Abstract
Candida albicans is a commensal fungus that asymptomatically colonizes the skin and mucosa of 60% of healthy individuals. Breaches in the cutaneous and mucosal barriers trigger candidiasis that ranges from asymptomatic candidemia and mucosal infections to fulminant sepsis with 70% mortality rates. Fungi influence at least several diseases, in part by mechanisms such as the production of pro-carcinogenic agents, molecular mimicking, and triggering of the inflammation cascade. These processes impact the interactions among human pathogenic and resident fungi, the bacteriome in various organs/tissues, and the host immune system, dictating the outcomes of invasive infections, metabolic diseases, and cancer. Although mechanistic investigations are at stages of infancy, recent studies have advanced our understanding of host–fungal interactions, their role in immune homeostasis, and their associated pathologies. This review summarizes the role of C. albicans and other opportunistic fungi, specifically their association with various diseases, providing a glimpse at the recent developments and our current knowledge in the context of inflammatory-bowel disease (IBD), cancers, and COVID-19. Two of the most common human diseases where fungal interactions have been previously well-studied are cancer and IBD. Here we also discuss the emerging role of fungi in the ongoing and evolving pandemic of COVID-19, as it is relevant to current health affairs.
1. Introduction
The past century has witnessed a significant increase in the wealth of knowledge on infectious diseases caused by viruses, bacteria, fungi, protozoa, and prions. Among the public health issues caused by microbes, fungal diseases are relatively neglected, owing to the low mortality rate of 1.5% globally [1]. Fungal diseases, also known as mycoses, although acknowledged in the late 1980s, have now captured greater attention than ever before because of the escalating population of high-risk elderly individuals, immunosuppressed individuals, transplant recipients, and premature neonates [2,3,4,5,6,7]. The average life expectancy has increased tremendously with our current scientific knowledge and advancements in medical treatments increasing the population size of individuals over 65 years of age. The United States census reports 39.6 million people aged 65 and over, which is expected to double by 2050, contributing to 21% of the population [8,9]. This population and other immunosuppressed individuals show a significant increase in the prevalence of fungal infections because of environmental factors as well as intrinsic degenerative immune and metabolic changes [10,11,12].
Among fungi, the Candida species have co-existed as the most common and innocuous commensals associated with human beings for quite a long time. They are frequently encountered on human skin, the mucosal surfaces of gastrointestinal and genitourinary tracts. Nonetheless, in immunologically weak, premature neonates, elderly people, and immunocompromised individuals they become opportunistic pathogens. Pathogenic adaptations by Candida manifest as local mucosal infections or systemic infections, occasionally resulting in infections spreading to major organs [13,14]. It ranks fourth as a causal factor among healthcare-associated infections and second among healthcare-related bloodstream infections in the United States [15,16,17,18].
Of the several known species of Candida, fifteen species distinctively cause human diseases. A majority of infections are caused by five different pathogenic strains, namely C. albicans, C. tropicalis, C. krusei (now known as Pichia kudriavzevii), C. glabrata, C. parapsilosis, and a more recently recognized emerging major pathogen, C. auris. The SENTRY Antimicrobial Surveillance Program study that was conducted for 20 years has shown that C. albicans represents almost half of the infectious Candida cases (47%) worldwide [19]. Furthermore, the same study has revealed a minor increase in non-albicans C. glabrata (19%), C. parapsilosis (16%), C. tropicalis (10%), C. krusei (3%), and 6.5% miscellaneous Candida species [19].
The first point of contact for invading pathogens is the epithelial barrier lining the skin, and the mucosa, followed by the cells of the host’s innate immune system. Antigen-presenting cells, namely dendritic cells (DCs) and macrophages are decisively positioned at the frontline of defense against these intruders. These cells essentially express the germline-encoded pattern-recognition receptors (PRRs) through which they sense several conserved architectures of the fungal cell wall and elicit innate immune responses [20]. These receptors including toll-like receptor-2, which is expressed on a wide variety of immune cells, have been extensively studied over the last two decades for their role in anti-fungal immunity. They sense fungal cell wall components including mannans, mannoproteins, β-glucans, and chitin [20,21,22]. C-type lectin receptors (CLRs) comprising Dendritic cell-associated C-type lectin-1 (Dectin-1), the Dectin-2 cluster (Dectin-2, Mcl and Mincle), Dectin-3, mannose receptor, and DC-specific ICAM-3-grabbing non-integrin (DC-SIGN) also recognize fungal derivatives and are mainly expressed on myeloid cells. Collectins are comprised of mannose-binding lectin and surfactant proteins A and B, and are additional players involved in anti-fungal immunity [23,24,25]. Upon ligation, PRRs induce multiple signaling cascades leading to the production of pro-inflammatory cytokines and antimicrobial proteins by resident macrophages, PMNs and epithelial cells [26]. Dectin-1 senses β-glucans present on the surface of fungi, and Dectin-2 and Dectin-3 sense α-mannans of fungal hyphae [24,25]. Upon the binding of Dectin-1, fungal β-glucans lead to the phosphorylation of the cytoplasmic domain of Dectin-1 by Src kinase and the docking and activation of spleen tyrosine kinase (Syk). These events induce the assembly of a scaffold comprising of caspase-recruitment domain 9 (CARD9), adaptor-protein B-cell lymophoma-10 (Bcl-10) and mucosa-associated lymphoid-tissue-lymphoma-translocation protein 1 (MALT1) [27,28,29]. The assembly and activation of the CARD9/Bcl-10/MALT1 complex activates the canonical NF-κB pathway leading to the release of various pro-inflammatory cytokines and reactive oxygen species (ROS) production, thereby triggering phagocytosis (Figure 1) [30,31].
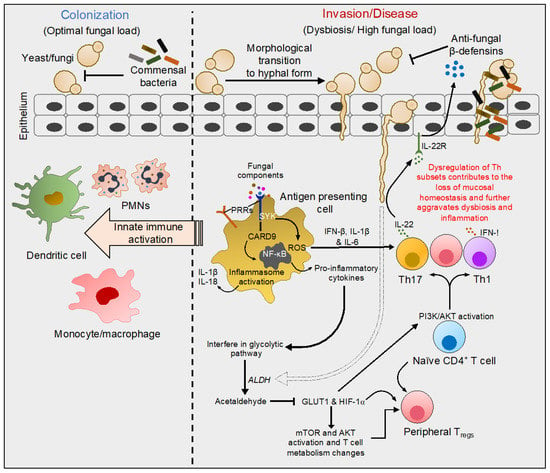
Figure 1.
Molecular events characteristic of interactions of fungi with mucosal surfaces. Fungi including C. albicans colonize healthy human skin and other mucosal surfaces in yeast form. In healthy individuals, yeast colonization does not induce epithelial damage or an intense cytokine response. During infection or dysbiosis in the context of diseases, yeast transform into hyphal form, gaining access to underlying host tissues (albeit hyphal transition does not occur in certain species including C. glabrata). Fungal components such as mannans, mannoproteins, β-glucans, and chitin are sensed by innate antigen-presenting cells through pattern-recognition receptors (PRRs) on their surface. Dectin-1 activation and the phosphorylation of the receptor at the cytoplasmic domain leads to the activation of spleen tyrosine kinase (Syk). Upon activation, a series of events leads to the assembly of a complex that activates the NF-kB pathway. NF-kB activation results in the release of pro-inflammatory cytokines and reactive oxygen species (ROS) triggering phagocytosis. These events also lead to inflammasome activation, triggering IL-1β and IL-18 production that further prime the innate immune activation. Some of the cytokines modulate PI3K/AKT and glycolytic pathways, instructing T-helper (Th)1, Th17, and regulatory T-cell (Treg) polarization and their functions. Th17 cells additionally promote the production of innate anti-microbial proteins. For example, pro-inflammatory cytokines secreted by macrophages induce a Th17 response releasing IL-22, which stimulates defensin secretion by epithelial cells. Besides the activation of the immune system, direct production of acetaldehyde resulting from Candida metabolism inhibits the expression of glucose transporter1 (GLUT1) and hypoxia-inducible factor-1α (HIF-1α), which subsequently may also control Th and Treg homeostasis. Thus, fungal dysbiosis modulates local immune milieu and alters Th functions, which significantly switches the balance between health and disease states.
Besides direct infections and morbidities, there is an accumulative concern about Candida colonization and invasion associated with several pathophysiological disorders including inflammatory-bowel disease (IBD), cancer, diabetes, metabolic diseases, and other infectious and inflammatory conditions. Notably, all of these are closely linked with underlying dysbiosis, i.e., changes in the resident microbiome. Although the initial research from 1998 focused solely on bacteriome appraising the bacterial community [32,33], some of the recent investigations have more comprehensively focused on characterization and exploration of the fungal biota. Thus, the fungal microbiome, namely the mycobiome, is an emerging integral component of the human microbiome but its precise function remains understudied [34,35,36]. Candida, being the highly represented genera in the mycobiome, is best-studied for its relationship with the host as an opportunistic pathogen as well as its non-pathogenic interactions and contributions to the host’s immune development [37]. The invasion by this yeast occurs through its morphological transition to the hyphal form thereby breaching mucosal barriers and gaining access to underlying host tissues (Figure 1) [38,39]. The mechanisms of barrier breach and the innate factors produced by the host are reviewed elsewhere [17,21,40,41,42]. This review sheds light on recent studies on how fungal components including those from Candida are mechanistically involved in human health and the diseases discussed here.
2. Association of Candida in Development and Progression of Cancer
Cancer is among the most prominent causes of death worldwide, posing an important threat to life expectancy in every country regardless of the level of economic development [43]. Based on the recent study by GLOBOCAN 2020, an estimate of 20 million new cancer cases have been reported and ~10 million deaths have occurred in 2020 alone [44]. Lately, the relationship between cancer and microbial infections has attracted enormous attention [45]. Transient immunosuppression is commonly seen in cancer patients undergoing chemotherapy, which consequently has been linked to bacterial and fungal infections. Recent studies provide insights on the association between the presence of microorganisms and the augmented risk of cancer development. The role of microbes and their involvement in diverse mechanisms including the initiation, establishment, and spread of cancer is reviewed elsewhere [46,47]. Here we will review in particular the contribution of colonization and infections by Candida species and their role in cancer.
Although Candida species are capable of fueling the initiation and propagation of cancerous processes, they are not by themselves oncogenic [48,49,50,51,52,53]. Candidiasis, although a predictor of cancer risk, may also result from cancer and their establishment could be favored by the immunosuppression resulting from cancer chemotherapy. Fungal infections modulate cancer development by impacting the host through diverse mechanisms: (1) perturbations in the DNA-damage response cause genetic mutations that accumulate inside the cell, modifying the oncogene expression involved in cell survival and proliferation; (2) oncogenic inflammation induced by DNA-damaging fungal toxins and their carcinogenic-inducing metabolites; and (3) fungal colonization or infection resulting in intense inflammation favoring the growth of primary tumors and metastases, making tumors resistant to chemotherapy drugs and suppressing the host’s anti-cancer immune responses [45].
In normal healthy individuals, Candida species are commonly found as a commensal colonizer in the skin and mucous membranes including the nose, mouth, gastrointestinal tract, reproductive organs, etc. [41,54]. Understandably, Candida burden has been shown to positively correlate with the progression of oral, esophageal, and colorectal cancer, which is consistent with their presence in these mucosa [55,56,57]. In a recent study of 100 patients inflicted with oral squamous-cell carcinoma (OSCC), 75% of the patients had Candida species [58]: 84% of those identified were C. albicans, and other non-albicans species varied between 1% and 8%. Patients with hematopoietic neoplasms, head and neck malignancies as well patients undergoing chemo/radiotherapy have also presented with oral candidiasis ranging from 7% to 52% [59,60]. Studies on animal models have also indicated that infections caused by C. albicans can contribute to carcinogenesis similar to known carcinogenic substances [45,48,55]. Several studies have shown substantial associations between candidiasis and dysplasia in the oral cavity, precancerous disorders, and OSCC [56,61,62].
A widely regarded hypothesis for the pro-carcinogenic effect of Candida species is the direct production of carcinogens and/or the metabolism of pro-carcinogens among other molecular mechanisms. Studies using Sprague–Dawley rats showed that nitrosamine production and release occurring through the hyphal invasion caused by oral microbiota dysbiosis leads to oral tumor growth and progression [63,64]. These findings are in line with the initial studies establishing that C. albicans can act as a promoter of carcinogenesis in the rat/mouse tongue following recurrent applications of nitroquinoline (4-nitroquinoline 1-oxide; 4-NQO), thereby mimicking the human neck and head cancer [65,66]. Furthermore, overexpression of Ki-67, P53, and COX-2 by the host cells following Candida infection appears to suggest the involvement of this fungus in the malignant transformation of the host cells [67]. Cell-proliferation markers (Ki-67 and P53) and their overexpression are well-described in several malignancies. Inflammatory marker COX-2, an enzyme that converts arachidonic acid to prostanoids (prostaglandins, thromboxanes and prostacyclins) are expressed in several cancers and precancerous lesions, thereby prompting cell proliferation, tumor invasion, and cell death [55].
Oral carcinogenesis begins as an epithelial dysplasia where the normal structure of epithelial cells is altered to an unusual proliferative state. Dysplasia usually results from a cell injury followed by chronic inflammation [68,69]. The characteristic feature of epithelial dysplasia is the altered proliferation of the damaged squamous cells on the epithelial surface leading to degradation of the basal membrane. These damaged cells undergo apoptosis or may transform into a malignant state, thereby resulting in local destruction and distant invasion [70].
The excessive production of pro-inflammatory cytokines during oral Candida infection such as IL-1α, IL-1β, IL-6, IL-8, TNF-α, IFN-γ, etc. suggests that alterations in metabolic pathways and dysfunction of the endothelium may negatively affect immune-related mechanisms leading to cancer development [8,71,72]. For instance, the production of acetaldehyde by Candida species, especially C. albicans, is mainly found in the oral cavity. Acetaldehyde is produced catabolically from its substrate ethanol/glucose by the action of alcohol dehydrogenases (ALDHs) and is found to be elevated in oral carcinomas compared to healthy individuals [55]. The fundamental characteristic features of acetaldehyde are genotoxicity, electrophilicity, effects on DNA repair, and induction of oxidative stress, thereby leading to the formation of protein and DNA adducts and gene mutations. The acetaldehyde-induced formation of DNA adducts interferes with DNA replication, thereby causing point mutations and chromosomal aberrations [73]. Synergistically, they also affect DNA repair and cytosine methylation, stimulating the activation of proto-oncogene and disturbances in the cell cycle, thereby resulting in tumor development. Glutathione, a tripeptide containing glutamate, cysteine, and glycine, functions as a potent anti-oxidant in the antioxidative system [74]. ROS produced during the aerobic metabolism via mitochondrial respiratory chain are well known to be elevated in cancer cells to back their rapid progression [75]. In human neuroblastoma, SH-SY5Y-cell acetaldehyde binds to the glutathione and thereby raises the intracellular ROS and calcium to arbitrate mitochondrial dysfunction [76]. Interestingly, apart from C. albicans, non-albicans such as C. tropicalis and C. parapsilosis are also able to produce significant amounts of acetaldehyde comparable to the carcinogenic levels (>100 μM), which is indicative of the carcinogenic nature of these organisms [77].
Glucose metabolism plays a crucial role in the regulation of T-cell activation and cytokine production. Conversion of excessive glucose to acetaldehyde is known to inhibit T cells by downregulating glucose-transporter-1 (Glut1) mRNA expression [78,79,80]. For example, exposure of cultured T cells to acetaldehyde (200 μM) reduces glucose uptake with a concomitant reduction in the expression of the Glut1 transcript. This process also involves the downregulation of hypoxia-inducible factor-1⍺ (Hif-1⍺) mRNA and subsequent suppression of downstream pathways including mammalian target of rapamycin (mTOR), Protein kinase B (PKB or AKT), and translation-initiation factor 4E-binding protein 1 (4E-BP1), all of which are involved in T-cell aerobic glycolysis [80]. Moreover, HIF-1⍺ is known to regulate FOXP3+-regulatory-T-cell (Treg) differentiation and accumulation [81,82]. Thus, it is clear that alterations in glucose metabolism by Candida can impact T-cell function including the suppressive activity by Tregs. In aging oral mucosa, immune dysfunction is associated with an augmented accumulation of dysfunctional Tregs during Candida infection (Figure 1) [83]. Similarly, the increased accrual of Tregs was also observed in human oral cancers as well in a mouse model of oral cancer [48]. Interestingly, the accumulation of dysfunctional Tregs observed in the oral tumor microenvironment somehow positively correlates with the Candida burden [48]. However, the role of acetaldehyde expression by Candida, metabolic changes in T cells, and their impact on Treg accumulation remains to be seen. Interestingly, Dectin-1, an intermediate in anti-fungal signaling, promotes carcinogenesis. Dectin-1-deficient mice exhibit lowered IL-1β, decreased infiltration of myeloid-derived suppressor cells (MDSC) in the mouse tongues as well as slower progression and a significantly reduced tumor burden. These outcomes significantly correlate with the lower percentage of Tregs in the oral tumor environment in these mice [48]. Nevertheless, in the context of tumors, how advanced aging influences Candida colonization and infection and alters Dectin-1 signaling remains an intriguing question to be addressed by future studies.
3. Role of Candida in IBD
Gastrointestinal diseases, predominantly IBD, have emerged as an important public health challenge by globally affecting over 1.5 million annually in North America [84,85]. The causes of IBD remain obscure, though studies on mice and clinical data demonstrate several factors including gut micro/mycobiota, environmental factors, the host’s genetic makeup, etc. that are known to promote disease susceptibility. Microbiota including bacteria, viruses, protozoa, and fungi colonize the mammalian intestine. An intact equilibrium between the resident microbial communities and homeostatic immune responses is indispensable for mammalian health. Recent discoveries have revealed the roles of the fungal community, i.e., mycobiota, in regulating gut immunity as well as the inflammatory origin of human gastrointestinal diseases in their development and progression [86,87].
Several gastrointestinal diseases are interconnected with fungal dysbiosis, which has been shown to contribute to disease sensitivity and severity [88,89]. Antibiotic exposure, genetic diversity, and diet are the underlying factors that promote fungal dysbiosis [87,90,91]. Fungal genera, mostly belonging to the Candida and Saccharomyces species have been shown to correlate with chronic idiopathic disorders such as ulcerative colitis (UC) and Crohn’s disease (CD), which together are known as IBD [87]. Candida species overgrowth in the gut due to antibacterial exposure has been known for a long time [86,87,91]. Antibiotic treatment in the context of oral fungal infection induces not only oral but also gut inflammation [88,89].
Recently, the fungal mycobiome has gained an expanded role in promoting human health and disease. Numerous studies have shed light on understanding the role of fungal dysbiosis and altered fungal immune responses in IBD [92,93,94]. In fact, early diagnosis of IBD relies on the prevalence of the anti-Saccharomyces cerevisiae antibody (ASCA) among the patients with confirmed IBD, indicating the clinical relevance of yeast in IBD. Explicitly, C. albicans and C. parapsilosis were consistently found in several IBD cohort studies. Conversely, Saccharomyces including S. cerevisiae have been found to be reduced in the feces of IBD patients with active inflammation [95,96,97].
The gut fungal composition in patients with CD, UC, and healthy subjects has been evaluated by Liguori et al., who showed that C. glabrata predominates during the disease state [97]. Additionally, non-candida fungi such as Filobasicium uniguttulatum and S. cerevisiae are found to be significantly elevated in the non-inflamed mucosa of CD. On the contrary, UC patients exhibit comparatively lowered fungal diversity [97,98,99]. This could be attributed to the inhibition of antimicrobial peptides against the bacteria in the small bowel modifying the bile-acid reabsorption, thereby benefiting the growth of fungi in patients with CD but not UC. These data also suggest that an increased fungal load of Candida species along with altered bacterial diversity could be associated with the pathogenic features of CD. In line with these findings, the 18S rRNA sequence analysis of a colonic biopsy of mucosa tissues and stool samples from patients with UC and healthy individuals revealed marked differences in the fungal communities. UC patients that were profoundly colonized with C. albicans displayed heightened mucosal injury and generation of ASCA [100,101,102]. Studies on dextran sodium sulfate (DSS)-induced colitis in C57BL/6J mice usually exhibited fungal invasion to colonic mucosa along with the expansion of Candida and Trichosporon and a decrease in non-pathogenic Saccharomyces spp., [94,102,103]. Numerous studies have shown a high abundance of colonization of Candida in ulcer and IBD patients, signifying the association of fungal dysbiosis with IBD [92,96,97,103]. A recent study by Sokol et al. involving the internal transcribed spacer2 (ITS2)-sequencing of rDNA also showed an increase in C. albicans and a decrease in S. cerevisiae abundance in IBD patients [92].
The relationship between intestinal inflammation and mycobiome dysbiosis appears to be a vicious cycle. Animal studies have also been employed to elucidate the relationship between IBD and the mycobiome. Studies involving the subcutaneous administration of cysteamine, an amino thiol to induce duodenal ulcers have revealed that rats receiving cysteamine and C. albicans inoculum progressed with perforated duodenal ulcers in contrast to the rats receiving cysteamine alone [104]. Seminal studies by Iliev et al. showed the protective role of Dectin-1 in colitis in a DSS-induced-colitis mouse model [94]. The study described the association of Dectin-1, which is encoded by the gene C-type lectin domain family 7 member A (CLEC7A), with the severity of UC. Mice with Dectin-1-deficiency (Clec7a -/-) displayed augmented severity of IBD symptoms, namely worse mucosal erosion, destruction of crypts, and inflammatory cell infiltration than wild-type mice. Clec7a -/- mice also showed an increased production of inflammatory cytokine TNF-α in the colon and an amplified production of IFN-γ and IL-17 in the intestines compared to wild-type littermate controls. The histological examination of colons from DSS-induced Clec7a -/- mice revealed the invasion of fungi in the inflamed tissues while they were localized to the lumens of the wild-type mice. This is in agreement with in vitro studies that showed intestinally conditioned Clec7a -/- DC were less effective in clearing C. tropicalis. The study also probed the role of C. tropicalis in colitis by comparing it with the non-pathogenic fungi S. fibuligeria, where both the yeasts grew in filamentous form and were sensed by Dectin-1. Clec7a -/- mice treated with C. tropicalis exhibited augmented colitis symptoms, IFN-γ, and IL-17 produced by T cells from mesenteric lymph nodes and colons compared with non-treated Clec7a -/- controls [86,94,102]. A recent study using Dectin-3-deficient (Clec4d -/-) mice showed their susceptibility to DSS-induced colitis. Clec4d -/- bone-marrow-derived macrophages were defective in NF-κB activation induced by C. tropicalis, thereby hindering their involvement in tissue repair during fungal invasion [26,105]. Taken together, fungal dysbiosis, impaired anti-fungal immunity and excessive overgrowth of C. albicans in the intestine escalate the IBD severity and hinder the gut’s wound-healing processes.
4. Candida and Other Fungal Infections in COVID-19
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly transmissible and pathogenic virus that emerged in late 2019. It has caused a global pandemic of coronavirus disease 2019 (COVID-19), which is an acute respiratory disease threatening human health and public safety [106,107]. As of 24 January 2022, ~349 million confirmed cases and ~5.59 million deaths have been reported globally [108]. SARS-CoV-2 spreads predominantly via respiratory droplets through close person-to-person contact, pre-symptomatic, asymptomatic, and symptomatic carriers. Infections vary from asymptomatic to a full-blown disease with a range of symptoms including upper-respiratory-tract infection or life-threatening sepsis with prolonged hospital stays [106,107,109]. COVID-19 patients show changes in their microbiota composition [110], due to secondary infections including those caused by bacteria and fungi [111]. Among the opportunistic infections in COVID-19 patients, a higher incidence of fungal co-infections was observed [112,113,114,115]. Most fungal-related co-infections in COVID-19 patients involved Aspergillus spp., C. albicans, C. auris, C. dubliniensis, C. glabrata, C. tropicalis, C. krusei, and C. parapsilosis sensu stricto [116,117].
While COVID vaccination has tremendously improved the morbidity and mortality of the disease, secondary fungal infections were common and had worsened the morbidity during the full-blown pandemic until 2021 [114,118]. SARS-CoV-2 triggers host immune dysregulation, which has been postulated to contribute to fungal co-infections increasing the morbidity and mortality associated with COVID-19 [119]. Candidiasis is one of the major complications of severe COVID-19, concomitant with a longer stay in the intensive care unit, a greater usage of broad-spectrum of antibiotics, and a higher mortality rate [115,120,121]. A study by Nucci et al. showed that COVID-19 patients with candidemia were more likely to be under mechanical ventilation compared to non-COVID-19 patients [122,123]. Moreover, Aspergillus spp., causes a life-threatening disease-invasive pulmonary aspergillosis (IPA) that is characteristically associated with immune-system dysregulation and affects the respiratory system. IPA chiefly arises due to the prolonged usage of glucocorticoids, immunosuppressive agents, chronic neutropenia, and in transplant recipients [124,125]. Case reports and observational studies have shown that COVID-19-associated pulmonary aspergillosis (CAPA) is of significant concern in hospitalized COVID-19 patients with complications [125,126].
Case reports show that patients treated with steroids during COVID-19 treatment often developed histoplasmosis caused by a dimorphic fungus Histoplasma capsulatum [127]. Fungus histoplasmosis is highly endemic and has been found in regions of the United States (California), Rio Grande, Southern Brazil, Buenos Aires, and Argentina [124]. This fungus is found in soil containing the excreta of birds or bats [127] and is associated with immunosuppressive conditions [127,128]. Histoplasmosis was previously shown to be associated with acquired immunodeficiency syndrome (AIDS) [129,130], another immune-dysfunction disease. It is noteworthy that this re-emerged as a co-infection in COVID-19 patients [131,132,133]. Another severe opportunistic infection in immune-compromised patients is Cryptococcus neoformans, which has a high risk of mortality. Studies have also shown that most COVID-19 patients with C. neoformans co-infection did not survive despite treatment with anti-fungal agents such as fluconazole and amphotericin B [114,134].
During or after weeks or months of recovery from COVID-19, patients who suffered lung complications have reported experiencing Mucormycosis, commonly known as COVID19-associated Mucormycosis (CAM). CAM was reported in many countries during the COVID-19 second wave including Brazil, Egypt, Austria, Iran, Italy, the United States, France, and India, constituting about 0.3% of COVID-19 infections [135,136]. Mucormycosis is characterized by the presence of hyphal infiltration of the sinus tissue and has a temporal course of roughly four weeks. The common species responsible for Mucormycosis belong to Rhizopus spp., Mucor spp., and Cunninghamella spp. Typical symptoms include complicated sinusitis, crusting nasal blockage, proptosis, facial pain, edema, ptosis, ophthalmoplegia with intracranial extension, and characteristically, a black eschar on the hard palate or in the nasal cavity [114]. For a short period during pandemic, an intensive increase in cases of CAM was observed in several patients requiring extreme surgical operations to treat the disease [114,137,138]. Other fungal co-infections were also described including those by Pneumocystis jirovecii and Coccidioides immitis [139,140,141]. Some of these co-infections, especially with P. jirovecii, are thought to be life threatening or have an adverse impact on COVID-19 [139,141].
The concurrent usage of steroids to control COVID-19 symptoms might contribute to the suppression of immunity, and consequently lead to these infections during different stages of the disease, but this possibility remains to be validated. Because steroids may promote these conditions [70,142], a sensible use of these drugs is critical while evaluating the treatment options for COVID-19 and its related co-infections. Additionally, a better understanding of the underlying COVID-19 immune dysfunction is imperative for the appropriate diagnosis and effective treatment of fungal co-infections.
5. Established Immune Mechanisms in Candida Associated Co-Morbidities
C. albicans, the most common member of the intestinal mycobiota, is known to be associated with numerous inflammatory conditions in humans [143,144]. Candida is known to poorly interact with epithelial cells. Conversely, its hyphae can enter the epithelial cells and induce numerous pro-inflammatory cytokines and host-defense peptides. The hyphal transition also reduces the colonization potential of Candida, typically via the anti-fungal immunity exerted by the augmented expression of hyphal-specific genes [86,145]. Notably, the formation of hyphae in C. albicans occurs by its adhesion to the epithelial cells, which is a strong inducer and complemented by hyphal-associated protein expression. Hwp1 (hyphal wall protein 1) and Als3 (agglutinin-like sequence 3) are the two essential proteins that stimulate epithelial adhesion [146,147,148]. These proteins are well-known to possess critical roles in adhesion, invasion, induction of damage, and immune activation/evasion.
Candidalysin, which is encoded solely by C. albicans, is a pore-forming peptide and an essential virulent factor associated with epithelial damage and host immune-cell activation [149]. This peptide stimulates the MPK1/MAPK/cFos-activation pathway, thereby leading to pro-inflammatory cytokine production such as IL-1α, IL-1β, IL-6, IL-8, GM-CSF, and G-CSF. Candidalysin by itself also induces in vitro cell damage in Caco-2 cells; however, ascertaining its effects on the gut epithelium needs additional studies [149,150,151]. Remarkably, candidalysin-mediated epithelial activation does not involve Toll-like receptors (TLRs) or CLRs, demonstrating that these cells utilize sensing mechanisms that are distinct from myeloid cells. This notion is consistent with previous studies that have shown that myeloid cells specifically sense Candida cell-wall moieties by β-glucans and mannans while epithelial-cell damage is mediated by the MPK1/MAPK/cFos pathway [147,152,153].
Innate activation through Dectin-1/CARD9 facilitating IL-1β, IL-6, and IL-23 secretion by dendritic cells is critical for disseminating adaptive antifungal Th17-cell responses [31]. A deficiency of Dectin-1 increases the risk of infectious diseases and thus reflects its pivotal role in immune defense [154]. In mice, Dectin-1 deficiency results in a significantly increased fungal burden and a low survival rate [155]. This is associated with a decrease in phagocytic cell recruitment and reduced pro-inflammatory-cytokine release at the site of infection in Dectin-1-knockout mice [155]. Oral candidiasis also showed augmented dissemination in Dectin-1-knockout mice [156]. A deficiency of CARD9, an important intermediate in the Dectin-1/Syk pathway, also results in augmented sensitivity to systemic candidiasis, further reiterating the significance of Dectin-1-mediated signaling in controlling inflammatory diseases involving fungi [28].
mTOR, a conserved Ser/Thr kinase, plays an essential role in the regulation of cell growth and metabolism. mTOR senses and combines several environmental cues, ultimately delivering them to the PI3K-AKT pathway, thereby activating two multiprotein complexes, namely complex1 (mTORC1) and complex2 (mTORC2) [157]. These complexes play divergent roles in T cells, especially in instructing the differentiation of Th cells. By modulating the autophagy/apoptosis pathway, the survival of CD4+ T cells during fungal sepsis, as well as CD8+-T-cell differentiation, the mTOR pathway regulates the aspergillosis prognosis in mice [158]. Compared with wild-type mice, T-cell-specific mTOR-knockout mice showed a higher survival rate after fungal sepsis, whereas after the T-cell-specific knockout of tuberous sclerosis complex 1(TSC1), which is a negative regulator of mTORC1, mice displayed lower survival rates after fungal sepsis [158,159]. These results show that mTOR activation in CD4+ T cells may have a detrimental role in exacerbating inflammation during fungal sepsis. Additionally, mTOR activation impairs CD8+-T-cell immunity via the augmentation of Eomesodermin in the context of invasive candidiasis [160]. Moreover, mTOR complexes possess dual and opposing roles in Tregs, either promoting or inhibiting inflammation depending on the context [161,162,163]. mTOR activation downstream of IL-1β and the myeloid-differentiation-primary-response-88 (MyD88) signaling are critical for Treg homeostasis in oral mucosa. Consequently, the mTOR/IL-1β/MyD88-signaling axis promotes the induction and expansion of an exclusive population of FOXP3+ cells expressing IL-17A, known as Treg17 [83]. However, the dysregulation of any node in this axis appears to diminish these cells and cause increased accumulation of dysfunctional Tregs. Additionally, it appears that aging and tumorigenesis may disrupt this signaling and potentially cause Candida dysbiosis and infection. Thus, mTOR appears to regulate immune dysfunction, dysbiosis, and Candida-associated oral inflammation [83]. The role of this signaling in gut dysbiosis or in relevance to other diseases remains unclear and warrants further investigation. Taken together, emerging roles of fungi in altering various mechanistic components including mTOR highlight their critical functions in skewing the regulation of T-cell metabolism/differentiation and mucosal homeostasis.
6. Concluding Remarks
Fungal dysbiosis and altered immune responses to fungi are linked to several human pathologies. Most fungal infections represent polymicrobial diseases in susceptible hosts and are more prevalent in immune-compromised and elderly individuals. Candida infections have steadily increased because of a higher incidence of systemic diseases including tumors, IBD, AIDS, disorders of the kidney and liver, the extensive development of interventional therapy, transplantations, and the over-exploitation of various antibiotics. Lymphocytopenia is commonly observed in immunocompromised/elderly population/COVID-19 patients, making them prone to persistent fungal infections or non-resolving inflammation associated with such infections. Over the last decade, a tremendous advancement has been made in understanding anti-fungal immunity. However, further understanding of the precise mechanistic interplay between fungal dysbiosis and mucosal immune system and its impact on the host in the context of diseases needs additional detailed investigations. Those studies will lead to potential new targets for reversing immune dysfunction in the context of fungal dysbiosis.
Author Contributions
P.P. drafted the framework for the review. S.S.M. wrote the preliminary drafts. S.S.M. and P.P. wrote and edited the final manuscript, and S.J. contributed to editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by NIH, NIDCR R01 DE026923.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi. 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, E.; Smith, P.B.; Jacqz-Aigrain, E.; Kaguelidou, F.; Cohen-Wolkowiez, M.; Manzoni, P.; Benjamin, D.K. Neonatal fungal infections: When to treat? Early Hum. Dev. 2012, 88 (Suppl. S2), S6–S10. [Google Scholar] [CrossRef]
- Eisi, H.; Ibraheem, S.; Hisham, T.; Al-Harbi, A.; Saidy, K.; Ali, I.; Nour, I.; Nasef, N. Risk factors and outcomes of deep tissue Candida invasion in neonates with invasive candidiasis. Mycoses 2021, 65, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef]
- Benedict, K.; Richardson, M.; Vallabhaneni, S.; Jackson, B.R.; Chiller, T. Emerging issues, challenges, and changing epidemiology of fungal disease outbreaks. Lancet Infect. Dis. 2017, 17, e403–e411. [Google Scholar] [CrossRef]
- Gavaldà, J.; Meije, Y.; Fortún, J.; Roilides, E.; Saliba, F.; Lortholary, O.; Muñoz, P.; Grossi, P.; Cuenca-Estrella, M.; ESCMID Study Group for Infections in Compromised Hosts. Invasive fungal infections in solid organ transplant recipients. Clin. Microbiol. Infect. 2014, 20 (Suppl. 7), 27–48. [Google Scholar] [CrossRef] [PubMed]
- Tönshoff, B. Immunosuppressants in Organ Transplantation. Handb. Exp. Pharmacol. 2020, 261, 441–469. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.; Collins, S.A.B.; Resneck, J.S.; Bolognia, J.L.; Hodge, J.A.; Rohrer, T.A.; Van Beek, M.J.; Margolis, D.J.; Sober, A.J.; Weinstock, M.A.; et al. The burden of skin disease in the United States. J. Am. Acad. Dermatol. 2017, 76, 958–972.e2. [Google Scholar] [CrossRef]
- Ortman, J.M.; Velkoff, V.A.; Hogan, H. An Aging Nation: The Older Population in the United States. Available online: https://www.census.gov/library/publications/2014/demo/p25-1140.html (accessed on 1 November 2021).
- Dekkers, B.G.J.; Veringa, A.; Marriott, D.J.E.; Boonstra, J.M.; van der Elst, K.C.M.; Doukas, F.F.; McLachlan, A.J.; Alffenaar, J.C. Invasive Candidiasis in the Elderly: Considerations for Drug Therapy. Drugs Aging 2018, 35, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kishi, M.; Suda, M.; Sakata, K.; Shimoda, H.; Miura, H.; Ogawa, A.; Kobayashi, S. Prevalence of Candida albicans and non-albicans on the tongue dorsa of elderly people living in a post-disaster area: A cross-sectional survey. BMC Oral Health 2017, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Barchiesi, F.; Orsetti, E.; Mazzanti, S.; Trave, F.; Salvi, A.; Nitti, C.; Manso, E. Candidemia in the elderly: What does it change? PLoS ONE 2017, 12, e0176576. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef]
- Kabir, M.A.; Ahmad, Z. Candida infections and their prevention. ISRN Prev. Med. 2013, 2013, 763628. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, A.A.; Harrison, L.H.; Farley, M.M.; Hollick, R.; Stein, B.; Chiller, T.M.; Lockhart, S.R.; Park, B.J. Declining incidence of candidemia and the shifting epidemiology of Candida resistance in two US metropolitan areas, 2008-2013: Results from population-based surveillance. PLoS ONE 2015, 10, e0120452. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.P.; White, C.M.; Pappas, P.G. Candidemia and Invasive Candidiasis. Infect. Dis. Clin. North. Am. 2021, 35, 389–413. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997–2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef] [PubMed]
- Patin, E.C.; Thompson, A.; Orr, S.J. Pattern recognition receptors in fungal immunity. Semin. Cell Dev. Biol. 2019, 89, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; van der Meer, J.W.; Kullberg, B.J.; van de Veerdonk, F.L. Immune defence against Candida fungal infections. Nat. Rev. Immunol. 2015, 15, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Vautier, S.; MacCallum, D.M.; Brown, G.D. C-type lectin receptors and cytokines in fungal immunity. Cytokine 2012, 58, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.; Gringhuis, S.I. Signalling through C-type lectin receptors: Shaping immune responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.; Gringhuis, S.I. C-type lectin receptors in the control of T helper cell differentiation. Nat. Rev. Immunol. 2016, 16, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Pan, D.; Zhou, Z.; You, Y.; Jiang, C.; Zhao, X.; Lin, X. Dectin-3 Deficiency Promotes Colitis Development due to Impaired Antifungal Innate Immune Responses in the Gut. PLoS Pathog. 2016, 12, e1005662. [Google Scholar] [CrossRef]
- Rogers, N.C.; Slack, E.C.; Edwards, A.D.; Nolte, M.A.; Schulz, O.; Schweighoffer, E.; Williams, D.L.; Gordon, S.; Tybulewicz, V.L.; Brown, G.D.; et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 2005, 22, 507–517. [Google Scholar] [CrossRef]
- Gross, O.; Gewies, A.; Finger, K.; Schäfer, M.; Sparwasser, T.; Peschel, C.; Förster, I.; Ruland, J. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 2006, 442, 651–656. [Google Scholar] [CrossRef]
- Gringhuis, S.I.; den Dunnen, J.; Litjens, M.; van der Vlist, M.; Wevers, B.; Bruijns, S.C.; Geijtenbeek, T.B. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat. Immunol. 2009, 10, 203–213. [Google Scholar] [CrossRef]
- LeibundGut-Landmann, S.; Gross, O.; Robinson, M.J.; Osorio, F.; Slack, E.C.; Tsoni, S.V.; Schweighoffer, E.; Tybulewicz, V.; Brown, G.D.; Ruland, J.; et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 2007, 8, 630–638. [Google Scholar] [CrossRef]
- Yu, M.; Song, X.T.; Liu, B.; Luan, T.T.; Liao, S.L.; Zhao, Z.T. The Emerging Role of Mast Cells in Response to Fungal Infection. Front. Immunol. 2021, 12, 688659. [Google Scholar] [CrossRef]
- Falk, P.G.; Hooper, L.V.; Midtvedt, T.; Gordon, J.I. Creating and maintaining the gastrointestinal ecosystem: What we know and need to know from gnotobiology. Microbiol. Mol. Biol. Rev. 1998, 62, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Angenent, L.T.; Gordon, J.I. Getting a grip on things: How do communities of bacterial symbionts become established in our intestine? Nat. Immunol. 2004, 5, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010, 6, e1000713. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Sendid, B.; Hoarau, G.; Colombel, J.F.; Poulain, D.; Ghannoum, M.A. Mycobiota in gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Wang, H.; Retuerto, M.; Zhang, H.; Burkey, B.; Ghannoum, M.A.; Eng, C. Bacteriome and mycobiome associations in oral tongue cancer. Oncotarget 2017, 8, 97273–97289. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D. Innate antifungal immunity: The key role of phagocytes. Annu. Rev. Immunol. 2011, 29, 1–21. [Google Scholar] [CrossRef]
- Berman, J.; Sudbery, P.E. Candida Albicans: A molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 2002, 3, 918–930. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, L.; Xu, Z.; Zhang, J.; Jiang, Y.Y.; Cao, Y.; Yan, T. Innate immune cell response upon Candida albicans infection. Virulence 2016, 7, 512–526. [Google Scholar] [CrossRef]
- Richardson, J.P.; Moyes, D.L. Adaptive immune responses to Candida albicans infection. Virulence 2015, 6, 327–337. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, L.; Lei, Y.L.; Ren, B.; Zhou, X. The Interactions between Candida albicans and mucosal immunity. Front. Microbiol. 2021, 12, 652725. [Google Scholar] [CrossRef]
- Villar, C.C.; Dongari-Bagtzoglou, A. Fungal diseases: Oral dysbiosis in susceptible hosts. Periodontol 2000 2021, 87, 166–180. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Garcia, A.; Rementeria, A.; Aguirre-Urizar, J.M.; Moragues, M.D.; Antoran, A.; Pellon, A.; Abad-Diaz-de-Cerio, A.; Hernando, F.L. Candida albicans and cancer: Can this yeast induce cancer development or progression? Crit. Rev. Microbiol. 2016, 42, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. Fighting cancer with microbes. Nature 2020, 577, S16–S18. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Athena Aktipis, C. The Role of the Microbiome in Cancer Initiation and Progression: How Microbes and Cancer Cells Utilize Excess Energy and Promote One Another’s Growth. Curr. Nutr. Rep. 2019, 8, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, N.; Jayaraman, S.; Quigley, C.; Mamileti, P.; Ghannoum, M.; Weinberg, A.; Thuener, J.; Pan, Q.; Pandiyan, P. The Role of Dectin-1 Signaling in Altering Tumor Immune Microenvironment in the Context of Aging. Front. Oncol. 2021, 11, 669066. [Google Scholar] [CrossRef] [PubMed]
- O’Higgins, C.; Ward, F.J.; Abu Eid, R. Deciphering the Role of Regulatory CD4 T Cells in Oral and Oropharyngeal Cancer: A Systematic Review. Front. Oncol. 2018, 8, 442. [Google Scholar] [CrossRef]
- Lee, C.H.; Chang, J.S.; Syu, S.H.; Wong, T.S.; Chan, J.Y.; Tang, Y.C.; Yang, Z.P.; Yang, W.C.; Chen, C.T.; Lu, S.C.; et al. IL-1β promotes malignant transformation and tumor aggressiveness in oral cancer. J. Cell Physiol. 2015, 230, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Al-Hebshi, N.N.; Perera, I.; Ipe, D.; Ulett, G.C.; Speicher, D.J.; Chen, T.; Johnson, N.W. A dysbiotic mycobiome dominated by. J. Oral Microbiol. 2017, 9, 1385369. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.M.; Liang, J.A.; Lin, C.L.; Sun, L.M.; Kao, C.H. Cancer risk in patients with candidiasis: A nationwide population-based cohort study. Oncotarget 2017, 8, 63562–63573. [Google Scholar] [CrossRef] [PubMed]
- Mellinghoff, S.C.; Thelen, M.; Bruns, C.; Garcia-Marquez, M.; Hartmann, P.; Lammertz, T.; Lehmann, J.; Nowag, A.; Stemler, J.; Wennhold, K.; et al. T-cells of invasive candidiasis patients show patterns of T-cell-exhaustion suggesting checkpoint blockade as treatment option. J. Infect. 2021. [Google Scholar] [CrossRef]
- Pellon, A.; Sadeghi Nasab, S.D.; Moyes, D.L. New Insights in Candida albicans Innate Immunity at the Mucosa: Toxins, Epithelium, Metabolism, and Beyond. Front. Cell Infect. Microbiol. 2020, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Di Cosola, M.; Cazzolla, A.P.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida albicans and Oral Carcinogenesis. A Brief Review. J. Fungi. 2021, 7, 476. [Google Scholar] [CrossRef] [PubMed]
- Alnuaimi, A.D.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Reynolds, E.C.; McCullough, M.J. Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: A matched case-control study. Oral Oncol. 2015, 51, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Siedlecka, K.; Dvořák, A.; Folwarski, M.; Daca, A.; Przewłócka, K.; Makarewicz, W. Fungal Gut Microbiota Dysbiosis and Its Role in Colorectal, Oral, and Pancreatic Carcinogenesis. Cancers 2020, 12, 1326. [Google Scholar] [CrossRef]
- Mäkinen, A.; Nawaz, A.; Mäkitie, A.; Meurman, J.H. Role of Non-Albicans Candida and Candida Albicans in Oral Squamous Cell Cancer Patients. J. Oral Maxillofac. Surg. 2018, 76, 2564–2571. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, A.L.; Katragadda, R.; Thyagarajan, R.; Vajravelu, L.; Manikesi, S.; Kaliappan, S.; Jayachandran, B. Oral Candidiasis among Cancer Patients Attending a Tertiary Care Hospital in Chennai, South India: An Evaluation of Clinicomycological Association and Antifungal Susceptibility Pattern. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 8758461. [Google Scholar] [CrossRef]
- Mäkinen, A.I.; Mäkitie, A.; Meurman, J.H. Candida prevalence in saliva before and after oral cancer treatment. Surgeon 2021, 19, e446–e451. [Google Scholar] [CrossRef] [PubMed]
- Hongal, B.P.; Kulkarni, V.V.; Deshmukh, R.S.; Joshi, P.S.; Karande, P.P.; Shroff, A.S. Prevalence of fungal hyphae in potentially malignant lesions and conditions-does its occurrence play a role in epithelial dysplasia? J. Oral Maxillofac. Pathol. 2015, 19, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Astekar, M.; Sapra, G.; Chitlangia, R.K.; Raj, N. Evaluation of candidal species among individuals with oral potentially malignant disorders and oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2019, 23, 302. [Google Scholar] [CrossRef] [PubMed]
- Isacco, C.G.; Ballini, A.; De Vito, D.; Nguyen, K.C.D.; Cantore, S.; Bottalico, L.; Quagliuolo, L.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; et al. Rebalancing the Oral Microbiota as an Efficient Tool in Endocrine, Metabolic and Immune Disorders. Endocr. Metab. Immune Disord. Drug. Targets 2021, 21, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Carlaio, R.G.; Bottalico, L. Does it make sense that diabetes is reciprocally associated with periodontal disease? Endocr. Metab. Immune Disord. Drug. Targets 2010, 10, 57–70. [Google Scholar] [CrossRef]
- Hawkins, B.L.; Heniford, B.W.; Ackermann, D.M.; Leonberger, M.; Martinez, S.A.; Hendler, F.J. 4NQO carcinogenesis: A mouse model of oral cavity squamous cell carcinoma. Head Neck 1994, 16, 424–432. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, J.F.; Reade, P.C. Candida albicans as a promoter of oral mucosal neoplasia. Carcinogenesis 1992, 13, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, K.; Fifita, S.F.; Kuyama, K. The Cytological Findings of Oral Inflammatory Lesions, Lichen Planus and Leukoplakia Coexisted with and without Candida: With Special Reference to Clinical, Histopathological, Immunohistochemical and Flow Cytometrical Analyses. Int. J. Oral-Med. Sci. 2007, 6, 81–90. [Google Scholar] [CrossRef][Green Version]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- Rivera, C.; Venegas, B. Histological and molecular aspects of oral squamous cell carcinoma (Review). Oncol. Lett. 2014, 8, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Engku Nasrullah Satiman, E.A.F.; Ahmad, H.; Ramzi, A.B.; Abdul Wahab, R.; Kaderi, M.A.; Wan Harun, W.H.A.; Dashper, S.; McCullough, M.; Arzmi, M.H. The role of Candida albicans candidalysin ECE1 gene in oral carcinogenesis. J. Oral Pathol. Med. 2020, 49, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Whiley, R.A.; Cruchley, A.T.; Gore, C.; Hagi-Pavli, E. Candida albicans strain-dependent modulation of pro-inflammatory cytokine release by in vitro oral and vaginal mucosal models. Cytokine 2012, 57, 89–97. [Google Scholar] [CrossRef]
- Gupta, S.R.; Gupta, N.; Sharma, A.; Xess, I.; Singh, G.; Mani, K. The association of Candida and antifungal therapy with pro-inflammatory cytokines in oral leukoplakia. Clin. Oral Investig. 2021, 25, 6287–6296. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zhao, Y. Acetaldehyde induces phosphorylation of dynamin-related protein 1 and mitochondrial dysfunction via elevating intracellular ROS and Ca2+ levels. Redox Biol. 2020, 28, 101381. [Google Scholar] [CrossRef]
- Stornetta, A.; Guidolin, V.; Balbo, S. Alcohol-Derived Acetaldehyde Exposure in the Oral Cavity. Cancers 2018, 10, 20. [Google Scholar] [CrossRef]
- Cohen, S.; Danzaki, K.; MacIver, N.J. Nutritional effects on T-cell immunometabolism. Eur. J. Immunol. 2017, 47, 225–235. [Google Scholar] [CrossRef] [PubMed]
- MacIver, N.J.; Michalek, R.D.; Rathmell, J.C. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 2013, 31, 259–283. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, Z.; Ren, T.; Kim, S.J.; He, Y.; Seo, W.; Guillot, A.; Ding, Y.; Wu, R.; Shao, S.; et al. Alcohol inhibits T-cell glucose metabolism and hepatitis in ALDH2-deficient mice and humans: Roles of acetaldehyde and glucocorticoids. Gut 2019, 68, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Dang, E.V.; Barbi, J.; Yang, H.Y.; Jinasena, D.; Yu, H.; Zheng, Y.; Bordman, Z.; Fu, J.; Kim, Y.; Yen, H.R.; et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 2011, 146, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Clambey, E.T.; McNamee, E.N.; Westrich, J.A.; Glover, L.E.; Campbell, E.L.; Jedlicka, P.; de Zoeten, E.F.; Cambier, J.C.; Stenmark, K.R.; Colgan, S.P.; et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc. Natl. Acad. Sci. USA 2012, 109, E2784–E2793. [Google Scholar] [CrossRef]
- Bhaskaran, N.; Faddoul, F.; Paes da Silva, A.; Jayaraman, S.; Schneider, E.; Mamileti, P.; Weinberg, A.; Pandiyan, P. IL-1β-MyD88-mTOR Axis Promotes Immune-Protective IL-17A. Front. Immunol. 2020, 11, 595936. [Google Scholar] [CrossRef] [PubMed]
- de Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Li, X.V.; Leonardi, I.; Iliev, I.D. Gut Mycobiota in Immunity and Inflammatory Disease. Immunity 2019, 50, 1365–1379. [Google Scholar] [CrossRef]
- Paterson, M.J.; Oh, S.; Underhill, D.M. Host-microbe interactions: Commensal fungi in the gut. Curr. Opin. Microbiol. 2017, 40, 131–137. [Google Scholar] [CrossRef]
- Bhaskaran, N.; Quigley, C.; Paw, C.; Butala, S.; Schneider, E.; Pandiyan, P. Role of Short Chain Fatty Acids in Controlling Tregs and Immunopathology During Mucosal Infection. Front. Microbiol. 2018, 9, 1995. [Google Scholar] [CrossRef]
- Pandiyan, P.; Bhaskaran, N.; Zou, M.; Schneider, E.; Jayaraman, S.; Huehn, J. Microbiome Dependent Regulation of Tregs and Th17 Cells in Mucosa. Front. Immunol. 2019, 10, 426. [Google Scholar] [CrossRef]
- Mason, K.L.; Erb Downward, J.R.; Mason, K.D.; Falkowski, N.R.; Eaton, K.A.; Kao, J.Y.; Young, V.B.; Huffnagle, G.B. Candida albicans and bacterial microbiota interactions in the cecum during recolonization following broad-spectrum antibiotic therapy. Infect. Immun. 2012, 80, 3371–3380. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Olshtain-Pops, K.; Krieger, M.; Oren, I.; Bishara, J.; Dan, M.; Wiener-Well, Y.; Weinberger, M.; Zimhony, O.; Chowers, M.; et al. Antibiotic exposure as a risk factor for fluconazole-resistant Candida bloodstream infection. Antimicrob. Agents Chemother. 2012, 56, 2518–2523. [Google Scholar] [CrossRef]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef]
- Wheeler, M.L.; Limon, J.J.; Bar, A.S.; Leal, C.A.; Gargus, M.; Tang, J.; Brown, J.; Funari, V.A.; Wang, H.L.; Crother, T.R.; et al. Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host Microbe 2016, 19, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Iliev, I.D.; Funari, V.A.; Taylor, K.D.; Nguyen, Q.; Reyes, C.N.; Strom, S.P.; Brown, J.; Becker, C.A.; Fleshner, P.R.; Dubinsky, M.; et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science 2012, 336, 1314–1317. [Google Scholar] [CrossRef] [PubMed]
- Chehoud, C.; Albenberg, L.G.; Judge, C.; Hoffmann, C.; Grunberg, S.; Bittinger, K.; Baldassano, R.N.; Lewis, J.D.; Bushman, F.D.; Wu, G.D. Fungal Signature in the Gut Microbiota of Pediatric Patients With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, C.; Tang, C.; He, Q.; Li, N.; Li, J. Dysbiosis of gut fungal microbiota is associated with mucosal inflammation in Crohn’s disease. J. Clin. Gastroenterol. 2014, 48, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.; Lamas, B.; Richard, M.L.; Brandi, G.; da Costa, G.; Hoffmann, T.W.; Di Simone, M.P.; Calabrese, C.; Poggioli, G.; Langella, P.; et al. Fungal Dysbiosis in Mucosa-associated Microbiota of Crohn’s Disease Patients. J. Crohns Colitis 2016, 10, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Hansen, R.; Meharg, C.; Thomson, J.M.; Russell, R.K.; Berry, S.H.; El-Omar, E.M.; Hold, G.L. The fungal microbiota of de-novo paediatric inflammatory bowel disease. Microbes Infect. 2015, 17, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Ng, S.C. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front. Microbiol. 2018, 9, 2247. [Google Scholar] [CrossRef]
- Ott, S.J.; Kühbacher, T.; Musfeldt, M.; Rosenstiel, P.; Hellmig, S.; Rehman, A.; Drews, O.; Weichert, W.; Timmis, K.N.; Schreiber, S. Fungi and inflammatory bowel diseases: Alterations of composition and diversity. Scand. J. Gastroenterol. 2008, 43, 831–841. [Google Scholar] [CrossRef]
- Colombel, J.F.; Sendid, B.; Jouault, T.; Poulain, D. Secukinumab failure in Crohn’s disease: The yeast connection? Gut 2013, 62, 800–801. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Yu, B.; He, J.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Tian, G.; Huang, Z.; et al. Fungi in Gastrointestinal Tracts of Human and Mice: From Community to Functions. Microb. Ecol. 2018, 75, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhang, F.; Yang, X.; Wu, N.; Jiang, W.; Li, X.; Liu, Y. Changes in the composition of intestinal fungi and their role in mice with dextran sulfate sodium-induced colitis. Sci. Rep. 2015, 5, 10416. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yoshida, M.; Ishikawa, H.; Kameyama, K.; Wakabayashi, G.; Otani, Y.; Shimazu, M.; Tanabe, M.; Kawachi, S.; Kumai, K.; et al. Candida albicans aggravates duodenal ulcer perforation induced by administration of cysteamine in rats. J. Gastroenterol. Hepatol. 2007, 22, 749–756. [Google Scholar] [CrossRef]
- Chiffoleau, E. C-Type Lectin-Like Receptors As Emerging Orchestrators of Sterile Inflammation Represent Potential Therapeutic Targets. Front. Immunol. 2018, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://covid19.who.int (accessed on 25 January 2022).
- Sharma, A.; Ahmad Farouk, I.; Lal, S.K. COVID-19: A Review on the Novel Coronavirus Disease Evolution, Transmission, Detection, Control and Prevention. Viruses 2021, 13, 202. [Google Scholar] [CrossRef]
- Soltani, S.; Zakeri, A.; Zandi, M.; Kesheh, M.M.; Tabibzadeh, A.; Dastranj, M.; Faramarzi, S.; Didehdar, M.; Hafezi, H.; Hosseini, P.; et al. The Role of Bacterial and Fungal Human Respiratory Microbiota in COVID-19 Patients. Biomed. Res. Int. 2021, 2021, 6670798. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Sharma, A. The lurking scourge of multidrug resistant Candida auris in times of COVID-19 pandemic. J. Glob. Antimicrob. Resist. 2020, 22, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Talento, A.F.; Hoenigl, M. Fungal Infections Complicating COVID-19: With the Rain Comes the Spores. J. Fungi. 2020, 6, 279. [Google Scholar] [CrossRef] [PubMed]
- Mulet Bayona, J.V.; Tormo Palop, N.; Salvador García, C.; Fuster Escrivá, B.; Chanzá Aviñó, M.; Ortega García, P.; Gimeno Cardona, C. Impact of the SARS-CoV-2 Pandemic in Candidaemia, Invasive Aspergillosis and Antifungal Consumption in a Tertiary Hospital. J. Fungi. 2021, 7, 440. [Google Scholar] [CrossRef]
- Roudbary, M.; Kumar, S.; Kumar, A.; Černáková, L.; Nikoomanesh, F.; Rodrigues, C.F. Overview on the Prevalence of Fungal Infections, Immune Response, and Microbiome Role in COVID-19 Patients. J. Fungi. 2021, 7, 720. [Google Scholar] [CrossRef] [PubMed]
- Moser, D.; Biere, K.; Han, B.; Hoerl, M.; Schelling, G.; Choukér, A.; Woehrle, T. COVID-19 Impairs Immune Response to Candida albicans. Front. Immunol. 2021, 12, 640644. [Google Scholar] [CrossRef]
- Chen, X.; Liao, B.; Cheng, L.; Peng, X.; Xu, X.; Li, Y.; Hu, T.; Li, J.; Zhou, X.; Ren, B. The microbial coinfection in COVID-19. Appl. Microbiol. Biotechnol. 2020, 104, 7777–7785. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.M.; Dinh, A.Q.; Tran, T.T.; Arenas, S.; Pronty, D.; Gershengorn, H.B.; Ferreira, T.; Arias, C.A.; Shukla, B.S. Invasive Infections during a COVID-19 Case Surge. Antimicrob. Agents Chemother. 2021, 65, e0114621. [Google Scholar] [CrossRef] [PubMed]
- Casalini, G.; Giacomelli, A.; Ridolfo, A.; Gervasoni, C.; Antinori, S. Invasive Fungal Infections Complicating COVID-19: A Narrative Review. J. Fungi. 2021, 7, 921. [Google Scholar] [CrossRef]
- Wong, L.R.; Perlman, S. Immune dysregulation and immunopathology induced by SARS-CoV-2 and related coronaviruses-are we our own worst enemy? Nat. Rev. Immunol. 2021, 22, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Carvalho, A.; Nguyen, M.H.; Hedayati, M.T.; Netea, M.G.; Perlin, D.S.; Hoenigl, M. COVID-19-Associated Candidiasis (CAC): An Underestimated Complication in the Absence of Immunological Predispositions? J. Fungi. 2020, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Gomaa, E.; Hockova, B.; Klugar, M. Oral candidiasis of COVID-19 patients: Case report and review of evidence. J. Cosmet. Dermatol. 2021, 20, 1580–1584. [Google Scholar] [CrossRef]
- Nucci, M.; Barreiros, G.; Guimarães, L.F.; Deriquehem, V.A.S.; Castiñeiras, A.C.; Nouér, S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses 2021, 64, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Katz, J. Prevalence of candidiasis and oral candidiasis in COVID-19 patients: A cross-sectional pilot study from the patients’ registry in a large health center. Quintessence Int. 2021, 52, 714–718. [Google Scholar] [CrossRef]
- Frías-De-León, M.G.; Pinto-Almazán, R.; Hernández-Castro, R.; García-Salazar, E.; Meza-Meneses, P.; Rodríguez-Cerdeira, C.; Arenas, R.; Conde-Cuevas, E.; Acosta-Altamirano, G.; Martínez-Herrera, E. Epidemiology of Systemic Mycoses in the COVID-19 Pandemic. J. Fungi. 2021, 7, 556. [Google Scholar] [CrossRef]
- Alanio, A.; Dellière, S.; Fodil, S.; Bretagne, S.; Mégarbane, B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir. Med. 2020, 8, e48–e49. [Google Scholar] [CrossRef]
- Gangneux, J.P.; Reizine, F.; Guegan, H.; Pinceaux, K.; Le Balch, P.; Prat, E.; Pelletier, R.; Belaz, S.; Le Souhaitier, M.; Le Tulzo, Y.; et al. Is the COVID-19 Pandemic a Good Time to Include Aspergillus Molecular Detection to Categorize Aspergillosis in ICU Patients? A Monocentric Experience. J. Fungi. 2020, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Wheat, L.J.; Azar, M.M.; Bahr, N.C.; Spec, A.; Relich, R.F.; Hage, C. Histoplasmosis. Infect. Dis. Clin. North. Am. 2016, 30, 207–227. [Google Scholar] [CrossRef]
- Azar, M.M.; Hage, C.A. Clinical Perspectives in the Diagnosis and Management of Histoplasmosis. Clin. Chest Med. 2017, 38, 403–415. [Google Scholar] [CrossRef]
- Messina, F.A.; Marin, E.; Caceres, D.H.; Romero, M.; Depardo, R.; Priarone, M.M.; Rey, L.; Vázquez, M.; Verweij, P.E.; Chiller, T.M.; et al. Coronavirus Disease 2019 (COVID-19) in a Patient with Disseminated Histoplasmosis and HIV-A Case Report from Argentina and Literature Review. J. Fungi. 2020, 6, 275. [Google Scholar] [CrossRef]
- Basso, R.P.; Poester, V.R.; Benelli, J.L.; Stevens, D.A.; Zogbi, H.E.; Vasconcellos, I.C.D.S.; Pasqualotto, A.C.; Xavier, M.O. COVID-19-Associated Histoplasmosis in an AIDS Patient. Mycopathologia 2021, 186, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, C.E.S.; Nigri, D.H.; Cardoso, F.R.; Mattos, R.S.A.R.; Gonçalves Martins, P.A.; Carvalho, A.R.S.; Altino de Almeida, S.; Rodrigues, R.S.; Rosado-de-Castro, P.H. Case Report: Incidental Finding of COVID-19 Infection after Positron Emission Tomography/CT Imaging in a Patient with a Diagnosis of Histoplasmosis and Recurring Fever. Am. J. Trop. Med. Hyg. 2021, 104, 1651. [Google Scholar] [CrossRef]
- De Macedo, P.M.; Freitas, A.D.; Bártholo, T.P.; Bernardes-Engemann, A.R.; Almeida, M.A.; Almeida-Silva, F.; Zancopé-Oliveira, R.M.; Almeida-Paes, R. Acute Pulmonary Histoplasmosis Following COVID-19: Novel Laboratorial Methods Aiding Diagnosis. J. Fungi. 2021, 7, 346. [Google Scholar] [CrossRef]
- Bertolini, M.; Mutti, M.F.; Barletta, J.A.; Falak, A.; Cuatz, D.; Sisto, A.; Ragusa, M.A.; Fernandez Claros, N.O.; Rolón, M.J. COVID-19 associated with AIDS-related disseminated histoplasmosis: A case report. Int. J. STD AIDS 2020, 31, 1222–1224. [Google Scholar] [CrossRef]
- Khatib, M.Y.; Ahmed, A.A.; Shaat, S.B.; Mohamed, A.S.; Nashwan, A.J. Cryptococcemia in a patient with COVID-19: A case report. Clin. Case Rep. 2021, 9, 853–855. [Google Scholar] [CrossRef]
- Aranjani, J.M.; Manuel, A.; Abdul Razack, H.I.; Mathew, S.T. COVID-19-associated mucormycosis: Evidence-based critical review of an emerging infection burden during the pandemic’s second wave in India. PLoS Negl. Trop. Dis. 2021, 15, e0009921. [Google Scholar] [CrossRef]
- Chao, C.M.; Lai, C.C.; Yu, W.L. COVID-19 associated mucormycosis—An emerging threat. J. Microbiol. Immunol. Infect. 2022. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Mang, S.; Kaddu-Mulindwa, D.; Metz, C.; Becker, A.; Seiler, F.; Smola, S.; Maßmann, A.; Becker, S.L.; Papan, C.; Bals, R.; et al. Pneumocystis jirovecii Pneumonia and Severe Acute Respiratory Syndrome Coronavirus 2 Coinfection in a Patient With Newly Diagnosed HIV-1 Infection. Clin. Infect. Dis. 2021, 72, 1487–1489. [Google Scholar] [CrossRef]
- Kim, D.; Kim, S.B.; Jeon, S.; Kim, S.; Lee, K.H.; Lee, H.S.; Han, S.H. No Change of Pneumocystis jirovecii Pneumonia after the COVID-19 Pandemic: Multicenter Time-Series Analyses. J. Fungi. 2021, 7, 990. [Google Scholar] [CrossRef]
- Viceconte, G.; Buonomo, A.R.; Lanzardo, A.; Pinchera, B.; Zappulo, E.; Scotto, R.; Schiano Moriello, N.; Vargas, M.; Iacovazzo, C.; Servillo, G.; et al. Pneumocystis jirovecii pneumonia in an immunocompetent patient recovered from COVID-19. Infect. Dis. 2021, 53, 382–385. [Google Scholar] [CrossRef]
- Nasrullah, A.; Javed, A.; Malik, K. Coronavirus Disease-Associated Pulmonary Aspergillosis: A Devastating Complication of COVID-19. Cureus 2021, 13, e13004. [Google Scholar] [CrossRef]
- Bacher, P.; Hohnstein, T.; Beerbaum, E.; Röcker, M.; Blango, M.G.; Kaufmann, S.; Röhmel, J.; Eschenhagen, P.; Grehn, C.; Seidel, K.; et al. Human Anti-fungal Th17 Immunity and Pathology Rely on Cross-Reactivity against Candida albicans. Cell 2019, 176, 1340–1355. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, I.; Li, X.; Semon, A.; Li, D.; Doron, I.; Putzel, G.; Bar, A.; Prieto, D.; Rescigno, M.; McGovern, D.P.B.; et al. CX3CR1+ mononuclear phagocytes control immunity to intestinal fungi. Science 2018, 359, 232–236. [Google Scholar] [CrossRef]
- Witchley, J.N.; Penumetcha, P.; Abon, N.V.; Woolford, C.A.; Mitchell, A.P.; Noble, S.M. Candida albicans Morphogenesis Programs Control the Balance between Gut Commensalism and Invasive Infection. Cell Host Microbe 2019, 25, 432–443.e436. [Google Scholar] [CrossRef] [PubMed]
- Staab, J.F.; Bradway, S.D.; Fidel, P.L.; Sundstrom, P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science 1999, 283, 1535–1538. [Google Scholar] [CrossRef]
- Naglik, J.R.; König, A.; Hube, B.; Gaffen, S.L. Candida albicans-epithelial interactions and induction of mucosal innate immunity. Curr. Opin. Microbiol. 2017, 40, 104–112. [Google Scholar] [CrossRef]
- Liu, Y.; Filler, S.G. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot. Cell 2011, 10, 168–173. [Google Scholar] [CrossRef]
- Moyes, D.L.; Wilson, D.; Richardson, J.P.; Mogavero, S.; Tang, S.X.; Wernecke, J.; Höfs, S.; Gratacap, R.L.; Robbins, J.; Runglall, M.; et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 2016, 532, 64–68. [Google Scholar] [CrossRef]
- Verma, A.H.; Richardson, J.P.; Zhou, C.; Coleman, B.M.; Moyes, D.L.; Ho, J.; Huppler, A.R.; Ramani, K.; McGeachy, M.J.; Mufazalov, I.A.; et al. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef]
- Allert, S.; Förster, T.M.; Svensson, C.M.; Richardson, J.P.; Pawlik, T.; Hebecker, B.; Rudolphi, S.; Juraschitz, M.; Schaller, M.; Blagojevic, M.; et al. Candida albicans-Induced Epithelial Damage Mediates Translocation through Intestinal Barriers. mBio 2018, 9, e00915-18. [Google Scholar] [CrossRef]
- Naglik, J.R.; Richardson, J.P.; Moyes, D.L. Candida albicans pathogenicity and epithelial immunity. PLoS Pathog. 2014, 10, e1004257. [Google Scholar] [CrossRef]
- Tang, S.X.; Moyes, D.L.; Richardson, J.P.; Blagojevic, M.; Naglik, J.R. Epithelial discrimination of commensal and pathogenic Candida albicans. Oral Dis. 2016, 22 (Suppl. 1), 114–119. [Google Scholar] [CrossRef]
- Kalia, N.; Singh, J.; Kaur, M. The role of dectin-1 in health and disease. Immunobiology 2021, 226, 152071. [Google Scholar] [CrossRef]
- Taylor, P.R.; Tsoni, S.V.; Willment, J.A.; Dennehy, K.M.; Rosas, M.; Findon, H.; Haynes, K.; Steele, C.; Botto, M.; Gordon, S.; et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 2007, 8, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hise, A.G.; Tomalka, J.; Ganesan, S.; Patel, K.; Hall, B.A.; Brown, G.D.; Fitzgerald, K.A. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 2009, 5, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 2012, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiao, Y.; Su, L.; Cui, N.; Liu, D. mTOR Modulates CD8+ T Cell Differentiation in Mice with Invasive Pulmonary Aspergillosis. Open Life Sci. 2018, 13, 129–136. [Google Scholar] [CrossRef]
- Wang, H.; Bai, G.; Cui, N.; Han, W.; Long, Y. T-cell-specific mTOR deletion in mice ameliorated CD4 + T-cell survival in lethal sepsis induced by severe invasive candidiasis. Virulence 2019, 10, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cui, N.; Wang, H.; Han, W.; Bai, G.; Cheng, W. Impact of mTOR signaling pathway on CD8+ T cell immunity through Eomesodermin in response to invasive candidiasis. J. Microbiol. Immunol. Infect. 2021, 54, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Pollizzi, K.N.; Patel, C.H.; Sun, I.H.; Oh, M.H.; Waickman, A.T.; Wen, J.; Delgoffe, G.M.; Powell, J.D. mTORC1 and mTORC2 selectively regulate CD8⁺ T cell differentiation. J. Clin. Investig. 2015, 125, 2090–2108. [Google Scholar] [CrossRef]
- Zhang, L.; Tschumi, B.O.; Lopez-Mejia, I.C.; Oberle, S.G.; Meyer, M.; Samson, G.; Rüegg, M.A.; Hall, M.N.; Fajas, L.; Zehn, D.; et al. Mammalian Target of Rapamycin Complex 2 Controls CD8 T Cell Memory Differentiation in a Foxo1-Dependent Manner. Cell Rep. 2016, 14, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Salmond, R.J. mTOR Regulation of Glycolytic Metabolism in T Cells. Front. Cell Dev. Biol. 2018, 6, 122. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).