Zoonotic Episodes of Scabies: A Global Overview
Abstract
:1. Introduction
2. Methodology
3. Overview of Zoonotic Scabies Episodes
4. Characterization of S. scabiei Mites in Zoonotic Scabies Episodes
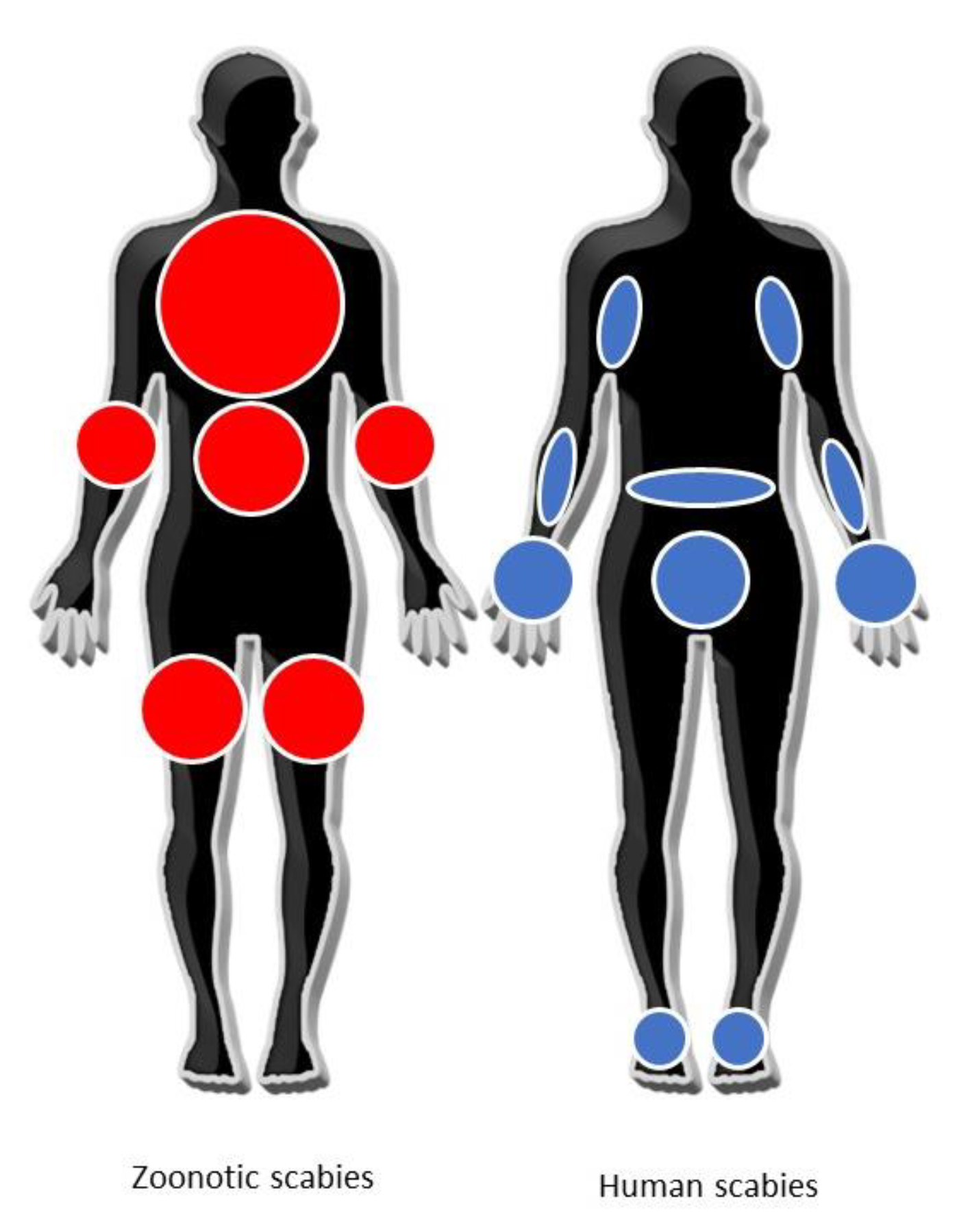
5. Diagnosis of Zoonotic Scabies
6. Treatment and Control
7. Knowledge Gaps and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fain, A. Epidemiological problems of scabies. Int. J. Dermatol. 1978, 17, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Karimkhani, C.; Colombara, D.V.; Drucker, A.M.; Norton, S.A.; Hay, R.; Engelman, D.; Steer, A.; Whitfeld, M.; Naghavi, M.; Dellavalle, R.P. The global burden of scabies: A cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2015, 17, 1247–1254. [Google Scholar] [CrossRef] [Green Version]
- Engelman, D.; Cantey, P.T.; Marks, M.; Solomon, A.W.; Chang, A.Y.; Chosidow, O.; Enbiale, W.; Engels, D.; Hay, R.J.; Hendrickx, D.; et al. The public health control of scabies: Priorities for research and action. Lancet 2019, 394, 81–92. [Google Scholar] [CrossRef]
- Rehmus, W.E.; Prendiville, J.S. Scabies and Pseudoscabies. In Harper’s Textbook of Pediatric Dermatology; Hoeger, P., Kinsler, V., Yan, A., Harper, J., Oranje, A., Bodemer, C., Larralde, M., Luk, D., Mendiratta, V., Purvis, D., Eds.; John Wiley& Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 711–722. [Google Scholar] [CrossRef]
- Burgess, I. Sarcoptes scabiei and scabies. Adv. Parasitol. 1993, 33, 235. [Google Scholar]
- Estes, S.A.; Kummel, B.; Arlian, L. Experimental canine scabies in humans. J. Am. Acad. Dermatol. 1983, 9, 397–401. [Google Scholar] [CrossRef]
- Delafond, H.M.O. Traité Pratique d’Entomologi.e., Et De Pathologi.e., Comparées de la Psore: Ou, Gale de l’Homme et des Animaux Domestiques; Imprimerie Impériale: Paris, France, 1862. [Google Scholar]
- Rabinowitz, P.M.; Gordon, Z. Outfoxing a Rash: Clinical Example of Human–Wildlife Interaction. Ecohealth 2004, 1, 404–407. [Google Scholar] [CrossRef] [Green Version]
- Gallegos, J.L.; Budnik, I.; Peña, A.; Canales, M.; Concha, M.; López, J. Sarna sarcóptica: Comunicación de un brote en un grupo familiar y su mascota. Rev. Chil. Infectol. 2014, 31, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Aydingöz, I.E.; Mansur, A.T. Canine scabies in humans: A case report and review of the literature. Dermatology 2011, 223, 104–106. [Google Scholar] [CrossRef]
- Graczyk, T.K.; Mudakikwa, A.B.; Cranfield, M.R.; Eilenberger, U. Hyperkeratotic mange caused by Sarcoptes scabiei (Acariformes: Sarcoptidae) in juvenile human-habituated mountain gorillas (Gorilla gorilla beringei). Parasitol. Res. 2001, 87, 1024–1028. [Google Scholar] [CrossRef]
- Escobar, L.E.; Carver, S.; Cross, P.C.; Rossi, L.; Almberg, E.S.; Yabsley, M.J.; Niedringhaus, K.D.; Van Wick, P.; Dominguez-Villegas, E.; Gakuya, F.; et al. Sarcoptic mange: An emerging panzootic in wildlife. Transbound. Emerg. Dis. 2021. [Google Scholar] [CrossRef]
- Valldeperes, M.; Moroni, B.; Rossi, L.; López-Olvera, J.R.; Velarde, R.; Molinar Min, A.R.; Mentaberre, G.; Serrano, E.; Angelone, S.; Lavín, S.; et al. First report of interspecific transmission of sarcoptic mange from Iberian ibex to wild boar. Parasit. Vectors 2021, 14, 481. [Google Scholar] [CrossRef] [PubMed]
- Roncalli, R.A. The history of scabies in veterinary and human medicine from biblical to modern times. Vet. Parasitol. 1987, 25, 193–198. [Google Scholar] [CrossRef]
- Burroughs, R.F.; Elston, D.M. What’s Eating You? Canine Scabies. Cutis 2003, 72, 21–23. [Google Scholar]
- Beck, A.L. Animal Scabies Affecting Man. Arch. Dermatol. 1965, 91, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, E.N.; Johnson, J.L. An Epidemic of Canine Scabies in Man. Arch. Dermatol. 1974, 110, 572–574. [Google Scholar] [CrossRef]
- Chun, B.M.; Park, J.H.; Her, Y.; Kim, C.W.; Kim, S.S. A case of human infestation of canine scabies. Korean J. Dermatol. 2009, 47, 104–107. [Google Scholar]
- EMDE, R.N. Sarcoptic Mange in the Human: A Report of an Epidemic of 10 Cases of Infection by Sarcoptes Scabiei, Variety Canis. Arch. Dermatol. 1961, 84, 633–636. [Google Scholar] [CrossRef]
- Kang, S.B.; Lee, J.Y.; Cho, B.K.; Houh, W. A case of human infestation of canine scabies. Korean J. Dermatol. 1988, 26, 570–574. [Google Scholar]
- Hewitt, M.; Walton, G.S.; Waterhouse, M. Pet animal infestations and human skin lesions. Br. J. Dermatol. 1971, 85, 215–225. [Google Scholar] [CrossRef]
- Larsson, M.H.M.A. Evidências epidemiológicas da ocorrência de escabiose, em humanos, causada pelo Sarcoptes scabiei (DeGeer, 1778) var. canis (Bourguignon, 1853). Rev. Saude Publica 1978, 12, 333–339. [Google Scholar] [CrossRef]
- Madison, J.F. Human infestation with canine scabies. J. Maine Med. Assoc. 1965, 56, 131. [Google Scholar] [PubMed]
- Morsy, T.A.; Bakr, M.E.; Ahmed, M.M.; Kotb, M.M. Human scabies acquired from a pet puppy. J. Egypt. Soc. Parasitol. 1994, 24, 305–308. [Google Scholar] [PubMed]
- Newton, D.K.; Gerrie, W. Sarcoptic scabies in a dog (with human involvement). Can. Vet. J. 1966, 7, 43. [Google Scholar] [PubMed]
- Norins, A.L. Canine Scabies in Children: “Puppy Dog” Dermatitis. Am. J. Dis. Child. 1969, 117, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Maldonado, R.; Tamayo, L.; Domiňguez, J. Norwegian scabies due to Sarcoptes scabiei var canis. Arch. Dermatol. 1977, 113, 1733. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.B.; Claypoole, T.F. Canine Scabies in Dogs and in Humans. JAMA J. Am. Med. Assoc. 1967, 199, 59–64. [Google Scholar] [CrossRef]
- Reddy, B.S.; Kumari, K.N. Canine Scabies-Its Therapeutic Management and Zoonotic importance. Intas Polivet. 2013, 14, 292–294. [Google Scholar]
- Tannenbaum, M.H. Canine scabies in man: A report of human mange. JAMA 1965, 193, 321–322. [Google Scholar] [CrossRef]
- Thomsett, L.R. Mite Infestations of Man Contracted from Dogs and Cats. Br. Med. J. 1968, 3, 93–95. [Google Scholar] [CrossRef] [Green Version]
- Warner, R.D. Occurrence and impact of zoonoses in pet dogs and cats at US Air Force bases. Am. J. Public Health 1984, 74, 1239–1243. [Google Scholar] [CrossRef] [Green Version]
- Birk, R.W.; Tebbe, B.; Schein, E.; Zouboulis, C.C.; Orfanos, C.E. Pseudoskabies durch rotfuchs ubertragen. Hautarzt 1999, 50, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Pisano, S.R.R.; Ryser-Degiorgis, M.P.; Rossi, L.; Peano, A.; Keckeis, K.; Roosje, P. Sarcoptic mange of fox origin in multiple farm animals and scabies in humans, Switzerland, 2018. Emerg. Infect. Dis. 2019, 25, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Moroni, B.; Dutto, M.; Barrasetas, M.; Molinar Min, A.; Rossi, L. Red Foxes as a Source of Zoonotic Scabies in a Rural Area of Piedmont, Italy. 2020. Available online: https://www.soipa2020.it/source/poster/moroni.pdf (accessed on 1 September 2021).
- Mörner, T. Sarcoptic mange in Swedish wildlife. Rev. Sci. Tech. Int. Epizoot. 1992, 11, 1115. [Google Scholar] [CrossRef] [Green Version]
- Mumcuoglu, Y.; Rufli, T. Human infestation by Sarcoptes scabiei var. bovis (cattle itch mite). Hautarzt 1979, 30, 423–426. [Google Scholar]
- Chakrabarti, A.; Chatterjee, A.; Chakrabarti, K.; Sengupta, D.N. Human scabies from contact with water buffaloes infested with Sarcoptes scabiei var. bubalis. Ann. Trop. Med. Parasitol. 1981, 75, 353–357. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Dutta, A.K.; Mandal, S.B.; Chakraborty, A.K.; Sengupta, D.N. Transmission of scabies from goats to human subjects. Indian J. Dermatol. 1981, 26, 7. [Google Scholar]
- Chakravorty, M.A.N.; Ghosh, S.; Banerjee, A.K. Case notes of scabies in a family transmitted from goats. Ind. Med. Gaz. 1953, 88, 153. [Google Scholar]
- Mahendra, P.; Pratibha, D. Human scabies contracted from a goat. Intas Polivet 2006, 7, 487–488. [Google Scholar]
- Mitra, M.; Mahanta, S.K.; Sen, S.; Ghosh, C.; Hati, A.K. Transmission of Sarcoptes scabiei from animal to man and its control. J. Indian Med. Assoc. 1995, 93, 142–143. [Google Scholar]
- Menzano, A.; Rambozzi, L.; Rossi, L. Outbreak of scabies in human beings, acquired from chamois (Rupicapra rupicapra). Vet. Rec. 2004, 155, 568. [Google Scholar] [CrossRef]
- Bazargani, T.T.; Hallan, J.A.; Nabian, S.; Rahbari, S. Sarcoptic mange of gazelle (Gazella subguttarosa) and its medical importance in Iran. Parasitol. Res. 2007, 101, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Sleutjens, J. Successful treatment of Sarcoptes scabiei in a 33-year-old pony with underlying pituitary pars intermedia dysfunction. Equine Vet. Educ. 2015, 27, 22–25. [Google Scholar] [CrossRef]
- Littlewood, J.D. Equine sarcoptic mange: Re-emergence of a previously notifiable disease? Equine Vet. Educ. 2011, 23, 24–26. [Google Scholar] [CrossRef]
- Skerratt, L.F.; Beveridge, I. Human scabies of wombat origin. Aust. Vet. J. 1999, 77, 607. [Google Scholar] [CrossRef]
- Twomey, D.F.; Birch, E.S.; Schock, A. Outbreak of sarcoptic mange in alpacas (Vicugna pacos) and control with repeated subcutaneous ivermectin injections. Vet. Parasitol. 2009, 159, 186–191. [Google Scholar] [CrossRef]
- Beck, W. Treatment of sarcoptic mange in llamas (Lama glama) and alpacas (Vicugna pacos) with repeated subcutaneous moxidectin injections. Vet. Parasitol. 2020, 283, 109190. [Google Scholar] [CrossRef]
- Choe, S.; Kim, S.; Na, K.J.; Nath, T.C.; Ndosi, B.A.; Kang, Y.; Bia, M.M.; Lee, D.; Park, H.; Eamudomkarn, C.; et al. First infestation case of sarcoptic mange from a pet rabbit Oryctolagus cuniculus in Republic of Korea. Korean J. Parasitol. 2020, 58, 315–319. [Google Scholar] [CrossRef]
- Li, J.; Yu, Y.; Zhang, W. Diagnosis and treatment for the cross infection of scabies mites between rabbit and human. Chin. J. Rabbit Farm 1999, 04, 38. [Google Scholar]
- Duan, H.F.; Wang, L.; Wang, J. A investigation report of an outbreak of human scabies caused by Sarcoptes scabies from rabbit factory in Harbin. Harbin Med. J. 2000, 20, 36–37. [Google Scholar]
- Goldman, L.; Feldman, M.D. Human infestation with scabies of monkeys. Arch. Derm. Syphilol. 1949, 59, 175–178. [Google Scholar] [CrossRef]
- Grahofer, A.; Bannoehr, J.; Nathues, H.; Roosje, P. Sarcoptes infestation in two miniature pigs with zoonotic transmission-A case report. BMC Vet. Res. 2018, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A. Pig Handler’s Itch. Int. J. Dermatol. 1990, 29, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.I.; Fox, M.T.; Loeffler, A.; Sinclair, G. Feline sarcoptic mange in the UK: A case report. Vet. Rec. Case Rep. 2012, 171, 351. [Google Scholar] [CrossRef]
- Iqomah, M.; Suwarno, N.; Yuliani, P. Cat Scabies at The Animal Health Clinic of Salatiga Agriculture Service on August to November 2020. J. Parasite Sci. 2020, 4, 45. [Google Scholar] [CrossRef]
- Huang, H.P.; Lien, Y.H. Feline sarcoptic mange in Taiwan: A case series of five cats. Vet. Dermatol. 2013, 24. [Google Scholar] [CrossRef]
- Malik, R.; McKellar Stewart, K.; Sousa, C.A.; Krockenberger, M.B.; Pope, S.; Ihrke, P.; Beatty, J.; Barrs, V.R.D.; Walton, S. Crusted scabies (sarcoptic mange) in four cats due to Sarcoptes scabiei infestation. J. Feline Med. Surg. 2006, 8, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Chee, J.; Kwon, J.; Cho, H.; Cho, K.; Lee, Y.; El-aty, A.M.A.B.D. A Survey of Ectoparasite Infestations in Stray Dogs of Gwang-ju City, Republic of Korea. Korean J. Parasitol. 2008, 46, 23–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rentería-Solís, Z.; Min, A.M.; Alasaad, S.; Müller, K.; Michler, F.U.; Schmäschke, R.; Wittstatt, U.; Rossi, L.; Wibbelt, G. Genetic epidemiology and pathology of raccoon-derived Sarcoptes mites from urban areas of Germany. Med. Vet. Entomol. 2014, 28, 98–103. [Google Scholar] [CrossRef]
- De Pennington, N.; Colles, K.M. Sarcoptes scabiei infestation of a donkey in the UK. Equine Vet. Educ. 2011, 23, 19–23. [Google Scholar] [CrossRef]
- Boch, J.; Schneidawind, H. Krankheiten des Jagdbaren Wildes: Mit 19 Tabellen; Parey: Hamburg, Germany, 1988. [Google Scholar]
- Haas, C.; Origgi, F.C.; Rossi, S.; López-Olvera, J.R.; Rossi, L.; Castillo-Contreras, R.; Malmsten, A.; Dalin, A.M.; Orusa, R.; Robetto, S.; et al. Serological survey in wild boar (Sus scrofa) in Switzerland and other European countries: Sarcoptes scabiei may be more widely distributed than previously thought. BMC Vet. Res. 2018, 14, 117. [Google Scholar] [CrossRef] [Green Version]
- Sannö, A.; Ander, M.; Ågren, E.; Troell, K. Sarcoptic mange in the wild boar, Sus scrofa, in Sweden. Curr. Res. Parasitol. Vector-Borne Dis. 2021, 1, 100060. [Google Scholar] [CrossRef]
- Rudd, J.L.; Clifford, D.L.; Cypher, B.L.; Hull, J.M.; Jane Riner, A.; Foley, J.E. Molecular epidemiology of a fatal sarcoptic mange epidemic in endangered San Joaquin kit foxes (Vulpes macrotis mutica). Parasites Vectors 2020, 13, 456. [Google Scholar] [CrossRef] [PubMed]
- Moroni, B.; Angelone, S.; Pérez, J.M.; Molinar Min, A.R.; Pasquetti, M.; Tizzani, P.; López-Olvera, J.R.; Valldeperes, M.; Granados, J.E.; Lavín, S.; et al. Sarcoptic mange in wild ruminants in Spain: Solving the epidemiological enigma using microsatellite markers. Parasit. Vectors 2021, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Cardells, J.; Lizana, V.; Martí-Marco, A.; Lavín, S.; Velarde, R.; Rossi, L.; Moroni, B. First description of sarcoptic mange in an Iberian hare (Lepus granatensis). Curr. Res. Parasitol. Vector-Borne Dis. 2021, 1, 100021. [Google Scholar] [CrossRef]
- Mofiz, E.; Seemann, T.; Bahlo, M.; Holt, D.; Currie, B.J.; Fischer, K.; Papenfuss, A.T. Mitochondrial Genome Sequence of the Scabies Mite Provides Insight into the Genetic Diversity of Individual Scabies Infections. PLoS Negl. Trop. Dis. 2016, 10, e0004384. [Google Scholar] [CrossRef] [PubMed]
- Mofiz, E.; Holt, D.C.; Seemann, T.; Currie, B.J.; Fischer, K.; Papenfuss, A.T. Genomic resources and draft assemblies of the human and porcine varieties of scabies mites, Sarcoptes scabiei var. hominis and var. suis. Gigascience 2016, 5, s13742-016-0129-2. [Google Scholar] [CrossRef] [Green Version]
- Walton, S.F.; Choy, J.L.; Bonson, A.; Valle, A.; McBroom, J.; Taplin, D.; Arlian, L.; Mathews, J.D.; Currie, B.; Kemp, D.J. Genetically distinct dog-derived and human-derived Sarcoptes scabiei in scabies-endemic communities in northern Australia. Am. J. Trop. Med. Hyg. 1999, 61, 542–547. [Google Scholar] [CrossRef]
- Andriantsoanirina, V.; Ariey, F.; Izri, A.; Bernigaud, C.; Fang, F.; Charrel, R.; Foulet, F.; Botterel, F.; Guillot, J.; Chosidow, O.; et al. Sarcoptes scabiei mites in humans are distributed into three genetically distinct clades. Clin. Microbiol. Infect. 2015, 21, 1107–1114. [Google Scholar] [CrossRef] [Green Version]
- Alasaad, S.; Soglia, D.; Maione, S.; Sartore, S.; Soriguer, R.C.; Pérez, J.M.; Rasero, R.; Rossi, L. Effectiveness of the postponed isolation (post-frozen isolation) method for PCR-quality Sarcoptes mite gDNA. Exp. Appl. Acarol. 2009, 47, 173–178. [Google Scholar] [CrossRef]
- Chosidow, O. Clinical practices. Scabies N. Engl. J. Med. 2006, 354, 1718–1727. [Google Scholar] [CrossRef]
- Engelman, D.; Yoshizumi, J.; Hay, R.J.; Osti, M.; Micali, G.; Norton, S.; Walton, S.; Boralevi, F.; Bernigaud, C.; Bowen, A.C.; et al. The 2020 International Alliance for the Control of Scabies Consensus Criteria for the Diagnosis of Scabies. Br. J. Dermatol. 2020, 183, 808–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandi, K.M.; Saikumar, C. Sarcoptic mange: A zoonotic ectoparasitic skin disease. J. Clin. Diagn. Res. 2013, 7, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, A.; Schanz, S.; Röcken, M.; Fierlbeck, G. A papulovesicular rash in a farmer and his wife. Clin. Infect. Dis. 2007, 45, 395–396. [Google Scholar] [CrossRef]
- Chiummo, R.; Petersen, I.; Plehn, C.; Zschiesche, E.; Roepke, R.; Thomas, E. Efficacy of orally and topically administered fluralaner (Bravecto®) for treatment of client-owned dogs with sarcoptic mange under field conditions. Parasites Vectors 2020, 13, 524. [Google Scholar] [CrossRef]
- Hampel, V.; Knaus, M.; Schäfer, J.; Beugnet, F.; Rehbein, S. Treatment of canine sarcoptic mange with afoxolaner (NexGard®) and afoxolaner plus milbemycin oxime (NexGard Spectra®) chewable tablets: Efficacy under field conditions in Portugal and Germany. Parasite 2018, 25, 63. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, V.; Tokano, K.; Nichols, D.; Martin, A.; Holme, R.; Phalen, D.; Mounsey, K.; Charleston, M.; Kreiss, A.; Pye, R.; et al. Fluralaner as a novel treatment for sarcoptic mange in the bare-nosed wombat (Vombatus ursinus): Safety, pharmacokinetics, efficacy and practicable use. Parasites Vectors 2021, 14, 18. [Google Scholar] [CrossRef]
- Deak, G.; Moroni, B.; Boncea, A.M.; Rambozzi, L.; Rossi, L.; Mihalca, A.D. Case Report: Successful Treatment of Sarcoptic Mange in European Camelids. Front. Vet. Sci. 2021, 8, 6–11. [Google Scholar] [CrossRef]
- Rowe, M.L.; Whiteley, P.L.; Carver, S. The treatment of sarcoptic mange in wildlife: A systematic review. Parasit. Vectors 2019, 12, 99. [Google Scholar] [CrossRef]
- Moroni, B.; Valldeperes, M.; Serrano, E.; López-Olvera, J.R.; Lavín, S.; Rossi, L. Comment on: “The treatment of sarcoptic mange in wildlife: A systematic review”. Parasites Vectors 2020, 13, 471. [Google Scholar] [CrossRef]
- Cafiero, M.A.; Camarda, A.; Circella, E.; Santagata, G.; Schino, G.; Lomuto, M. Pseudoscabies caused by Dermanyssus gallinae in Italian city dwellers: A new setting for an old dermatitis. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1380–1382. [Google Scholar] [CrossRef]
- Moroni, B.; Barlaam, A.; Misia, A.L.; Peano, A.; Rossi, L.; Giangaspero, A. Dermanyssus gallinae in non-avian hosts: A case report in a dog and review of the literature. Parasitol. Int. 2021, 84, 102378. [Google Scholar] [CrossRef] [PubMed]

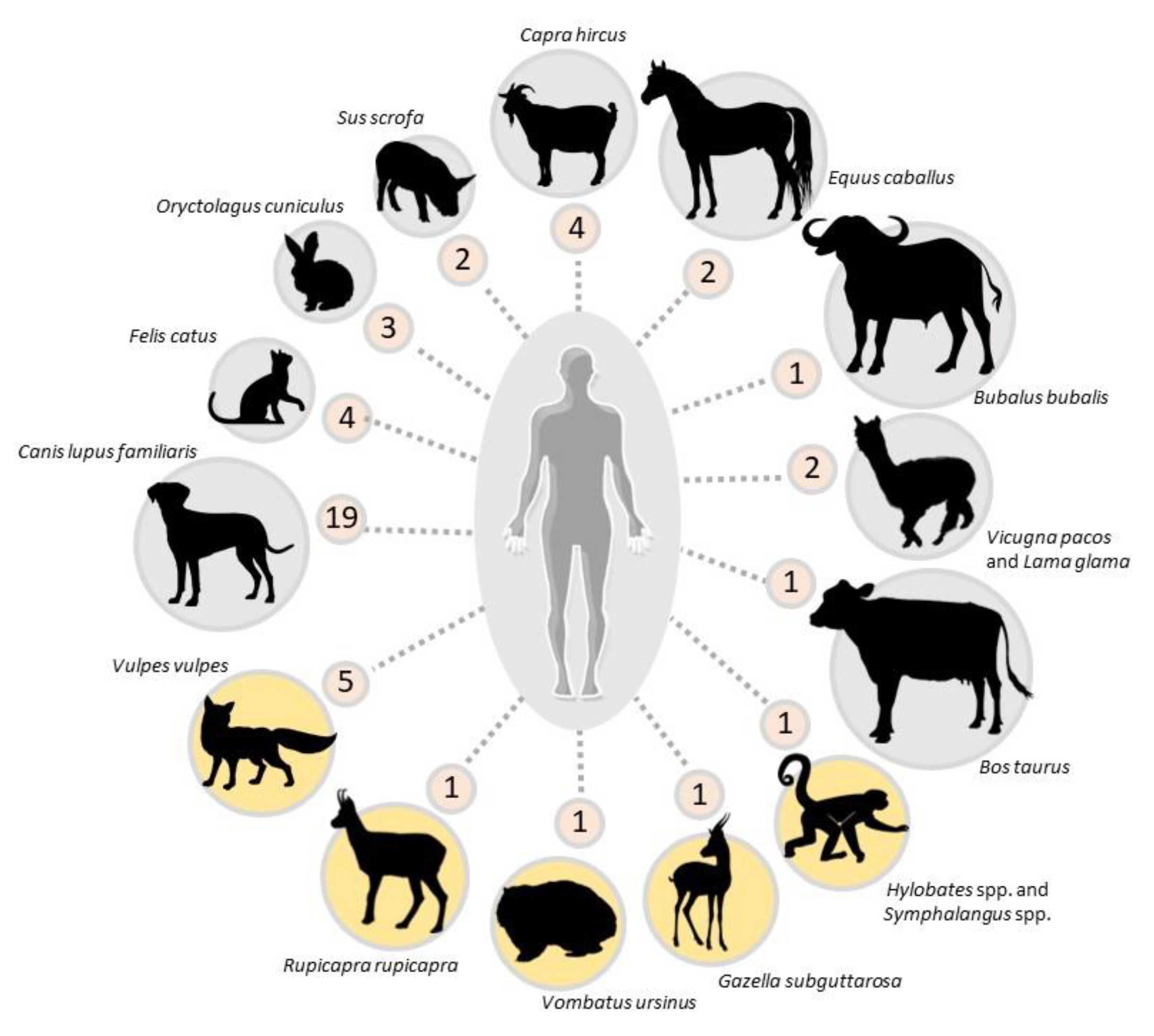
| Country | Epidemic Aspect (Number of People Infected) | Diagnosisin Humans | References | |
|---|---|---|---|---|
| Contact with dogs (Canis lupus familiaris) | ||||
| Turkey | no | Dermoscopy | [10] | |
| USA | yes (n = 9) | Clinical | [16] | |
| USA | yes (n = 15) | Skin scraping | [17] | |
| South Korea | no | Skin scraping | [18] | |
| USA | yes (n = 10) | Clinical | [19] | |
| Chile | yes (n = 7) | Skin scraping | [9] | |
| South Korea | No | Skin scraping | [20] | |
| UK | No | Clinical | [21] | |
| Brazil | yes (n = 58 *) | Skin scraping/clinical | [22] | |
| USA | - | - | [23] | |
| Egypt | - | - | [24] | |
| USA | yes (n = 4) | Skin scraping | [25] | |
| USA | - | Skin scraping | [26] | |
| Mexico | No | Skin scraping | [27] | |
| USA | yes (n = 22 *) | Clinical/ex- juvantibus | [28] | |
| India | No | - | [29] | |
| USA | yes (n = 7) | Skin scraping | [30] | |
| UK | No | Clinical | [31] | |
| USA | yes (n = 67 *) | - | [32] | |
| Contact with red foxes (Vulpes vulpes) | ||||
| Germany | No | Clinical/ex-juvantibus | [33] | |
| Switzerland | yes (n = 4) | Dermoscopy | [34] | |
| USA | No | Clinical/ex-juvantibus | [8] | |
| Italy | No | Dermoscopy | [35] | |
| Sweden | - | - | [36] | |
| Contact with Bovidae | ||||
| Cow (Bos taurus) | - | No | Skin scraping | [37] |
| Water buffalo (Bubalus bubalis) | India | yes (n = 35) | Skin scraping and clinical | [38] |
| Goat (Capra hircus) | India | yes | - | [39] |
| India | yes (n = 13) | Skin scraping | [40] | |
| India | No | Skin scraping | [41] | |
| India | yes | - | [42] | |
| Chamois (Rupicapra rupicapra) | Italy | yes (n = 7) | Clinical | [43] |
| Goitrerd gazelle (Gazella subgutturosa) | Iran | yes (n = 6) | Skin scraping | [44] |
| Contact with horses (Equus caballus) | ||||
| UK | No | Clinical | [45] | |
| UK | No | Clinical | [46] | |
| Contact with wombats (Vombatus ursinus) | ||||
| Australia | yes (n = 3) | Clinical | [47] | |
| Contact with Camelidae | ||||
| Alpaca (Vicugna pacos) | UK | No | Clinical/ex-juvantibus | [48] |
| Llama, alpaca (Lama glama, Vicugna pacos) | Germany | No | Clinical | [49] |
| Contact with rabbits (Oryctolagus cuniculus) | ||||
| South Korea | No | Ex-juvantibus | [50] | |
| China | - | - | [51] | |
| China | - | - | [52] | |
| Contact with primates | ||||
| Gibbon (Hylobates leuciscos) | UK | yes (n = 10) | Dermoscopy | [53] |
| Contact with pigs (Sus scrofa) | ||||
| Switzerland | No | Clinical | [54] | |
| India | yes (n = 30) | Skin scraping/clinical | [55] | |
| Contact with cats (Felis catus) | ||||
| UK | No | Clinical | [56] | |
| Indonesia | No | Clinical | [57] | |
| Taiwan | yes (n = 5) | Clinical | [58] | |
| Australia | No | Clinical | [59] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moroni, B.; Rossi, L.; Bernigaud, C.; Guillot, J. Zoonotic Episodes of Scabies: A Global Overview. Pathogens 2022, 11, 213. https://doi.org/10.3390/pathogens11020213
Moroni B, Rossi L, Bernigaud C, Guillot J. Zoonotic Episodes of Scabies: A Global Overview. Pathogens. 2022; 11(2):213. https://doi.org/10.3390/pathogens11020213
Chicago/Turabian StyleMoroni, Barbara, Luca Rossi, Charlotte Bernigaud, and Jacques Guillot. 2022. "Zoonotic Episodes of Scabies: A Global Overview" Pathogens 11, no. 2: 213. https://doi.org/10.3390/pathogens11020213
APA StyleMoroni, B., Rossi, L., Bernigaud, C., & Guillot, J. (2022). Zoonotic Episodes of Scabies: A Global Overview. Pathogens, 11(2), 213. https://doi.org/10.3390/pathogens11020213