Patients with Obesity and a History of Metformin Treatment Have Lower Influenza Mortality: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Data
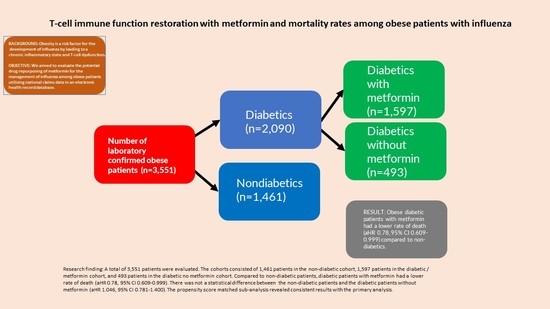
2.2. Cohort Selection
2.3. Study Outcome
2.4. Medication Exposure
2.5. Baseline Data
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global influenza strategy 2019–2030. 2019. Available online: https://apps.who.int/iris/handle/10665/311184. (accessed on 26 January 2022).
- CDC. Pandemic Basics. 2017. Available online: https://www.cdc.gov/flu/pandemic-resources/basics/faq.html (accessed on 26 January 2022).
- Morgan, O.W.; Bramley, A.; Fowlkes, A.; Freedman, D.S.; Taylor, T.H.; Gargiullo, P.; Belay, B.; Jain, S.; Cox, C.; Kamimoto, L.; et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS ONE 2010, 5, e9694. [Google Scholar] [CrossRef] [PubMed]
- Louie, J.K.; Acosta, M.; Samuel, M.C.; Schechter, R.; Vugia, D.J.; Harriman, K.; Matyas, B.T. A novel risk factor for a novel virus: Obesity and 2009 pandemic influenza a (H1N1). Clin. Infect. Dis. 2011, 52, 301–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neidich, S.D.; Green, W.D.; Rebeles, J.; Karlsson, E.A.; Schultz-Cherry, S.; Noah, T.L.; Chakladar, S.; Hudgens, M.G.; Weir, S.S.; Beck, M.A. Increased risk of influenza among vaccinated adults who are obese. Int. J. Obes. 2017, 41, 1324–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheridan, P.A.; Paich, H.A.; Handy, J.; Karlsson, E.A.; Hudgens, M.G.; Sammon, A.B.; Holland, L.A.; Weir, S.; Noah, T.L.; Beck, M.A. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012, 36, 1072–1077. [Google Scholar] [CrossRef] [Green Version]
- Green, W.D.; Beck, M.A. Obesity impairs the adaptive immune response to influenza virus. Ann. Am. Thorac. Soc. 2017, 14, S406–S409. [Google Scholar] [CrossRef]
- Paich, H.A.; Sheridan, P.A.; Handy, J.; Karlsson, E.A.; Schultz-Cherry, S.; Hudgens, M.G.; Noah, T.L.; Weir, S.S.; Beck, M.A. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 Influenza a virus. Obesity 2013, 21, 2377–2386. [Google Scholar] [CrossRef]
- Alwarawrah, Y.; Nichols, A.G.; Green, W.D.; Eisner, W.; Kiernan, K.; Warren, J.; Hale, L.P.; Beck, M.A.; MacIver, N.J. Targeting T-cell oxidative metabolism to improve influenza survival in a mouse model of obesity. Int. J. Obes. 2020, 44, 2419–2429. [Google Scholar] [CrossRef]
- Rebeles, J.; Green, W.D.; Alwarawrah, Y.; Nichols, A.G.; Eisner, W.; Danzaki, K.; MacIver, N.J.; Beck, M.A. Obesity-induced changes in T-cell metabolism are associated with impaired memory T-cell response to influenza and are not reversed with weight loss. J. Infect. Dis. 2019, 219, 1652–1661. [Google Scholar] [CrossRef]
- Whitley, R.J.; Monto, A.S. Prevention and treatment of influenza in high-risk groups: Children, pregnant women, immunocompromised hosts, and nursing home residents. J. Infect. Dis. 2006, 194, S133–S138. [Google Scholar] [CrossRef] [Green Version]
- Madan, R.P.; Tan, M.; Fernandez-Sesma, A.; Moran, T.M.; Emre, S.; Campbell, A.; Herold, B.C. A prospective, comparative study of the immune response to inactivated influenza vaccine in pediatric liver transplant recipients and their healthy siblings. Clin. Infect. Dis. 2008, 46, 712–718. [Google Scholar] [CrossRef] [Green Version]
- He, X.-S.; Holmes, T.H.; Zhang, C.; Mahmood, K.; Kemble, G.W.; Lewis, D.B.; Dekker, C.L.; Greenberg, H.B.; Arvin, A.M. Cellular Immune Responses in Children and Adults Receiving Inactivated or Live Attenuated Influenza Vaccines. J. Virol. 2006, 80, 11756–11766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huttunen, R.; Syrjänen, J. Obesity and the risk and outcome of infection. Int. J. Obes. 2013, 37, 333–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milner, J.J.; Beck, M.A. The impact of obesity on the immune response to infection. Nutr. Soc. 2012, 71, 298–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honce, R.; Schultz-Cherry, S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front. Immunol. 2019, 10, 1071. [Google Scholar] [CrossRef]
- Benedetti, F.; Sorrenti, V.; Buriani, A.; Fortinguerra, S.; Scapagnini, G.; Zella, D. Resveratrol, rapamycin and metformin as modulators of antiviral pathways. Viruses 2020, 12, 1458. [Google Scholar] [CrossRef]
- Ganeshan, K.; Chawla, A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014, 32, 609–634. [Google Scholar] [CrossRef] [Green Version]
- Alwarawrah, Y.; Kiernan, K.; MacIver, N.J. Changes in nutritional status impact immune cell metabolism and function. Front. Immunol. 2018, 9, 1055. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Desai, R.J.; Handy, D.E.; Wang, R.; Schneeweiss, S.; Barabási, A.L.; Loscalzo, J. Network-based approach to prediction and population-based validation of in silico drug repurposing. Nat. Commun. 2018, 9, 2691. [Google Scholar] [CrossRef] [Green Version]
- Jordakieva, G.; Kundi, M.; Untersmayr, E.; Pali-Schöll, I.; Reichardt, B.; Jensen-Jarolim, E. Country-wide medical records infer increased allergy risk of gastric acid inhibition. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Schneeweiss, S.; Eichler, H.G.; Garcia-Altes, A.; Chinn, C.; Eggimann, A.V.; Garner, S.; Goettsch, W.; Lim, R.; Löbker, W.; Martin, D.; et al. Real World Data in Adaptive Biomedical Innovation: A Framework for Generating Evidence Fit for Decision-Making. Clin. Pharmacol. Ther. 2016, 100, 633–646. [Google Scholar] [CrossRef]
- Schneeweiss, S.; Avorn, J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J. Clin. Epidemiol. 2005, 58, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, S.; Rassen, J.A.; Glynn, R.J.; Avorn, J.; Mogun, H.; Brookhart, M.A. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 2009, 20, 512–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brilliant, M.H.; Vaziri, K.; Connor, T.B.; Schwartz, S.G.; Carroll, J.J.; McCarty, C.A.; Schrodi, S.J.; Hebbring, S.J.; Kishor, K.S.; Flynn, H.W.; et al. Mining Retrospective Data for Virtual Prospective Drug Repurposing: L-DOPA and Age-related Macular Degeneration. Am. J. Med. 2016, 129, 292–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellers, S.A.; Hagan, R.S.; Hayden, F.G.; Fischer, W.A. The hidden burden of influenza: A review of the extra-pulmonary complications of influenza infection. Influenza Other Respi. Viruses 2017, 11, 372–393. [Google Scholar] [CrossRef]
- Warren-Gash, C.; Bhaskaran, K.; Hayward, A.; Leung, G.M.; Lo, S.V.; Wong, C.M.; Ellis, J.; Pebody, R.; Smeeth, L.; Cowling, B.J. Circulating influenza virus, climatic factors, and acute myocardial infarction: A time series study in England and Wales and Hong Kong. J. Infect. Dis. 2011, 203, 1710–1718. [Google Scholar] [CrossRef]
- Warren-Gash, C.; Hayward, A.C.; Hemingway, H.; Denaxas, S.; Thomas, S.L.; Timmis, A.D.; Whitaker, H.; Smeeth, L. Influenza infection and risk of acute myocardial infarction in england and wales: A CALIBER self-controlled case series study. J. Infect. Dis. 2012, 206, 1652–1659. [Google Scholar] [CrossRef] [Green Version]
- Warren-Gash, C.; Smeeth, L.; Hayward, A.C. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: A systematic review. Lancet Infect. Dis. 2009, 9, 601–610. [Google Scholar] [CrossRef]
- Murillo, J.E.; McNeil, C.; Van Nest, K. Abstract 9819: Real World Data: Off-Label Metformin in Patients with Prediabetes is Associated with Improved Cardiovascular Outcomes. Circulation 2021, 144, A9819. [Google Scholar]
- Nojima, I.; Eikawa, S.; Tomonobu, N.; Hada, Y.; Kajitani, N.; Teshigawara, S.; Miyamoto, S.; Tone, A.; Uchida, H.A.; Nakatsuka, A.; et al. Dysfunction of CD8 + PD-1 + T cells in type 2 diabetes caused by the impairment of metabolism-immune axis. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Johnson, J.A.; Majumdar, S.R.; Simpson, S.H.; Toth, E.L. Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care 2002, 25, 2244–2248. [Google Scholar] [CrossRef] [Green Version]
- Roussel, R.; Travert, F.; Pasquet, B.; Wilson, P.W.F.; Smith, S.C.; Goto, S.; Ravaud, P.; Marre, M.; Porath, A.; Bhatt, D.L.; et al. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch. Intern. Med. 2010, 170, 1892–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bramante, C.T.; Ingraham, N.E.; Murray, T.A.; Marmor, S.; Hovertsen, S.; Gronski, J.; McNeil, C.; Feng, R.; Guzman, G.; Abdelwahab, N.; et al. Metformin and risk of mortality in patients hospitalised with COVID-19: A retrospective cohort analysis. Lancet Healthy Longev. 2021, 2, e34–e41. [Google Scholar] [CrossRef]
| Diabetes | ||||||
|---|---|---|---|---|---|---|
| Variable | No Metformin N = 493 | Metformin Exposed N = 1597 | Non-Diabetes N = 1461 | p-Value * | Standardized Difference | |
| Sex | Female | 26(5.27) | 103(6.45) | 172(11.77) | <0.001 | 0.234 |
| Male | 467(94.73) | 1494(93.55) | 1289(88.23) | 0.234 | ||
| Race | Black | 98(19.88) | 313(19.6) | 244(16.7) | 0.248 | 0.082 |
| Other/Unknown | 29(5.88) | 83(5.2) | 81(5.54) | 0.030 | ||
| White | 366(74.24) | 1201(75.2) | 1136(77.75) | 0.082 | ||
| Age (mean, standard deviation) | 69.02(11) | 66.55(10.34) | 60.63(15.34) | <0.001 | 0.629 | |
| Charlson comorbidity (mean, standard deviation) | 3.26(2.75) | 3.63(2.52) | 1.16(1.74) | <0.001 | 1.140 | |
| Hypercholesterolemia | 11(2.23) | 55(3.44) | 38(2.6) | 0.236 | 0.073 | |
| Hyperlipidemia | 303(61.46) | 985(61.68) | 622(42.57) | <0.001 | 0.390 | |
| Hypertriglyceridemia | 65(13.18) | 320(20.04) | 183(12.53) | <0.001 | 0.204 | |
| Hypertension | 387(78.5) | 1382(86.54) | 868(59.41) | <0.001 | 0.642 | |
| Smoking | 88(17.85) | 243(15.22) | 216(14.78) | 0.255 | 0.083 | |
| Ischemic Heart Disease | 156(31.64) | 516(32.31) | 257(17.59) | <0.001 | 0.345 | |
| Non-ischemic Heart Disease | 144(29.21) | 375(23.48) | 183(12.53) | <0.001 | 0.419 | |
| Metformin MPR*100 (mean, standard deviation) HbA1c (mean, standard deviation) | --(--) | 0.73(0.28) | --(--) | --(--) | --(--) | |
| 6.38(1.16) | 7.64(1.63) | --(--) | <0.001 | 0.898 | ||
| Index year (mean, standard deviation) | 2017.3(1.64) | 2017.39(1.59) | 2017.37(1.61) | 0.587 | 0.053 | |
| MPR = Medication procession ratio | ||||||
| Diabetics with No Metformin | Diabetics with Metformin | No Diabetes | p-Value | Standardized Difference | |
|---|---|---|---|---|---|
| Overall Death Number of patients (%) | 92(18.66) | 198(12.4) | 118(8.08) | <0.001 | 0.315 |
| Mortality | |
|---|---|
| Variables | HR (95% CI) |
| Diabetic status (reference = Non-Diabetic) | |
| Diabetic no metformin | 1.046 (0.781–1.400) |
| Diabetic with metformin | 0.780 (0.609–0.999) |
| Sex (reference = Female) | |
| Male | 1.223 (0.735–2.034) |
| Age | 1.048 (1.039–1.057) |
| Race (reference = White) | |
| Black | 0.761 (0.576–1.007) |
| Other/Unknown | 0.675 (0.378–1.204) |
| Charlson comorbidity | 1.264 (1.221–1.308) |
| Pure Hypercholesterolemia | 0.819 (0.459–1.461) |
| Hyperlipidemia | 0.757 (0.612–0.936) |
| Hypertriglyceridemia | 0.830 (0.629–1.094) |
| Hypertension | 1.072 (0.803–1.433) |
| Smoking | 1.365 (1.083–1.721) |
| Ischemic Heart Disease | 0.974 (0.779–1.218) |
| Non-ischemic Heart Disease | 1.397 (1.110–1.758) |
| Index year | 0.896 (0.845–0.950) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cummings, T.H.; Magagnoli, J.; Hardin, J.W.; Sutton, S.S. Patients with Obesity and a History of Metformin Treatment Have Lower Influenza Mortality: A Retrospective Cohort Study. Pathogens 2022, 11, 270. https://doi.org/10.3390/pathogens11020270
Cummings TH, Magagnoli J, Hardin JW, Sutton SS. Patients with Obesity and a History of Metformin Treatment Have Lower Influenza Mortality: A Retrospective Cohort Study. Pathogens. 2022; 11(2):270. https://doi.org/10.3390/pathogens11020270
Chicago/Turabian StyleCummings, Tammy H., Joseph Magagnoli, James W. Hardin, and S. Scott Sutton. 2022. "Patients with Obesity and a History of Metformin Treatment Have Lower Influenza Mortality: A Retrospective Cohort Study" Pathogens 11, no. 2: 270. https://doi.org/10.3390/pathogens11020270
APA StyleCummings, T. H., Magagnoli, J., Hardin, J. W., & Sutton, S. S. (2022). Patients with Obesity and a History of Metformin Treatment Have Lower Influenza Mortality: A Retrospective Cohort Study. Pathogens, 11(2), 270. https://doi.org/10.3390/pathogens11020270