Rabbit Haemorrhagic Disease Virus 2 (RHDV2; GI.2) in Ireland Focusing on Wild Irish Hares (Lepus timidus hibernicus): An Overview of the First Outbreaks and Contextual Review
Abstract
1. Introduction
1.1. The Pathogen and Its Close Relatives
1.2. Disease, Clinical Signs and Pathology
1.3. Epidemiology and Transmission
1.4. Ecological Impact
1.5. Hosts in Ireland—Distribution, Abundance, and Autecology
2. RHDV2 on the Island of Ireland (2016–2019)
2.1. Domestic/Pet Animals
2.2. Wild Animals
2.3. Summary of Laboratory Findings
3. Ecological Risk to Irish Hares of the Emergence of RHDV2
3.1. How Susceptible Are Hares to RHDV2?
3.2. Is Hare-to-Hare Transmission Possible?
3.3. Does RHDV2 Exhibit Lowered Virulence in Hares, Relative to Other RHD Strains and Hosts?
3.4. Are Hares a Reservoir of Infection?
3.5. Can Hares Transmit Infection into Other Host Populations (for Example, Rabbits)?
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Gall-Reculé, G.; Lavazza, A.; Marchandeau, S.; Bertagnoli, S.; Zwingelstein, F.; Cavadini, P.; Martinelli, N.; Lombardi, G.; Guérin, J.-L.; Lemaitre, E.; et al. Emergence of a new lagovirus related to rabbit haemorrhagic disease virus. Vet. Res. 2013, 44, 81. [Google Scholar] [CrossRef] [PubMed]
- Neimanis, A.S.; Ahola, H.; Pettersson, U.L.; Lopes, A.M.; Abrantes, J.; Zohari, S.; Esteves, P.J.; Gavier-Widén, D. Overcoming species barriers: An outbreak of Lagovirus europaeus GI. 2/RHDV2 in an isolated population of Mountain Hares (Lepus timidus). BMC Vet. Res. 2018, 14, 367. [Google Scholar] [CrossRef] [PubMed]
- Rouco, C.; Aguayo-Adán, J.A.; Santoro, S.; Abrantes, J.; Delibes-Mateos, M. Worldwide rapid spread of the novel rabbit haemorrhagic disease virus (GI. 2/RHDV2/b). Transbound. Emerg. Dis. 2019, 66, 1762–1764. [Google Scholar] [CrossRef] [PubMed]
- IUCN 2019, Red List-Lagomorpha. Available online: https://www.iucnredlist.org/search?taxonomies=100228&searchType=species (accessed on 22 December 2021).
- Ohlinger, V.F.; Haas, B.; Meyers, G.; Weiland, F.; Thiel, H.J. Identification and characterization of the virus causing rabbit hemorrhagic disease. J. Virol. 1990, 64, 3331–3336. [Google Scholar] [CrossRef]
- Le Pendu, J.; Abrantes, J.; Bertagnoli, S.; Guitton, J.-S.; Le Gall-Reculé, G.; Lopes, A.M.; Marchandeau, S.; Alda, F.; Almeida, T.; Célio, A.P.; et al. Proposal for a unified classification system and nomenclature of lagoviruses. J. Gen. Virol. 2017, 98, 1658–1666. [Google Scholar] [CrossRef]
- Lavazza, A.; Cavadini, P.; Barbieri, I.; Tizzani, P.; Pinheiro, A.; Abrantes, J.; Esteves, P.J.; Grilli, G.; Gioia, E.; Zanoni, M.; et al. Field and experimental data indicate that the eastern cottontail (Sylvilagus floridanus) is susceptible to in-fection with European brown hare syndrome (EBHS) virus and not with rabbit haemorrhagic disease (RHD) virus. Vet. Res. 2015, 46, 13. [Google Scholar] [CrossRef]
- Le Gall-Reculé, G.; Lemaitre, E.; Bertagnoli, S.; Hubert, C.; Top, S.; Decors, A.; Marchandeau, S.; Guitton, J.-S. Large-scale lagovirus disease outbreaks in European brown hares (Lepus europaeus) in France caused by RHDV2 strains spatially shared with rabbits (Oryctolagus cuniculus). Vet. Res. 2017, 48, 1–9. [Google Scholar] [CrossRef]
- Rocchi, M.; Maley, M.; Dagleish, M.; Boag, B. Rabbit haemorrhagic disease virus type 2 in hares in Scotland. Vet. Rec. 2019, 185, 23. [Google Scholar] [CrossRef]
- Neimanis, A. Lagovirus europaeus GI. 2/RHDV2 (Rabbit Haemorrhagic Disease Virus 2). Ph.D. Thesis, Swedish University of Agricultural Sciences (SLU), Uppsala, Sweden, 2013. [Google Scholar]
- Velarde, R.; Cavadini, P.; Neimanis, A.; Cabezón, O.; Chiari, M.; Gaffuri, A.; Lavin, S.; Grilli, G.; Gavier-Widén, D.; Lavazza, A.; et al. Spillover Events of Infection of Brown Hares (Lepus europaeus) with Rabbit Haemorrhagic Disease Type 2 Virus (RHDV 2) Caused Sporadic Cases of an European Brown Hare Syndrome-Like Disease in Italy and Spain. Transbound. Emerg. Dis. 2017, 64, 1750–1761. [Google Scholar] [CrossRef]
- Kennedy, A.; Britton, L.; Byrne, A.W.; Byrne, C.; Casey, M.; Flynn, O.; Lozano, J.M.; Marnell, F.; McElroy, M.; Reid, N.; et al. First detected case of rabbit Haemorrhagic disease virus 2 (RHDV2) in the Irish hare (Lepus timidus hibernicus). Ir. Vet. J. 2021, 74, 1–5. [Google Scholar] [CrossRef]
- Lankton, J.S.; Knowles, S.; Keller, S.; Shearn-Bochsler, V.I.; Ip, H.S. Pathology of Lagovirus europaeus GI. 2/RHDV2/b (Rabbit Hemorrhagic Disease Virus 2) in Native North American Lagomorphs. J. Wildl. Dis. 2021, 57, 694–700. [Google Scholar] [PubMed]
- Velarde, R.; Abrantes, J.; Lopes, A.M.; Estruch, J.; Côrte-Real, J.V.; Esteves, P.J.; García-Bocanegra, I.; Ruiz-Olmo, J.; Rouco, C. Spillover event of recombinant Lagovirus europaeus/GI. 2 into the Iberian hare (Lepus granatensis) in Spain. Transbound. Emerg. Dis. 2021, 68, 3187–3193. [Google Scholar] [CrossRef] [PubMed]
- Asin, J.; Rejmanek, D.; Clifford, D.L.; Mikolon, A.B.; Henderson, E.E.; Nyaoke, A.C.; Macías-Rioseco, M.; Streitenberger, N.; Beingesser, J.; Woods, L.W.; et al. Early circulation of rabbit hemorrhagic disease virus type 2 (RHDV2) in domestic and wild lagomorphs in southern California, USA (2020-2021). Transbound. Emerg. Dis. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Asin, J.; Nyaoke, A.C.; Moore, J.D.; Gonzalez-Astudillo, V.; Clifford, D.L.; Lantz, E.L.; Mikolon, A.B.; Dodd, K.A.; Crossley, B.; Uzal, F.A. Outbreak of rabbit hemorrhagic disease virus 2 in the southwestern United States: First detections in southern California. J. Vet. Diagn. Investig. 2021, 33, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wei, H.; Fan, Z.; Song, Y.; Chen, M.; Qiu, R.; Zhu, W.; Xu, W.; Xue, J.; Wang, F. Emergence of rabbit haemorrhagic disease virus 2 in China in 2020. Vet. Med. Sci. 2021, 7, 236–239. [Google Scholar] [CrossRef]
- Happi, A.N.; Ogunsanya, O.A.; Oguzie, J.U.; Oluniyi, P.E.; Olono, A.S.; Heeney, J.L.; Happi, C.T. Microbial meta-genomic approach uncovers the first rabbit haemorrhagic disease virus genome in Sub-Saharan Africa. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Rouco, C.; Abrantes, J.; Serronha, A.; Lopes, A.; Maio, E.; Magalhães, M.J.; Blanco, E.; Barcena, J.; Esteves, P.; Santos, N.; et al. Epidemiology of RHDV2 (Lagovirus europaeus/GI.2) in free-living wild European rabbits in Portugal. Transbound. Emerg. Dis. 2017, 65, e373–e382. [Google Scholar] [CrossRef]
- Hall, R.N.; King, T.; O’Connor, T.; Read, A.J.; Arrow, J.; Trought, K.; Duckworth, J.; Piper, M.; Strive, T. Age and infectious dose significantly affect disease progression after RHDV2 infection in naive domestic rabbits. Viruses 2021, 13, 1184. [Google Scholar] [CrossRef]
- Xu, W.Y. Viral haemorrhagic disease of rabbits in the People’s Republic of China: Epidemiology and virus characterisation. Rev. Sci. Et Tech. Int. Off. Epizoot. 1991, 10, 393–408. [Google Scholar]
- Kerr, P.J.; Kitchen, A.; Holmes, E. Origin and Phylodynamics of Rabbit Hemorrhagic Disease Virus. J. Virol. 2009, 83, 12129–12138. [Google Scholar] [CrossRef]
- Abrantes, J.; Van Der Loo, W.; Le Pendu, J.; Esteves, P.J. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): A review. Vet. Res. 2012, 43, 12. [Google Scholar] [CrossRef] [PubMed]
- Strive, T.; Cox, T.E. Lethal biological control of rabbits–the most powerful tools for landscape-scale mitigation of rabbit impacts in Australia. Aust. Zool. 2019, 40, 118–128. [Google Scholar] [CrossRef]
- Elfekih, S.; Metcalfe, S.; Walsh, T.K.; Cox, T.E.; Strive, T. Genomic insights into a population of introduced European rabbits Oryctolagus cuniculus in Australia and the development of genetic resistance to rabbit hemorrhagic disease virus. Transbound. Emerg. Dis. 2021. [Google Scholar] [CrossRef]
- Kerr, P.J.; Hall, R.N.; Strive, T. Viruses for landscape-scale therapy: Biological control of rabbits in Australia. Methods Mol Biol. 2021, 2225, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Neimanis, A.; Pettersson, U.L.; Huang, N.; Gavier-Widén, D.; Strive, T. Elucidation of the pathology and tissue distribution of Lagovirus europaeus GI. 2/RHDV2 (rabbit haemorrhagic disease virus 2) in young and adult rabbits (Oryctolagus cuniculus). Vet. Res. 2018, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Harcourt-Brown, N.; Silkstone, M.; Whitbread, T.J.; Harcourt-Brown, F.M. RHDV2 epidemic in UK pet rabbits. Part 1: Clinical features, gross post mortem and histopathological findings. J. Small Anim. Pract. 2020, 61, 419–427. [Google Scholar] [CrossRef]
- Hall, R.N.; King, T.; O’Connor, T.W.; Read, A.J.; Vrankovic, S.; Piper, M.; Strive, T. Passive Immunisation against RHDV2 Induces Protection against Disease but Not Infection. Vaccines 2021, 9, 1197. [Google Scholar] [CrossRef]
- Duarte, M.D.; Carvalho, C.L.; dos Santos, F.A.; Monteiro, J.; Monteiro, M.; Carvalho, P.M.; Mendonça, P.; Santos, P.T.; Melo, P.C. The Health and Future of the Six Hare Species in Europe: A Closer Look at the Iberian Hare. In Lagomorpha Characteristics; IntechOpen: London, UK, 2020. [Google Scholar]
- Puggioni, G.; Cavadini, P.; Maestrale, C.; Scivoli, R.; Botti, G.; Ligios, C.; Le Gall-Reculé, G.; Lavazza, A.; Capucci, L. The new French 2010 Rabbit Hemorrhagic Disease Virus causes an RHD-like disease in the Sardinian Cape hare (Lepus capensis mediterraneus). Vet. Res. 2013, 44, 96. [Google Scholar] [CrossRef]
- Mohamed, F.; Gidlewski, T.; Berninger, M.L.; Petrowski, H.M.; Bracht, A.J.; de Rueda, C.B.; Barrette, R.W.; Grady, M.; O’Hearn, E.S.; Lewis, C.E.; et al. Comparative susceptibility of eastern cottontails and New Zealand white rabbits to classical rabbit hemorrhagic disease virus (RHDV) and RHDV2. Transbound. Emerg. Dis. 2021. [Google Scholar] [CrossRef]
- OIE. Rabbit Haemorrhagic Disease. In OIE Terrestrial Handbook; Office International des Epizooties: Paris, France, 2018; pp. 1389–1406. [Google Scholar]
- Henning, J.; Meers, J.; Davies, P.R.; Morris, R.S. Survival of rabbit haemorrhagic disease virus (RHDV) in the environ-ment. Epidemiol. Infect. 2005, 133, 719–730. [Google Scholar] [CrossRef]
- Morisse, J.P.; Le Gall, G.; Boilletot, E. Hepatitis of viral origin in Leporidae: Introduction and aetiological hypotheses. Rev. Sci. Et Tech. De L’oie 1991, 10, 269–310. [Google Scholar] [CrossRef]
- Asgari, S.; Hardy, J.R.; Sinclair, R.G.; Cooke, B.D. Field evidence for mechanical transmission of rabbit haemorrhagic disease virus (RHDV) by flies (Diptera: Calliphoridae) among wild rabbits in Australia. Virus Res. 1998, 54, 123–132. [Google Scholar] [CrossRef]
- Merchán, T.; Rocha, G.; Alda, F.; Silva, E.; Thompson, G.; de Trucios, S.H.; Pagés, A. Detection of rabbit haem-orrhagic disease virus (RHDV) in nonspecific vertebrate hosts sympatric to the European wild rabbit (Oryctolagus cuniculus). Infect. Genet. Evol. 2011, 11, 1469–1474. [Google Scholar] [CrossRef] [PubMed]
- Calvete, C.; Mendoza, M.; Sarto, M.P.; de Bagüés, M.P.J.; Luján, L.; Molín, J.; Calvo, A.J.; Monroy, F.; Calvo, J.H. Detection of Rabbit Hemorrhagic Disease Virus GI. 2/RHDV2/B in the Mediterranean Pine Vole (Microtus duodecimcostatus) and White-Toothed Shrew (Crocidura russula). J. Wildl. Dis. 2019, 55, 467–472. [Google Scholar]
- dos Santos, F.A.A.; Pinto, A.; Burgoyne, T.; Dalton, K.P.; Carvalho, C.L.; Ramilo, D.W.; Carneiro, C.; Carvalho, T.; Peleteiro, M.C.; Parra, F.; et al. Spillover events of rabbit haemorrhagic disease virus 2 (recombinant GI.4P-GI.2) from Lagomorpha to Eurasian badger. Transbound. Emerg. Dis. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Bao, S.; An, K.; Liu, C.; Xing, X.; Fu, X.; Xue, H.; Wen, F.; He, X.; Wang, J. Rabbit Hemorrhagic Disease Virus Isolated from Diseased Alpine Musk Deer (Moschus sifanicus). Viruses 2020, 12, 897. [Google Scholar] [CrossRef]
- Chong, R.; Shi, M.; Grueber, C.E.; Holmes, E.C.; Hogg, C.J.; Belov, K.; Barrs, V.R. Fecal Viral Diversity of Captive and Wild Tasmanian Devils Characterized Using Virion-Enriched Metagenomics and Metatranscriptomics. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Campbell, S.J.; Ashley, W.; Gil-Fernandez, M.; Newsome, T.M.; Di Giallonardo, F.; Ortiz-Baez, A.S.; E Mahar, J.; Towerton, A.L.; Gillings, M.; Holmes, E.C.; et al. Red fox viromes in urban and rural landscapes. Virus Evol. 2020, 6, veaa065. [Google Scholar] [CrossRef]
- E Fa, J.; Sharples, C.M.; Bell, D.J.; DeAngelis, D. An individual-based model of rabbit viral haemorrhagic disease in European wild rabbits (Oryctolagus cuniculus). Ecol. Model. 2001, 144, 121–138. [Google Scholar] [CrossRef]
- Chiari, M.; Ferrari, N.; Giardiello, D.; Avisani, D.; Zanoni, M.; Alborali, G.L.; Lanfranchi, P.; Guberti, V.; Lorenzo, C.; Antonio, L. Temporal dynamics of European brown hare syndrome infection in Northern Italian brown hares (Lepus europaeus). Eur. J. Wildl. Res. 2014, 60, 891–896. [Google Scholar] [CrossRef]
- Salvioli, M.; Pasquali, S.; Lavazza, A.; Zanoni, M.; Guberti, V.; Chiari, M.; Gilioli, G. EBHS in European brown hares (Lepus europaeus): Disease dynamics and control. Hystrix Ital. J. Mammal. 2017, 28, 202–207. [Google Scholar]
- Cooke, B. Rabbit haemorrhagic disease: Field epidemiology and the management of wild rabbit populations. Rev. Sci. Et Tech. De L’oie 2002, 21, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Schirrmeier, H.; Reimann, I.; Köllner, B.; Granzow, H. Pathogenic, antigenic and molecular properties of rabbit haemorrhagic disease virus (RHDV) isolated from vaccinated rabbits: Detection and characterization of antigenic variants. Arch. Virol. 1999, 144, 719–735. [Google Scholar] [CrossRef] [PubMed]
- Henzell, R.P.; Cunningham, R.B.; Neave, H.M. Factors affecting the survival of Australian wild rabbits exposed to rabbit haemorrhagic disease. Wildl. Res. 2002, 29, 523–542. [Google Scholar] [CrossRef][Green Version]
- Aguayo-Adán, J.A.; Rouco, C.; Delibes-Mateos, M.; Santoro, S. Lack of evidence for differences in the spread of classic (Lagovirus europaeus /GI.1) and novel ( Lagovirus europaeus /GI.2) rabbit haemorrhagic disease viruses in Europe and North Africa. Vet. Rec. 2021, 190, e1067. [Google Scholar] [CrossRef] [PubMed]
- Ambagala, A.; Ababio, P.; Lamboo, L.; Goolia, M.; Lung, O.; Berhane, Y.; Odoom, T. Outbreak of Rabbit Hemorrhagic Disease Virus 2 Infections, Ghana. Emerg. Infect. Dis. 2021, 27, 1999–2002. [Google Scholar] [CrossRef]
- Ramsey, D.S.; Cox, T.; Strive, T.; Forsyth, D.M.; Stuart, I.; Hall, R.; Elsworth, P.; Campbell, S. Emerging RHDV2 suppresses the impact of endemic and novel strains of RHDV on wild rabbit populations. J. Appl. Ecol. 2020, 57, 630–641. [Google Scholar] [CrossRef]
- Bębnowska, D.; Niedźwiedzka-Rystwej, P. Characteristics of a new variant of rabbit haemorrhagic disease virus—RHDV. Acta Biol. 2019, 15, 83–97. [Google Scholar] [CrossRef]
- Peacock, D.; Kovaliski, J.; Sinclair, R.; Mutze, G.; Iannella, A.; Capucci, L. RHDV2 overcoming RHDV immunity in wild rabbits (Oryctolagus cuniculus ) in Australia. Vet. Rec. 2017, 180, 280. [Google Scholar] [CrossRef]
- Camarda, A.; Pugliese, N.; Cavadini, P.; Circella, E.; Capucci, L.; Caroli, A.; Legretto, M.; Mallia, E.; Lavazza, A. Detec-tion of the new emerging rabbit haemorrhagic disease type 2 virus (RHDV2) in Sicily from rabbit (Oryctolagus cuniculus) and Italian hare (Lepus corsicanus). Res. Vet. Sci. 2014, 97, 642–645. [Google Scholar] [CrossRef]
- Dalton, K.P.; Nicieza, I.; Balseiro, A.; Muguerza, M.A.; Rosell, J.M.; Casais, R.; Álvarez, Á.L.; Parra, F. Variant Rabbit Hemorrhagic Disease Virus in Young Rabbits, Spain. Emerg. Infect. Dis. 2012, 18, 2009–2012. [Google Scholar] [CrossRef]
- Taggart, P.L.; Hall, R.N.; Cox, T.E.; Kovaliski, J.; McLeod, S.R.; Strive, T. Changes in virus transmission dynamics following the emergence of RHDV2 shed light on its competitive advantage over previously circulating variants. Transbound. Emerg. Dis. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Mahar, E.J.; Jenckel, M.; Huang, N.; Smertina, E.; Holmes, E.C.; Strive, T.; Hall, R.N. Frequent intergenotypic recombination between the non-structural and structural genes is a major driver of epidemiological fitness in caliciviruses. Virus Evol. 2021, 7. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Silvério, D.; Lopes, A.; Melo-Ferreira, J.; Magalhães, M.J.; Monterroso, P.; Serronha, A.; Maio, E.; Alves, P.C.; Esteves, P.J.; Abrantes, J. Insights into the evolution of the new variant rabbit haemorrhagic disease virus (GI.2) and the identification of novel recombinant strains. Transbound. Emerg. Dis. 2018, 65, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Capucci, L.; Cavadini, P.; Schiavitto, M.; Lombardi, G.; Lavazza, A. Increased pathogenicity in rabbit haemorrhagic disease virus type 2 (RHDV2). Vet. Rec. 2017, 180, 426. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.N.; Mahar, J.E.; Haboury, S.; Stevens, V.; Holmes, E.C.; Strive, T. Emerging Rabbit Hemorrhagic Disease Virus 2 (RHDVb), Australia. Emerg. Infect. Dis. 2015, 21, 2276–2278. [Google Scholar] [CrossRef] [PubMed]
- Calvete, C.; Capucci, L.; Lavazza, A.; Sarto, M.P.; Calvo, A.J.; Monroy, F.; Calvo, J.H. Changes in European wild rabbit population dynamics and the epidemiology of rabbit haemorrhagic disease in response to artificially increased viral transmission. Transbound. Emerg. Dis. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Schwensow, N.I.; Detering, H.; Pederson, S.; Mazzoni, C.; Sinclair, R.; Peacock, D.; Kovaliski, J.; Cooke, B.; Fickel, J.; Sommer, S. Resistance to RHD virus in wild Australian rabbits: Comparison of susceptible and resistant individuals using a genomewide approach. Mol. Ecol. 2017, 26, 4551–4561. [Google Scholar] [CrossRef] [PubMed]
- Delibes-Mateos, M.A.; Olivero, J.; Márquez, A.L.; Vargas, J.M. Long-Term Changes in Game Species Over a Long Period of Transformation in the Iberian Mediterranean Landscape. Environ. Manag. 2009, 43, 1256–1268. [Google Scholar] [CrossRef]
- Fedriani, J.M.; Ferreras, P.; Delibes, M. Dietary response of the Eurasian badger, Meles meles, to a decline of its main prey in the Doñana National Park. J. Zool. 1998, 245, 214–218. [Google Scholar]
- McIntosh, M.T.; Behan, S.C.; Mohamed, F.M.; Lu, Z.; Moran, K.E.; Burrage, T.G.; Neilan, J.G.; Ward, G.B.; Botti, G.; Capucci, L.; et al. A pandemic strain of calicivirus threatens rabbit industries in the Americas. Virol. J. 2007, 4, 1–13. [Google Scholar] [CrossRef]
- Guerrero-Casado, J.; Carpio, A.J.; Tortosa, F.S. Recent negative trends of wild rabbit populations in southern Spain after the arrival of the new variant of the rabbit hemorrhagic disease virus RHDV2. Mamm. Biol. 2016, 81, 361–364. [Google Scholar] [CrossRef]
- Carro, F.; Ortega, M.; Soriguer, R. Is restocking a useful tool for increasing rabbit densities? Glob. Ecol. Conserv. 2019, 17, e00560. [Google Scholar] [CrossRef]
- Cooke, B.D. Swamp wallaby (Wallabia bicolor) distribution has dramatically increased following sustained biological control of rabbits. Aust. Mammal. 2020, 42, 321. [Google Scholar] [CrossRef]
- Pedler, R.D.; Brandle, R.; Read, J.L.; Southgate, R.; Bird, P.; Moseby, K. Rabbit biocontrol and landscape-scale recovery of threatened desert mammals. Conserv. Biol. 2016, 30, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Lysaght, L.; Marnell, F. Atlas of Mammals in Ireland 2010; National Biodiversity Data Centre: Waterford, Ireland, 2016. [Google Scholar]
- McGowan, N.E.; McDermott, N.; Stone, R.; Lysaght, L.; Dingerkus, S.K.; Caravaggi, A.; Kerr, I.; Reid, N. National Hare Survey & Population Assessment 2017-Irish Wildlife Manuals No. 113; National Parks and Wildlife Service, Department of Culture, Heritage and the Gaeltacht: Dublin, Ireland, 2019. [Google Scholar]
- Reid, N.; McDonald, R.A.; Montgomery, W.I. Homogeneous habitat can fulfil the discrete and varied resource requirements of hares but may set an ecological trap. Biol. Conserv. 2010, 143, 1701–1706. [Google Scholar] [CrossRef]
- Reid, N.; Brommer, J.E.; Stenseth, N.C.; Marnell, F.; McDonald, R.A.; Montgomery, W.I. Regime shift tipping point in hare population collapse associated with climatic and agricultural change during the very early 20th century. Glob. Chang. Biol. 2021, 27, 3732–3740. [Google Scholar] [CrossRef]
- Wolfe, A.; Hayden, T.J. Home range sizes of Irish Mountain Hares on coastal grassland. Biol. Environ. Proc. Royal Ir. Acad. 1996, 96B, 141–146. [Google Scholar]
- Hamill, R.M.; Doyle, D.; Duke, E.J. Microsatellite Analysis of Mountain Hares (Lepus timidus hibernicus): Low Genetic Differentiation and Possible Sex-Bias in Dispersal. J. Mammal. 2007, 88, 784–792. [Google Scholar] [CrossRef]
- Reid, N.; Harrison, A.T. Post-release GPS tracking of hand-reared Irish hare Lepus timidus hibernicus leverets, Slemish, Co. Antrim, Northern Ireland. Conserv. Evid. 2010, 7, 32–38. [Google Scholar]
- Hewson, R. Behavior, population-changes and dispersal of Mountain Hares (Lepus timidus L.) in Scotland. J. Zool. 1990, 220, 287–309. [Google Scholar] [CrossRef]
- Rocchi, M.; Maley, M.; Dagleish, M.; Vick, C.; Ryan, D.; Lee, A.; Jahns, H. RHDV-2 on the Isle of Man and in the Republic of Ireland. Vet. Rec. 2016, 179, 389–390. [Google Scholar] [CrossRef]
- OIE, World Health Organization. Outbreak RHDV2 2016: Cases of RHDV2 in Ireland. Available online: http://www.oie.int/wahis_2/temp/reports/en_imm_0000021302_20161021_105659.pdf (accessed on 28 September 2018).
- All-Island Disease Report, DAFM & AFBI-NI. Available online: https://www.afbini.gov.uk/sites/afbini.gov.uk/files/publications/All%20Island%20Disease%20Surveillance%20Report%202016.pdf (accessed on 22 December 2021).
- Hall, R.N.; Peacock, D.E.; Kovaliski, J.; Mahar, J.E.; Mourant, R.; Piper, M.; Strive, T. Detection of RHDV2 in European brown hares (Lepus europaeus ) in Australia. Vet. Rec. 2017, 180, 121. [Google Scholar] [CrossRef]
- Calvete, C.; Mendoza, M.; Alcaraz, A.; Sarto, M.P.; Jiménez-De-Bagüéss, M.P.; Calvo, A.J.; Monroy, F.; Calvo, J.H. Rabbit haemorrhagic disease: Cross-protection and comparative pathogenicity of GI.2/RHDV2/b and GI.1b/RHDV lagoviruses in a challenge trial. Vet. Microbiol. 2018, 219, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Sillero, L.; Caballero-Gómez, J.; Gómez-Guillamón, F.; Martínez-Padilla, A.; Agüero, M.; Miguel, E.S.; Zorrilla, I.; Rayas, E.; Talavera, V.; García-Bocanegra, I. Monitoring of the novel rabbit haemorrhagic disease virus type 2 (GI.2) epidemic in European wild rabbits (Oryctolagus cuniculus) in southern Spain, 2013–2017. Vet. Microbiol. 2019, 237, 108361. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.J.; Davis, J.P.; Gardner, M.; Barlow, A.M.; Rocchi, M.; Gentil, M.; Wilson, R.J. Rabbit haemorrhagic disease virus type 2 in hares in England. Vet. Rec. 2019, 184, 127. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.W.; Paterson, R.A.; Townsend, C.R.; Poulin, R.; Tompkins, D.M. Parasite spillback: A neglected concept in invasion ecology? Ecology 2009, 90, 2047–2056. [Google Scholar] [CrossRef]
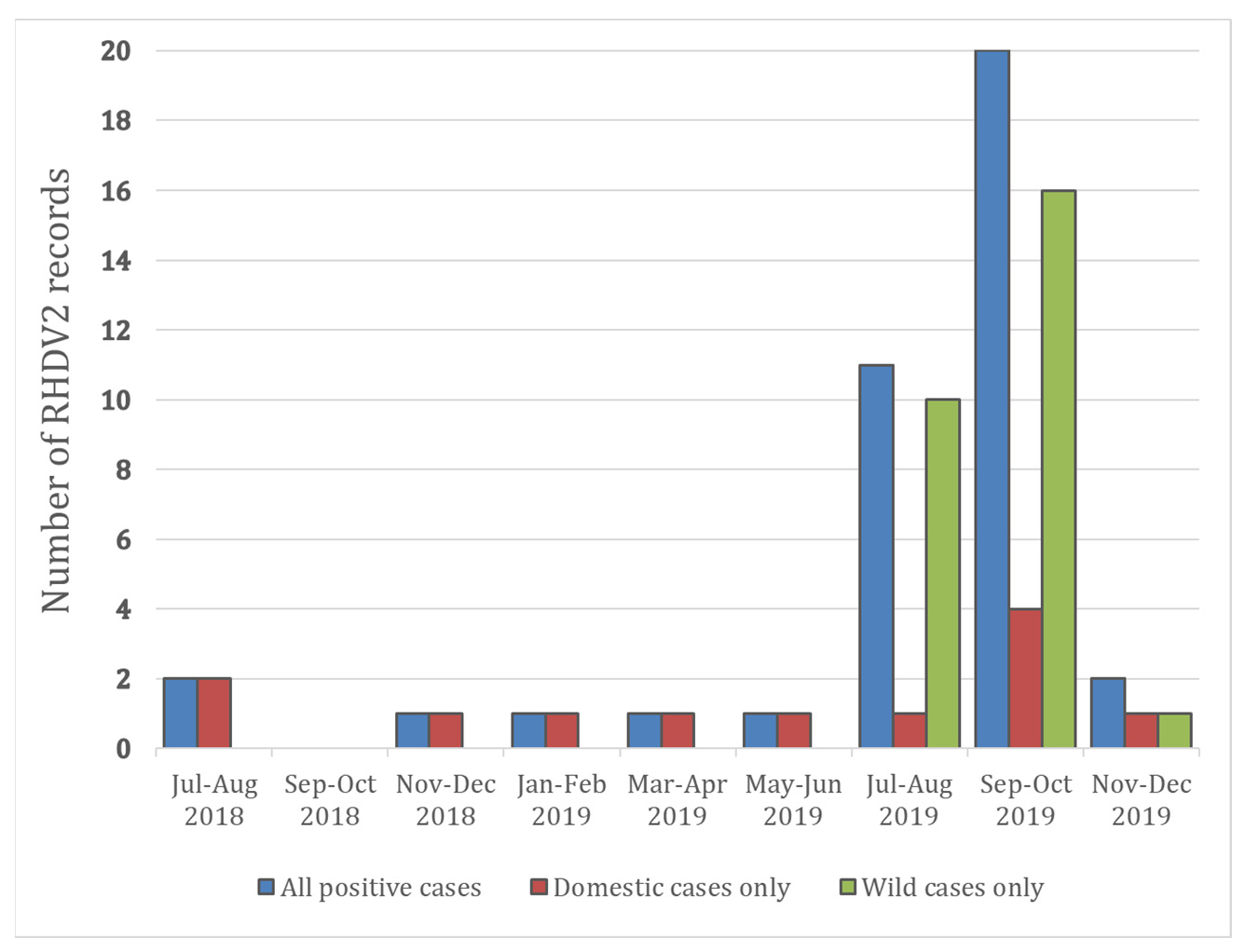
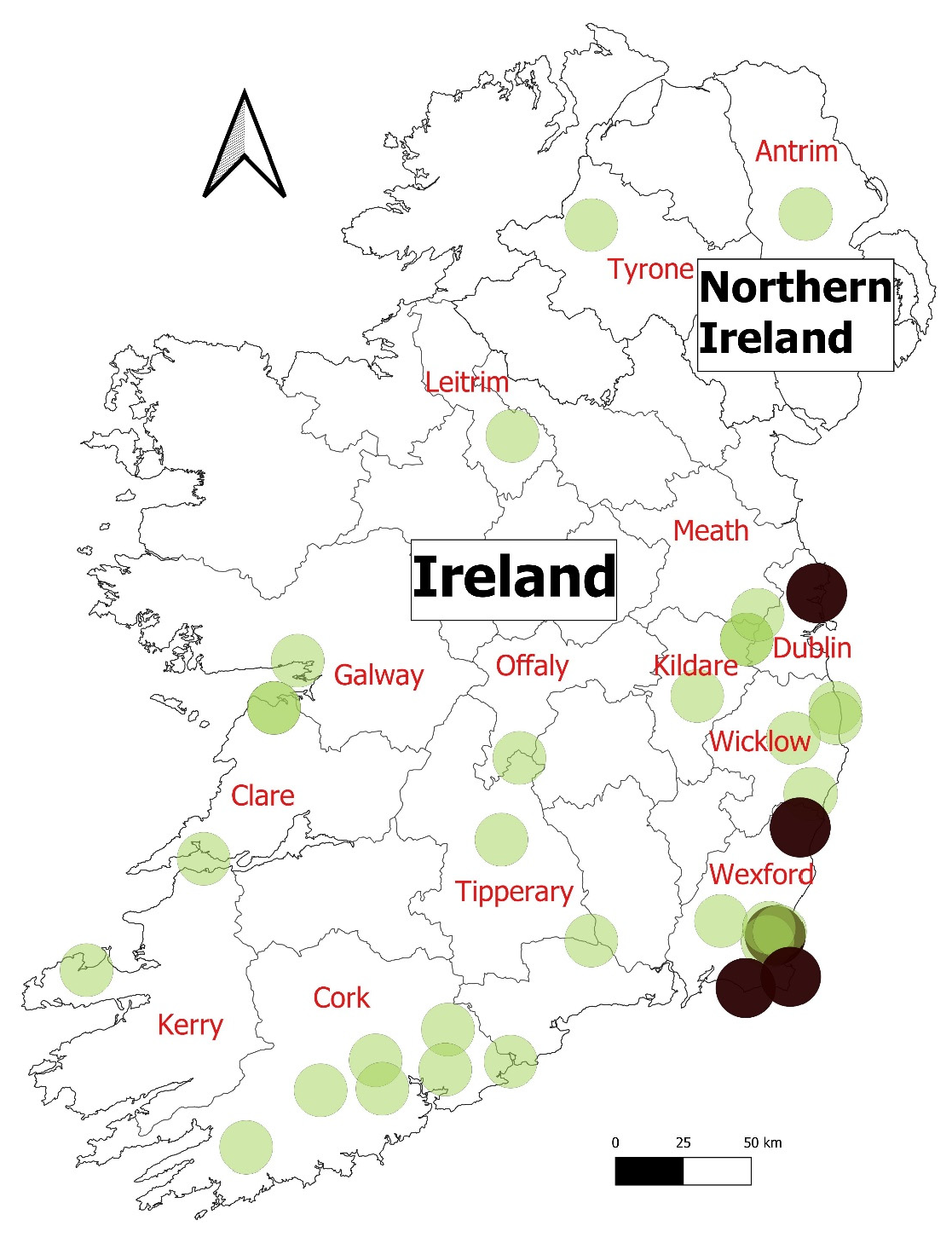
| Case | Date | Laboratory | Pet/Wild | County | Animal Type | PM Known |
|---|---|---|---|---|---|---|
| 1 | 20 August 2018 | Limerick | Pet | Tipperary | Rabbit | Carcass |
| 2 | 21 August 2018 | Cork | Pet | Cork | Rabbit | Carcass |
| 3 | 4 December 2018 | GV | Pet | Wicklow | Rabbit | Diagnostic |
| 4 | 25 January 2019 | AFBI-NI | Pet | Tyrone | Rabbit | Carcass |
| 5 | 15 April 2019 | Cork | Pet | Cork | Rabbit | Carcass |
| 6 | 20 June 2019 | Cork | Pet | Cork | Rabbit | Carcass |
| 7 | 10 July 2019 | Limerick | Wild | Clare | Rabbit | Carcass |
| 8 | 11 July 2019 | GV | Wild | Wicklow | Rabbit | Diagnostic |
| 9 | 25 July 2019 | Kilkenny | Wild | Wexford | Hare | Carcass |
| 10 | 12 August 2019 | Limerick | Wild | Clare | Rabbit | Carcass |
| 11 | 14 August 2019 | Kilkenny | Wild | Wexford | Hare | Carcass |
| 12 | 15 August 2019 | Sligo | Wild | Leitrim | Rabbit | Carcass |
| 13 | 26 August 2019 | Limerick | Wild | Clare | Rabbit | Carcass |
| 14 | 27 August 2019 | Cork | Wild | Cork | Rabbit | Carcass |
| 15 | 30 August 2019 | Athlone | Wild | Kildare | Rabbit | Carcass |
| 16 | 30 August 2019 | Athlone | Wild | Offaly | Rabbit | Carcass |
| 17 | 4 September 2019 | Kilkenny | Wild | Wexford | Rabbit | Carcass |
| 18 | 10 September 2019 | GV | Pet | Kildare | Rabbit | Diagnostic |
| 19 | 19 September 2019 | Kilkenny | Pet | Tipperary | Rabbit | Carcass |
| 20 | 26 September 2019 | Dublin | Wild | Wicklow | Rabbit | Carcass |
| 21 | 27 September 2019 | Kilkenny | Wild | Wexford | Rabbit | Carcass |
| 22 | 27 September 2019 | Kilkenny | Wild | Wexford | Rabbit | Carcass |
| 23 | 30 September 2019 | Dublin | Wild | Kildare | Rabbit | Carcass |
| 24 | 1 October 2019 | Dublin | Wild | Dublin | Hare | Carcass |
| 25 | 1 October 2019 | Kilkenny | Wild | Wexford | Hare | Carcass |
| 26 | 7 October 2019 | Kilkenny | Wild | Wicklow | Rabbit | Carcass |
| 27 | 8 October 2019 | Dublin | Wild | Meath | Rabbit | Carcass |
| 28 | 9 October 2019 | Limerick | Wild | Tipperary | Rabbit | Carcass |
| 29 | 16 October 2019 | Cork | Wild | Cork | Rabbit | Carcass |
| 30 | 17 October 2019 | Kilkenny | Wild | Wexford | Rabbit | Carcass |
| 31 | 17 October 2019 | Athlone | Pet | Galway | Rabbit | Carcass |
| 32 | 18 October 2019 | Dublin | Wild | Wexford | Hare | Carcass |
| 33 | 21 October 2019 | GV | Pet | Unknown | Rabbit | Diagnostic |
| 34 | 22 October 2019 | Cork | Wild | Kerry | Rabbit | Carcass |
| 35 | 23 October 2019 | Kilkenny | Wild | Wexford | Rabbit | Carcass |
| 36 | 29 October 2019 | Kilkenny | Wild | Wexford | Hare | Carcass |
| 37 | 1 November 2019 | Cork | Wild | Cork | Rabbit | Carcass |
| 38 | 29 November 2019 | AFBI-NI | Pet | Antrim | Rabbit | Carcass |
| 39 | 16 December 2019 | Cork | Pet | Cork | Rabbit | Carcass |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byrne, A.W.; Marnell, F.; Barrett, D.; Reid, N.; Hanna, R.E.B.; McElroy, M.C.; Casey, M. Rabbit Haemorrhagic Disease Virus 2 (RHDV2; GI.2) in Ireland Focusing on Wild Irish Hares (Lepus timidus hibernicus): An Overview of the First Outbreaks and Contextual Review. Pathogens 2022, 11, 288. https://doi.org/10.3390/pathogens11030288
Byrne AW, Marnell F, Barrett D, Reid N, Hanna REB, McElroy MC, Casey M. Rabbit Haemorrhagic Disease Virus 2 (RHDV2; GI.2) in Ireland Focusing on Wild Irish Hares (Lepus timidus hibernicus): An Overview of the First Outbreaks and Contextual Review. Pathogens. 2022; 11(3):288. https://doi.org/10.3390/pathogens11030288
Chicago/Turabian StyleByrne, Andrew W., Ferdia Marnell, Damien Barrett, Neil Reid, Robert E. B. Hanna, Máire C. McElroy, and Mícheál Casey. 2022. "Rabbit Haemorrhagic Disease Virus 2 (RHDV2; GI.2) in Ireland Focusing on Wild Irish Hares (Lepus timidus hibernicus): An Overview of the First Outbreaks and Contextual Review" Pathogens 11, no. 3: 288. https://doi.org/10.3390/pathogens11030288
APA StyleByrne, A. W., Marnell, F., Barrett, D., Reid, N., Hanna, R. E. B., McElroy, M. C., & Casey, M. (2022). Rabbit Haemorrhagic Disease Virus 2 (RHDV2; GI.2) in Ireland Focusing on Wild Irish Hares (Lepus timidus hibernicus): An Overview of the First Outbreaks and Contextual Review. Pathogens, 11(3), 288. https://doi.org/10.3390/pathogens11030288







