.e., 4 and 7 days), and between time points for each Heterobasidion species. The above comparisons were carried out with unbiased recursive partitioning tree models [44,45] set as described in Lione et al. [46].
The same model was used to test whether the concentration of PLT (i.e., C, response variable) produced by monocultures of P. protegens (strain DSMZ 13134) are significantly associated with any of the following factors (i.e., covariates): culture medium, incubation temperature, and incubation period (i.e., 24 h and 7 days). The c statistics and its related p-value were calculated for each covariate [44]. Since none of the covariates was significantly associated with the response variable (see results), the concentration of PLT produced by monocultures of P. protegens (strain DSMZ 13134) was analyzed as such by estimating its upper tail probability distribution function. For any concentration of PLT, this function provides an estimate of the probability that monocultures of P. protegens (strain DSMZ 13134) can release PLT with a concentration x higher than a given threshold C. The equation of the function was obtained through the fit of the distribution types 0-VII included in the Pearson system of generalized frequency curves [47,48,49]. The fit was performed through maximum likelihood, and for each distribution type, the Akaike Information Criterion (AIC) was calculated [50,51]. The distribution type minimizing AIC was selected as the optimal Pearson curve [52]. The probabilities of retrieving PLT from monocultures of P. protegens (strain DSMZ 13134) with a concentration over 0 and up to 3.77 mg/L (i.e., mean concentration measured in the CFF, see results) were calculated from the optimal curve equation.
The concentration of PLT produced by P. protegens (strain DSMZ 13134) growing in co-culture with the genotype of either species of Heterobasidion on KB broth was analyzed through a further unbiased recursive partitioning tree model set as described above. The model was fitted to test if the production of PLT released by the bacterium in co-cultures was significantly influenced by the co-culture type (i.e., P. protegens (strain DSMZ 13134) and the genotype of either H. abietinum, H. annosum, H. irregulare, or H. parviporum) and the incubation temperature.
The 95% confidence intervals (95% CI) of the average values of MGI and PLT concentration were calculated with the bootstrap bias-corrected and accelerated (BCa) method [53,54] based on the setting parameters reported in Lione et al. [46]. Statistical analyses were conducted with R version 3.6.0 [55] and with the associated packages bootstrap [56], strucchange [57], partykit [45], and PearsonDS [58]. The significance threshold was set to 0.05 for all tests.
Statistical analyses were not performed on data of concentrations of 2,4-DAPG and PRN, because such compounds were either under the LOQ or under the LOD (see results).
5. Conclusions
The current study revealed the presence of the antifungal compound PLT at a concentration of 3.77 mg/L in the CFF of P. protegens (strain DSMZ 13134). This concentration of PLT has a remarkable antifungal activity in vitro against Heterobasidion spp., although all lines of evidence suggest that antibiosis does not rely on a single constitutively produced metabolite, but rather on a plethora of secondary metabolites. The instability and the loss of efficacy of PLT over time may be a constraint for using this molecule as a pesticide against Heterobasidion spp. We did not determine the optimal fermentation conditions for the production of 2,4-DAPG, PLT, or PRN by P. protegens (strain DSMZ 13134), but the ability of the bacterium to produce PLT and 2,4-DAPG was demonstrated. Finally, an increased production of PLT was observed when the bacterium was grown in co-culture with Heterobasidion spp. at 4 °C. This finding may suggest that Proradix® could perform better when stump treatments are performed at low temperatures or if the product is kept at low temperatures before use. In more general terms, the availability of further effective products for stump treatments against Heterobasidion species is relevant considering that the approved ones, including the chemical product urea and biological products based on Phlebiopsis gigantea (Fr.) Jülich, are either close to the expiration date [59] or not registered for use in several southern EU member states.
Author Contributions
Conceptualization, M.P. and P.G.; methodology, M.P. and E.P.; formal analysis, G.L.; investigation, M.P. and E.P.; resources, L.C. and P.G.; data curation, M.P. and G.L.; writing—original draft preparation, M.P.; writing—review and editing, M.P., E.P., G.L., L.C. and P.G.; visualization, G.L.; supervision, G.L., L.C. and P.G.; project administration, P.G.; funding acquisition, L.C. and P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SP Sourcon Padena GmbH.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are reported in the manuscript.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Raaijmakers, J.M.; Mazzola, M. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu. Rev. Phytopathol. 2012, 50, 403–424. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Arora, N.K. Secondary metabolites of fluorescent pseudomonads in biocontrol of phytopathogens for sustainable agriculture. Appl. Soil Ecol. 2018, 125, 35–45. [Google Scholar] [CrossRef]
- Shahid, I.; Malik, K.A.; Mehnaz, S. A decade of understanding secondary metabolism in Pseudomonas spp. for sustainable agriculture and pharmaceutical applications. J. Environ. Sustain. 2018, 1, 3–17. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [Green Version]
- Cesa-Luna, C.; Baez, A.; Aguayo-Acosta, A.; Llano-Villarreal, R.C.; Juárez-González, V.R.; Gaytán, P.; Bustillos-Cristales, M.R.; Rivera-Urbalejo, A.; Muñoz-Rojas, J.; Quintero-Hernández, V. Growth inhibition of pathogenic microorganisms by Pseudomonas protegens EMM-1 and partial characterization of inhibitory substances. PLoS ONE 2020, 15, e0240545. [Google Scholar] [CrossRef]
- Shahid, I.; Han, J.; Hardie, D.; Baig, D.N.; Malik, K.A.; Borchers, C.H.; Mehnaz, S. Profiling of antimicrobial metabolites of plant growth promoting Pseudomonas spp. isolated from different plant hosts. 3 Biotech 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Garbelotto, M.; Gonthier, P. Biology, epidemiology, and control of Heterobasidion species worldwide. Annu. Rev. Phytopathol. 2013, 51, 39–59. [Google Scholar] [CrossRef] [Green Version]
- Gonthier, P.; Nicolotti, G.; Linzer, R.; Guglielmo, F.; Garbelotto, M. Invasion of European pine stands by a North American forest pathogen and its hybridization with a native interfertile taxon. Mol. Ecol. 2007, 16, 1389–1400. [Google Scholar] [CrossRef]
- EPPO. Pest Risk Analysis for Heterobasidion Irregulare; EPPO: Paris, France, 2015; Available online: http://www.eppo.int/QUARANTINE/Pest_Risk_Analysis/PRA_intro.htm (accessed on 27 February 2022).
- Gžibovska, Z. Evaluation of Phlebiopsis gigantea and Pseudomonas spp. for Biocontrol of Heterobasidion spp. in Norway Spruce. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2016. [Google Scholar]
- Rönnberg, J.; Magazniece, Z. Potential “new” protective agents for biocontrol of Heterobasidion spp. on Norway spruce. In Proceedings of the LIFE + ELMIAS Ash and Elm, and IUFRO WP 7.02.01 Program & Book of Abstracts Root and Stem Rots Conference (LIFE-IUFRO), Uppsala and Visby, Visby, Sweden, 26 August–1 September 2018; p. 27. [Google Scholar]
- Pellicciaro, M.; Lione, G.; Giordano, L.; Gonthier, P. Biocontrol potential of Pseudomonas protegens against Heterobasidion species attacking conifers in Europe. Biol. Control 2021, 157, 104583. [Google Scholar] [CrossRef]
- Pellicciaro, M.; Lione, G.; Ongaro, S.; Gonthier, P. Comparative efficacy of state-of-the-art and new biological stump treatments in forests infested by the native and the alien invasive Heterobasidion species present in Europe. Pathogens 2021, 10, 1272. [Google Scholar] [CrossRef]
- Roberti, R.; Veronesi, A.; Flamigni, F. Evaluation of microbial products for the control of zucchini foot and root rot caused by Fusarium solani f. sp. cucurbitae race 1. Phytopathol. Mediterr. 2012, 51, 317–331. [Google Scholar] [CrossRef]
- Raymaekers, K.; Ponet, L.; Holtappels, D.; Berckmans, B.; Cammue, B.P. Screening for novel biocontrol agents applicable in plant disease management—A review. Biol. Control 2020, 144, 104240. [Google Scholar] [CrossRef]
- Pawar, S.; Chaudhari, A.; Prabha, R.; Shukla, R.; Singh, D.P. Microbial pyrrolnitrin: Natural metabolite with immense practical utility. Biomolecules 2019, 9, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, R. Pseudomonas pigments. III. Derivatives of pyoluteorin. Bull. Agric. Chem. Soc. Jpn. 1959, 23, 126–130. [Google Scholar] [CrossRef]
- Kidarsa, T.A.; Goebel, N.C.; Zabriskie, T.M.; Loper, J.E. Phloroglucinol mediates cross-talk between the pyoluteorin and 2,4-diacetylphloroglucinol biosynthetic pathways in Pseudomonas fluorescens Pf-5. Mol. Microbiol. 2011, 81, 395–414. [Google Scholar] [CrossRef]
- Quecine, M.C.; Kidarsa, T.A.; Goebel, N.C.; Shaffer, B.T.; Henkels, M.D.; Zabriskie, T.M.; Loper, J.E. An interspecies signaling system mediated by fusaric acid has parallel effects on antifungal metabolite production by Pseudomonas protegens strain Pf-5 and antibiosis of Fusarium spp. Appl. Environ. Microbiol. 2016, 82, 1372–1382. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, W.; Lu, X.; Xu, Y.; Zhang, X. The stability and degradation of a new biological pesticide, pyoluteorin. Pest Manag. Sci. 2010, 66, 248–252. [Google Scholar] [CrossRef]
- Scherlach, K.; Hertweck, C. Triggering cryptic natural product biosynthesis in microorganisms. Org. Biomol. Chem. 2009, 7, 1753–1760. [Google Scholar] [CrossRef]
- Abdelwahab, M.F.; Kurtán, T.; Mándi, A.; Müller, W.E.; Fouad, M.A.; Kamel, M.S.; Liu, Z.; Ebrahim, W.; Daletos, G.; Proksch, P. Induced secondary metabolites from the endophytic fungus Aspergillus versicolor through bacterial co-culture and OSMAC approaches. Tetrahedron Lett. 2018, 59, 2647–2652. [Google Scholar] [CrossRef] [Green Version]
- Ramette, A.; Frapolli, M.; Fischer-Le Saux, M.; Gruffaz, C.; Meyer, J.M.; Défago, G.; Sutrab, L.; Moënne-Loccoz, Y. Pseudomonas protegens sp. nov., widespread plant-protecting bacteria producing the biocontrol compounds 2, 4-diacetylphloroglucinol and pyoluteorin. Syst. Appl. Microbiol. 2011, 34, 180–188. [Google Scholar] [CrossRef]
- Yasmin, S.; Hafeez, F.Y.; Mirza, M.S.; Rasul, M.; Arshad, H.M.; Zubair, M.; Iqbal, M. Biocontrol of bacterial leaf blight of rice and profiling of secondary metabolites produced by rhizospheric Pseudomonas aeruginosa BRp3. Front. Microbiol. 2017, 8, 1895. [Google Scholar] [CrossRef] [Green Version]
- Prigigallo, M.I.; De Stradis, A.; Anand, A.; Mannerucci, F.; L’Haridon, F.; Weisskopf, L.; Bubici, G. Basidiomycetes are particularly sensitive to bacterial volatile compounds: Mechanistic insight into the case study of Pseudomonas protegens volatilome against Heterobasidion abietinum. Front. Microb. 2021, 12, 684664. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Thompson, B.; Gould, S.J.; Kraus, J.; Loper, J.E. Production of 2,4-diacetylphloroglucinol by the biocontrol agent Pseudomonas fluorescens Pf-5. Can. J. Microbiol. 1994, 40, 1064–1066. [Google Scholar] [CrossRef]
- Kraus, J.; Loper, J.E. Characterization of a genomic region required for production of the antibiotic pyoluteorin by the biological control agent Pseudomonas fluorescens Pf-5. Appl. Environ. Microbiol. 1995, 61, 849–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, B.K.; Défago, G. Environmental factors modulating antibiotic and siderophore biosynthesis by Pseudomonas fluorescens biocontrol strains. Appl. Environ. Microbiol. 1999, 65, 2429–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodhagen, M.; Henkels, M.D.; Loper, J.E. Positive autoregulation and signaling properties of pyoluteorin, an antibiotic produced by the biological control organism Pseudomonas fluorescens Pf-5. Appl. Environ. Microbiol. 2004, 70, 1758–1766. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Philmus, B.; Chang, J.H.; Loper, J.E. Novel mechanism of metabolic co-regulation coordinates the biosynthesis of secondary metabolites in Pseudomonas protegens. eLife 2017, 6, e22835. [Google Scholar] [CrossRef]
- Biessy, A.; Filion, M. Phloroglucinol Derivatives in Plant-Beneficial Pseudomonas spp.: Biosynthesis, Regulation, and Functions. Metabolites 2021, 11, 182. [Google Scholar] [CrossRef]
- Marmann, A.; Aly, A.H.; Lin, W.; Wang, B.; Proksch, P. Co-cultivation—A powerful emerging tool for enhancing the chemical diversity of microorganisms. Mar. Drugs 2014, 12, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Moussa, M.; Ebrahim, W.; Bonus, M.; Gohlke, H.; Mándi, A.; Kurtán, T.; Hartmann, R.; Kalscheuer, R.; Lin, W.; Liu, Z.; et al. Co-culture of the fungus Fusarium tricinctum with Streptomyces lividans induces production of cryptic naphthoquinone dimers. RSC Adv. 2019, 9, 1491–1500. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Tang, J.; Karuppiah, V.; Li, Y.; Xu, N.; Chen, J. Co-culture of Trichoderma atroviride SG3403 and Bacillus subtilis 22 improves the production of antifungal secondary metabolites. Biol. Control 2020, 140, 104122. [Google Scholar] [CrossRef]
- Shanahan, P.; O’Sullivan, D.J.; Simpson, P.; Glennon, J.D.; O’Gara, F. Isolation of 2, 4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl. Environ. Microbiol. 1992, 58, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raaijmakers, J.M.; Vlami, M.; de Souza, J.T. Antibiotic production by bacterial biocontrol agents. Antonie Van Leeuwenhoek 2002, 81, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Charyulu, E.M.; Gnanamani, A. Condition stabilization for Pseudomonas aeruginosa MTCC 5210 to yield high titers of extra cellular antimicrobial secondary metabolite using response surface methodology. Curr. Res. Bacteriol. 2010, 4, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Horak, I.; Engelbrecht, G.; van Rensburg, P.J.; Claassens, S. Microbial metabolomics: Essential definitions and the importance of cultivation conditions for utilizing Bacillus species as bionematicides. J. Appl. Microbiol. 2019, 127, 326–343. [Google Scholar] [CrossRef] [Green Version]
- Tomm, H.A.; Ucciferri, L.; Ross, A.C. Advances in microbial culturing conditions to activate silent biosynthetic gene clusters for novel metabolite production. J. Ind. Microbiol. 2019, 46, 1381–1400. [Google Scholar] [CrossRef]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar]
- Lee, J.Y.; Moon, S.S.; Hwang, B.K. Isolation and antifungal and antioomycete activities of aerugine produced by Pseudomonas fluorescens strain MM-B16. Appl. Environ. Microbiol. 2003, 69, 2023–2031. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, A.; Singh, B.; Joshi, S.; Johri, B.N. Production and characterization of an antifungal compound from Pseudomonas protegens strain W45. Proc. Natl. Acad. Sci. India B Biol. Sci. 2018, 88, 1081–1089. [Google Scholar] [CrossRef]
- Ruiz, B.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D.; Sánchez, B.; Rodríguez-Sanoja, R.; Sánchez, S.; et al. Production of microbial secondary metabolites: Regulation by the carbon source. Crit. Rev. Microbiol. 2010, 36, 146–167. [Google Scholar] [CrossRef]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased recursive partitioning: A conditional inference framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef] [Green Version]
- Hothorn, T.; Zeileis, A. partykit: A modular toolkit for recursive partytioning in R. J. Mach. Learn. Res. 2015, 16, 3905–3909. Available online: http://jmlr.org/papers/v16/hothorn15a.html (accessed on 31 August 2021).
- Lione, G.; Giordano, L.; Turina, M.; Gonthier, P. Hail-induced infections of the chestnut blight pathogen Cryphonectria parasitica depend on wound size and may lead to severe diebacks. Phytopathology 2020, 110, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K. Contributions to the mathematical theory of evolution.-II: Skew variation in homogeneous material. Philos. Trans. R. Soc. Lond. 1895, 186, 343–414. [Google Scholar] [CrossRef] [Green Version]
- Rietz, H.L. Mathematical Statistics; The Open Court Publishing Company: Chicago, IL, USA, 1927. [Google Scholar]
- Lachene, B. On Pearson families of distributions and its applications. Afr. J. Math. Comput. Sci. Res. 2013, 6, 108–117. [Google Scholar] [CrossRef]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Proceedings of the 2nd International Symposium on Information Theory, Tsahkadsor, Armenia, 2–8 September 1971; Petrov, C., Ed.; Akadémiai Kiadó: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Cousineau, D.; Brown, S.; Heathcote, A. Fitting distributions using maximum likelihood: Methods and packages. Behav. Res. Meth. Instrum. Comput. 2004, 36, 742–756. [Google Scholar] [CrossRef]
- Crawley, M.J. The R Book, 2nd ed.; John Wiley & Sons: Chichester, UK, 2013. [Google Scholar]
- DiCiccio, T.J.; Efron, B. Bootstrap confidence intervals. Stat. Sci. 1996, 11, 189–228. [Google Scholar] [CrossRef]
- Carsey, T.M.; Harden, J.J. Monte Carlo Simulation and Resampling Methods for Social Science; SAGE Publications Inc.: Thousand Oaks, CA, USA, 2014. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 31 August 2021).
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap, S original, from StatLib and by Rob Tibshirani. R port by Friedrich Leisch. 2019. Bootstrap: Functions for the Book “An Introduction to the Bootstrap”; R package version 2019.6; CRC Press: Boca Raton, FL, USA, 1994; Available online: https://CRAN.R-project.org/package=bootstrap (accessed on 31 August 2021).
- Zeileis, A.; Leisch, F.; Hornik, K.; Kleiber, C. strucchange: An R Package for Testing for Structural Change in Linear Regression Models. J. Stat. Softw. 2002, 7, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Becker, M.; Klößner, S. PearsonDS: Pearson Distribution System; R package Version 0.97. 2013. Available online: http://CRAN.R-project.org/package=PearsonDS (accessed on 22 January 2016).
- European Union Commission. Commission Implementing Regulation (EU) No 2021/745 of 6 May 2021 Amending Implementing Regulation (EU) No 540/2011; European Union Commission: Brussels, Belgium, 2021. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).