Low Risk of Occult Hepatitis B Infection among Vietnamese Blood Donors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Serological Assays
2.3. Nucleic Acid Isolation
2.4. HBV Screening and Sequencing
2.5. Statistical and Phylogenetic Analysis
3. Results
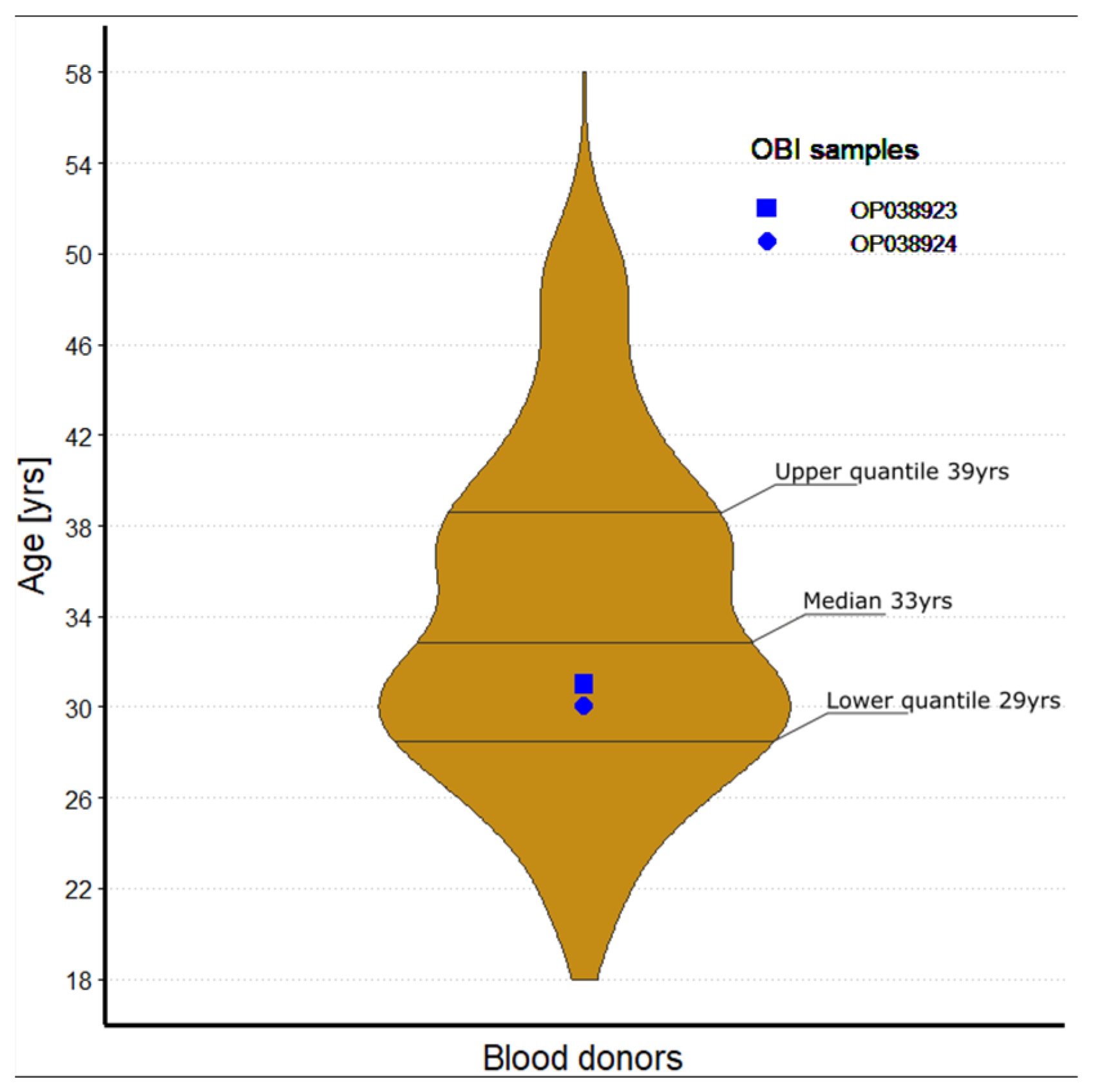
3.1. Baseline Characteristics
3.2. HBV Serology and Nucleic Acid Detection
3.3. OBI Genotyping
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Croagh, C.M.N.; Lubel, J.S. Natural history of chronic hepatitis B: Phases in a complex relationship. World J. Gastroenterol. 2014, 20, 10395–10404. [Google Scholar] [CrossRef] [PubMed]
- Rajbhandari, R.; Chung, R.T. Treatment of Hepatitis B: A Concise Review. Clin. Transl. Gastroenterol. 2016, 7, e190. [Google Scholar] [CrossRef]
- Juszczyk, J. Clinical course and consequences of hepatitis B infection. Vaccine 2000, 18 (Suppl. S1), S23–S25. [Google Scholar] [CrossRef] [PubMed]
- Allweiss, L.; Dandri, M. The Role of cccDNA in HBV Maintenance. Viruses 2017, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Suk-Fong Lok, A. Hepatitis B Treatment: What We Know Now and What Remains to Be Researched. Hepatol. Commun. 2019, 3, 8–19. [Google Scholar] [CrossRef]
- Sarin, S.K.; Kumar, M.; Lau, G.K.; Abbas, Z.; Chan, H.L.; Chen, C.J.; Chen, D.S.; Chen, H.L.; Chen, P.J.; Chien, R.N.; et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int. 2016, 10, 1–98. [Google Scholar] [CrossRef]
- Oluyinka, O.O.; Tong, H.V.; Bui Tien, S.; Fagbami, A.H.; Adekanle, O.; Ojurongbe, O.; Bock, C.T.; Kremsner, P.G.; Velavan, T.P. Occult Hepatitis B Virus Infection in Nigerian Blood Donors and Hepatitis B Virus Transmission Risks. PLoS ONE 2015, 10, e0131912. [Google Scholar] [CrossRef]
- Esposito, A.; Sabia, C.; Iannone, C.; Nicoletti, G.F.; Sommese, L.; Napoli, C. Occult Hepatitis Infection in Transfusion Medicine: Screening Policy and Assessment of Current Use of Anti-HBc Testing. Transfus. Med. Hemother. 2017, 44, 263–272. [Google Scholar] [CrossRef]
- Olotu, A.A.; Oyelese, A.O.; Salawu, L.; Audu, R.A.; Okwuraiwe, A.P.; Aboderin, A.O. Occult Hepatitis B virus infection in previously screened, blood donors in Ile-Ife, Nigeria: Implications for blood transfusion and stem cell transplantation. Virol. J. 2016, 13, 76. [Google Scholar] [CrossRef]
- Raimondo, G.; Locarnini, S.; Pollicino, T.; Levrero, M.; Zoulim, F.; Lok, A.S.; Taormina Workshop on Occult HBV Infection Faculty Members. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J. Hepatol. 2019, 71, 397–408. [Google Scholar] [CrossRef]
- Im, Y.R.; Jagdish, R.; Leith, D.; Kim, J.U.; Yoshida, K.; Majid, A.; Ge, Y.; Ndow, G.; Shimakawa, Y.; Lemoine, M. Prevalence of occult hepatitis B virus infection in adults: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 932–942. [Google Scholar] [CrossRef]
- Lazarevic, I.; Banko, A.; Miljanovic, D.; Cupic, M. Immune-Escape Hepatitis B Virus Mutations Associated with Viral Reactivation upon Immunosuppression. Viruses 2019, 11, 778. [Google Scholar] [CrossRef]
- Kwak, M.S.; Kim, Y.J. Occult hepatitis B virus infection. World J. Hepatol. 2014, 6, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Mak, L.Y.; Wong, D.K.; Pollicino, T.; Raimondo, G.; Hollinger, F.B.; Yuen, M.F. Occult hepatitis B infection and hepatocellular carcinoma: Epidemiology, virology, hepatocarcinogenesis and clinical significance. J. Hepatol. 2020, 73, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Waheed, Y.; Siddiq, M.; Jamil, Z.; Najmi, M.H. Hepatitis elimination by 2030: Progress and challenges. World J. Gastroenterol. 2018, 24, 4959–4961. [Google Scholar] [CrossRef]
- Ye, X.; Zhao, Y.; Li, R.; Li, T.; Zheng, X.; Xiong, W.; Zeng, J.; Xu, M.; Chen, L. High Frequency Occult Hepatitis B Virus Infection Detected in Non-Resolved Donations Suggests the Requirement of Anti-HBc Test in Blood Donors in Southern China. Front. Immunol. 2021, 12, 699217. [Google Scholar] [CrossRef]
- Flower, B.; Du Hong, D.; Vu Thi Kim, H.; Pham Minh, K.; Geskus, R.B.; Day, J.; Cooke, G.S. Seroprevalence of Hepatitis B, C and D in Vietnam: A systematic review and meta-analysis. Lancet Reg. Health West. Pac. 2022, 24, 100468. [Google Scholar] [CrossRef]
- Huy Do, S. Epidemiology of Hepatitis B and C Virus Infections and Liver Cancer in Vietnam. Euroasian J. Hepatogastroenterol. 2015, 5, 49–51. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, J.H.; Cowie, B.C. Hepatitis B virus epidemiology. Cold Spring Harb. Perspect. Med. 2015, 5, a021410. [Google Scholar] [CrossRef] [PubMed]
- Elizalde, M.M.; Tadey, L.; Mammana, L.; Quarleri, J.F.; Campos, R.H.; Flichman, D.M. Biological Characterization of Hepatitis B virus Genotypes: Their Role in Viral Replication and Antigen Expression. Front. Microbiol. 2021, 12, 758613. [Google Scholar] [CrossRef]
- Hoan, N.X.; Hoechel, M.; Tomazatos, A.; Anh, C.X.; Pallerla, S.R.; Linh, L.T.K.; Binh, M.T.; Sy, B.T.; Toan, N.L.; Wedemeyer, H.; et al. Predominance of HBV Genotype B and HDV Genotype 1 in Vietnamese Patients with Chronic Hepatitis. Viruses 2021, 13, 346. [Google Scholar] [CrossRef]
- Velkov, S.; Ott, J.J.; Protzer, U.; Michler, T. The Global Hepatitis B Virus Genotype Distribution Approximated from Available Genotyping Data. Genes 2018, 9, 495. [Google Scholar] [CrossRef]
- Loh, K.; Kew, S. Interpreting hepatitis B serology. Malays Fam. Physician 2007, 2, 31–32. [Google Scholar] [PubMed]
- Said, Z.N. An overview of occult hepatitis B virus infection. World J. Gastroenterol. 2011, 17, 1927–1938. [Google Scholar] [CrossRef] [PubMed]
- Gish, R.G.; Basit, S.A.; Ryan, J.; Dawood, A.; Protzer, U. Hepatitis B Core Antibody: Role in Clinical Practice in 2020. Curr. Hepatol. Rep. 2020, 19, 254–265. [Google Scholar] [CrossRef]
- de Almeida, N.A.A.; de Paula, V.S. Occult Hepatitis B virus (HBV) infection and challenges for hepatitis elimination: A literature review. J. Appl. Microbiol. 2022, 132, 1616–1635. [Google Scholar] [CrossRef] [PubMed]
- Jutavijittum, P.; Andernach, I.E.; Yousukh, A.; Samountry, B.; Samountry, K.; Thammavong, T.; Keokhamphue, J.; Toriyama, K.; Muller, C.P. Occult hepatitis B infections among blood donors in Lao PDR. Vox Sang. 2014, 106, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ye, X.; Zhang, L.; Wang, W.; Shuai, L.; Wang, A.; Zeng, J.; Candotti, D.; Allain, J.P.; Li, C. Characterization of occult hepatitis B virus infection from blood donors in China. J. Clin. Microbiol. 2011, 49, 1730–1737. [Google Scholar] [CrossRef]
- Biswas, A.; Panigrahi, R.; Chandra, P.K.; Banerjee, A.; Datta, S.; Pal, M.; Chakraborty, S.; Bhattacharya, P.; Chakrabarti, S.; Chakravarty, R. Characterization of the occult hepatitis B virus variants circulating among the blood donors from eastern India. Sci. World J. 2013, 2013, 212704. [Google Scholar] [CrossRef]
- Hudu, S.A.; Harmal, N.S.; Saeed, M.I.; Alshrari, A.S.; Malik, Y.A.; Niazlin, M.T.; Hassan, R.; Sekawi, Z. Molecular and serological detection of occult hepatitis B virus among healthy hepatitis B surface antigen-negative blood donors in Malaysia. Afr. Health Sci. 2016, 16, 677–683. [Google Scholar] [CrossRef][Green Version]
- Ji, D.Z.; Pang, X.Y.; Shen, D.T.; Liu, S.N.; Goyal, H.; Xu, H.G. Global prevalence of occult hepatitis B: A systematic review and meta-analysis. J. Viral Hepat. 2022, 29, 317–329. [Google Scholar] [CrossRef]
- Schmeltzer, P.; Sherman, K.E. Occult hepatitis B: Clinical implications and treatment decisions. Dig. Dis. Sci. 2010, 55, 3328–3335. [Google Scholar] [CrossRef]
- Tosun, S.; Aygün, O.; Özdemir, H.; Korkmaz, E.; Özdemir, D. The impact of economic and social factors on the prevalence of hepatitis B in Turkey. BMC Public Health 2018, 18, 649. [Google Scholar] [CrossRef]
- Araujo, N.M.; Teles, S.A.; Spitz, N. Comprehensive Analysis of Clinically Significant Hepatitis B Virus Mutations in Relation to Genotype, Subgenotype and Geographic Region. Front. Microbiol. 2020, 11, 616023. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.-H.; Chen, P.-J.; Chen, D.-S. Chapter 2—Recent Advances in the Research of Hepatitis B Virus-Related Hepatocellular Carcinoma: Epidemiologic and Molecular Biological Aspects. In Advances in Cancer Research; Vande Woude, G.F., Klein, G., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 108, pp. 21–72. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Zeng, J.; Du, P.; Zheng, X.; Ye, X.; Zhu, W.; Fu, Y.; Candotti, D.; Allain, J.P.; et al. Occurrence of occult hepatitis B virus infection associated with envelope protein mutations according to anti-HBs carriage in blood donors. Int. J. Infect. Dis. 2020, 92, 38–45. [Google Scholar] [CrossRef]
- Yu, D.M.; Li, X.H.; Mom, V.; Lu, Z.H.; Liao, X.W.; Han, Y.; Pichoud, C.; Gong, Q.M.; Zhang, D.H.; Zhang, Y.; et al. N-glycosylation mutations within hepatitis B virus surface major hydrophilic region contribute mostly to immune escape. J. Hepatol. 2014, 60, 515–522. [Google Scholar] [CrossRef]
- Shi, Y.; Wei, F.; Hu, D.; Li, Q.; Smith, D.; Li, N.; Chen, D. Mutations in the major hydrophilic region (MHR) of hepatitis B virus genotype C in North China. J. Med. Virol. 2012, 84, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.C.; Kok, C.C.; Baleriola, C.; Robertson, P.; Rawlinson, W.D. Investigation of occult hepatitis B virus infection in anti-hbc positive patients from a liver clinic. PLoS ONE 2015, 10, e0117275. [Google Scholar] [CrossRef]
- Ponde, R.A.; Cardoso, D.D.; Ferro, M.O. The underlying mechanisms for the ‘anti-HBc alone’ serological profile. Arch. Virol. 2010, 155, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, G.P.; Abate, M.L.; Tandoi, F.; Ciancio, A.; Amoroso, A.; Salizzoni, M.; Saracco, G.M.; Rizzetto, M.; Romagnoli, R.; Smedile, A. Quantitation of HBV cccDNA in anti-HBc-positive liver donors by droplet digital PCR: A new tool to detect occult infection. J. Hepatol. 2018, 69, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Allain, J.P. Occult hepatitis B virus infection: Implications in transfusion. Vox Sang. 2004, 86, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Allain, J.P.; Candotti, D. Diagnostic algorithm for HBV safe transfusion. Blood Transfus. 2009, 7, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.M.; Welge, J.A.; Rouster, S.D.; Shata, M.T.; Sherman, K.E.; Blackard, J.T. Mutations associated with occult hepatitis B virus infection result in decreased surface antigen expression in vitro. J. Viral Hepat. 2012, 19, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Grund, S.; Verheyen, J.; Esser, S.; Chen, X.; Lu, M. Spontaneous reactivation of hepatitis B virus replication in an HIV coinfected patient with isolated anti-Hepatitis B core antibodies. Virol. J. 2014, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, M.; Huang, J.; Xu, R.; Liao, Q.; Shan, Z.; Zheng, Y.; Rong, X.; Tang, X.; Li, T.; et al. Novel hepatitis B virus surface antigen mutations associated with occult genotype B hepatitis B virus infection affect HBsAg detection. J. Viral Hepat. 2020, 27, 915–921. [Google Scholar] [CrossRef]
- Kim, H.S.; Chen, X.; Xu, M.; Yan, C.; Liu, Y.; Deng, H.; Hoang, B.H.; Thuy, P.T.T.; Wang, T.; Yan, Y.; et al. Frequency of hepatitis B surface antigen variants (HBsAg) in hepatitis B virus genotype B and C infected East- and Southeast Asian patients: Detection by the Elecsys((R)) HBsAg II assay. J. Clin. Virol. 2018, 103, 48–56. [Google Scholar] [CrossRef]
- Wang, H.Y.; Chien, M.H.; Huang, H.P.; Chang, H.C.; Wu, C.C.; Chen, P.J.; Chang, M.H.; Chen, D.S. Distinct hepatitis B virus dynamics in the immunotolerant and early immunoclearance phases. J. Virol. 2010, 84, 3454–3463. [Google Scholar] [CrossRef]
- Wang, M.; Xu, R.; Huang, J.; Liao, Q.; Tang, X.; Shan, Z.; Zhong, H.; Rong, X.; Fu, Y. Molecular characteristics of the full-length genome of occult hepatitis B virus from blood donors in China. Sci. Rep. 2022, 12, 8194. [Google Scholar] [CrossRef]


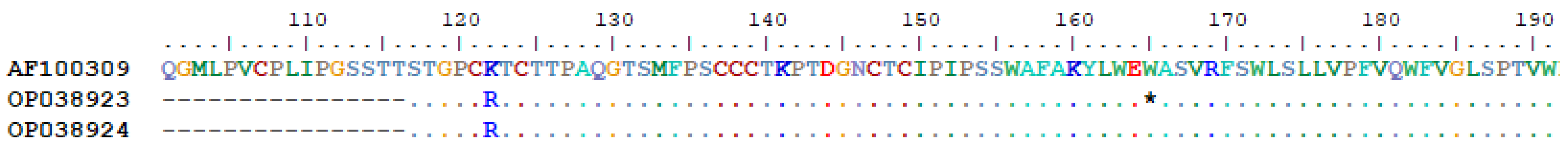

| Serology | Number (%) |
|---|---|
| Anti-HBc-positive | 242 (39) |
| Anti-HB-positive | 434 (70) |
| Anti-HB-negative and anti-HBc-positive | 19 (3) |
| Anti-HB-positive and anti-HBc-negative | 211 (34) |
| Anti-HB-positive and anti-HBc-positive | 223 (36) |
| Anti-HB-negative and anti-HBc-negative | 170 (27) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tung, T.T.; Schmid, J.; Nghia, V.X.; Cao, L.C.; Linh, L.T.K.; Rungsung, I.; Sy, B.T.; My, T.N.; The, N.T.; Hoan, N.X.; et al. Low Risk of Occult Hepatitis B Infection among Vietnamese Blood Donors. Pathogens 2022, 11, 1524. https://doi.org/10.3390/pathogens11121524
Tung TT, Schmid J, Nghia VX, Cao LC, Linh LTK, Rungsung I, Sy BT, My TN, The NT, Hoan NX, et al. Low Risk of Occult Hepatitis B Infection among Vietnamese Blood Donors. Pathogens. 2022; 11(12):1524. https://doi.org/10.3390/pathogens11121524
Chicago/Turabian StyleTung, Tran Thanh, Jürgen Schmid, Vu Xuan Nghia, Le Chi Cao, Le Thi Kieu Linh, Ikrormi Rungsung, Bui Tien Sy, Truong Nhat My, Nguyen Trong The, Nghiem Xuan Hoan, and et al. 2022. "Low Risk of Occult Hepatitis B Infection among Vietnamese Blood Donors" Pathogens 11, no. 12: 1524. https://doi.org/10.3390/pathogens11121524
APA StyleTung, T. T., Schmid, J., Nghia, V. X., Cao, L. C., Linh, L. T. K., Rungsung, I., Sy, B. T., My, T. N., The, N. T., Hoan, N. X., Meyer, C. G., Wedemeyer, H., Kremsner, P. G., Toan, N. L., Song, L. H., Bock, C.-T., & Velavan, T. P. (2022). Low Risk of Occult Hepatitis B Infection among Vietnamese Blood Donors. Pathogens, 11(12), 1524. https://doi.org/10.3390/pathogens11121524











