Molecular Characterization of Leishmania Species among Patients with Cutaneous Leishmaniasis in Asir Province, Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
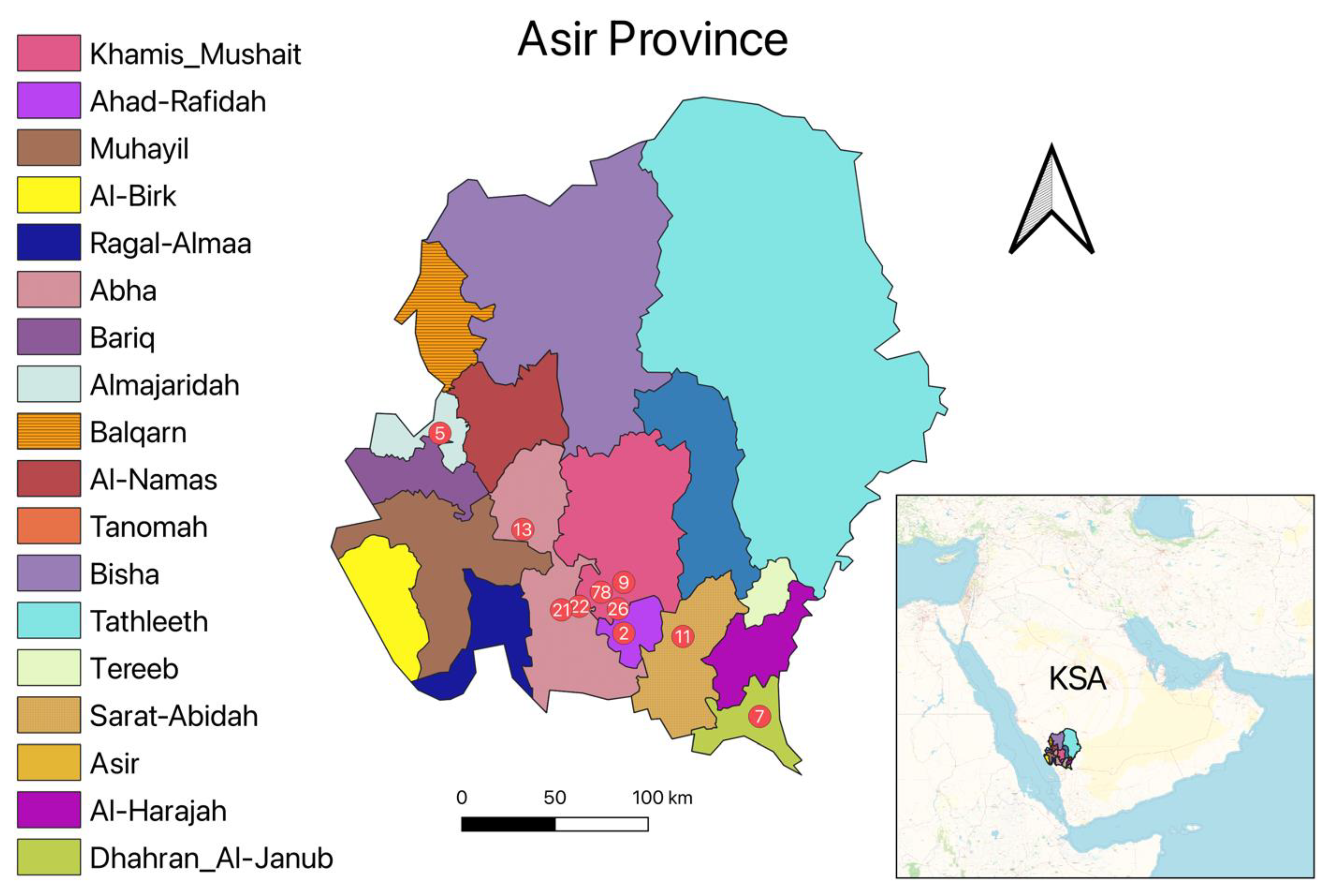
2.1. Study Area and Sample Collection
2.2. DNA Extraction
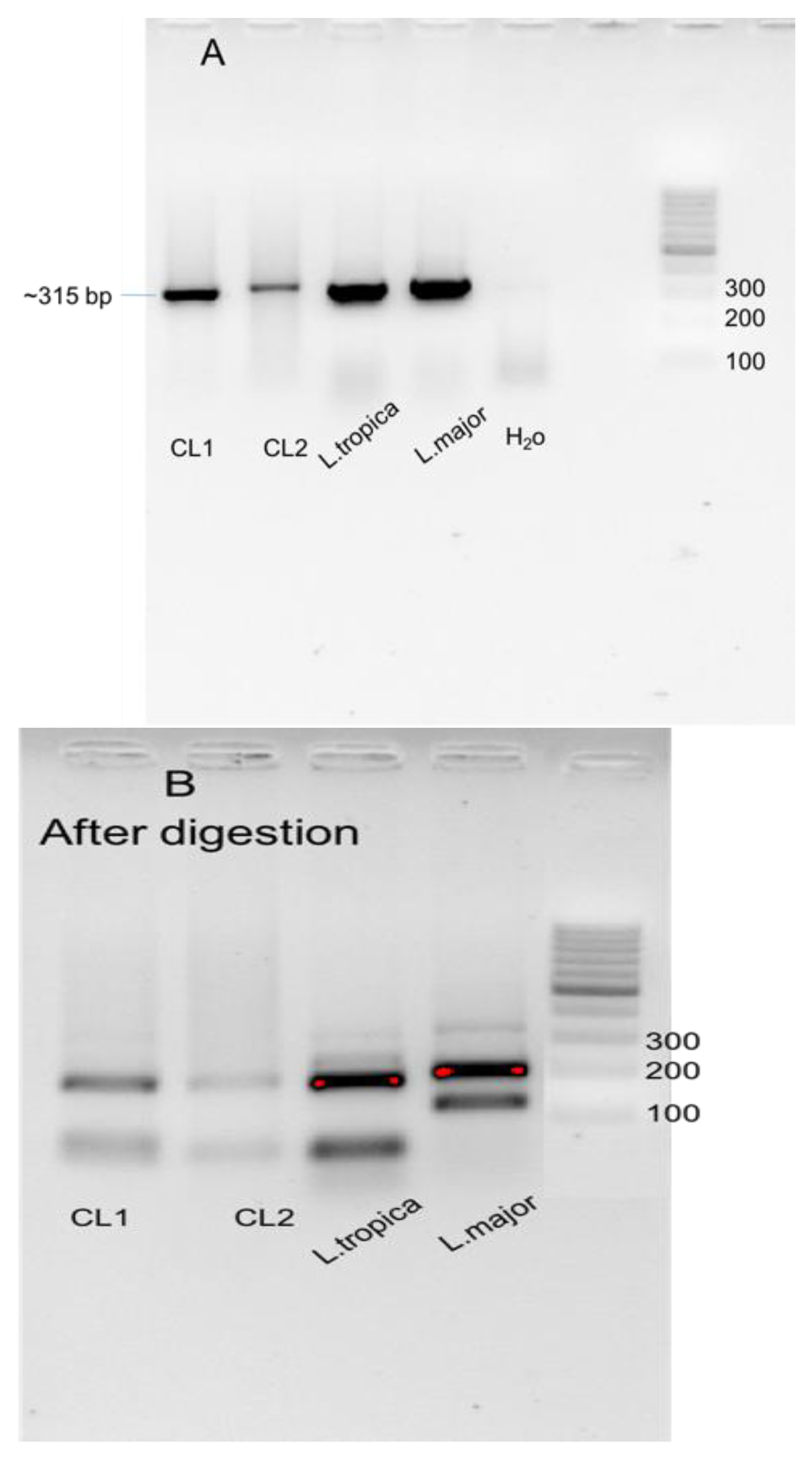
2.3. Leishmania Identification Using PCR-RFLP Assay
2.4. Leishmania Identification Using Real-Time PCR-HRM Assay
2.5. Data Analysis
2.6. Phylogenetic Analysis
2.7. Ethical Approval
3. Results
3.1. Socio-Epidemiological Features
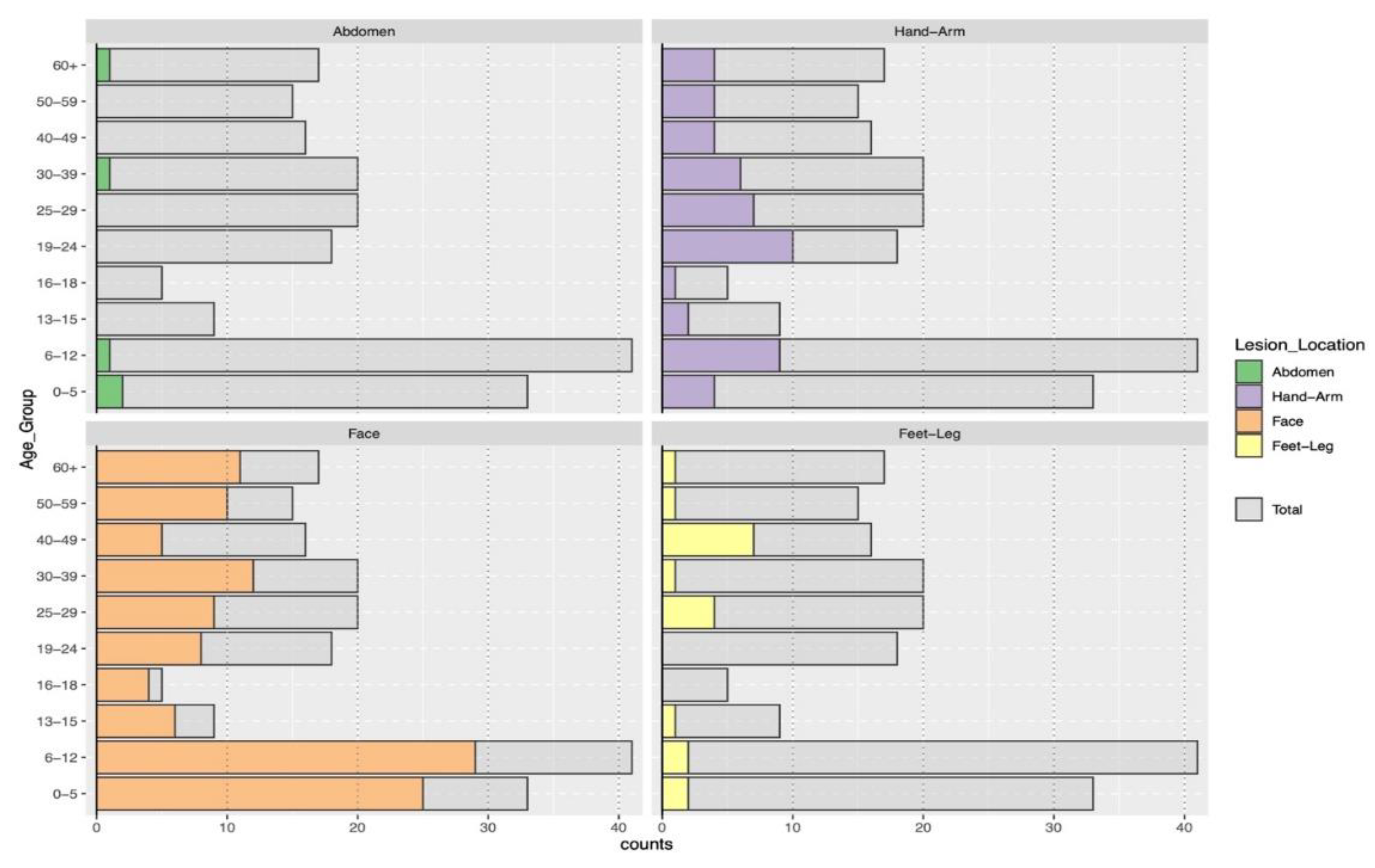
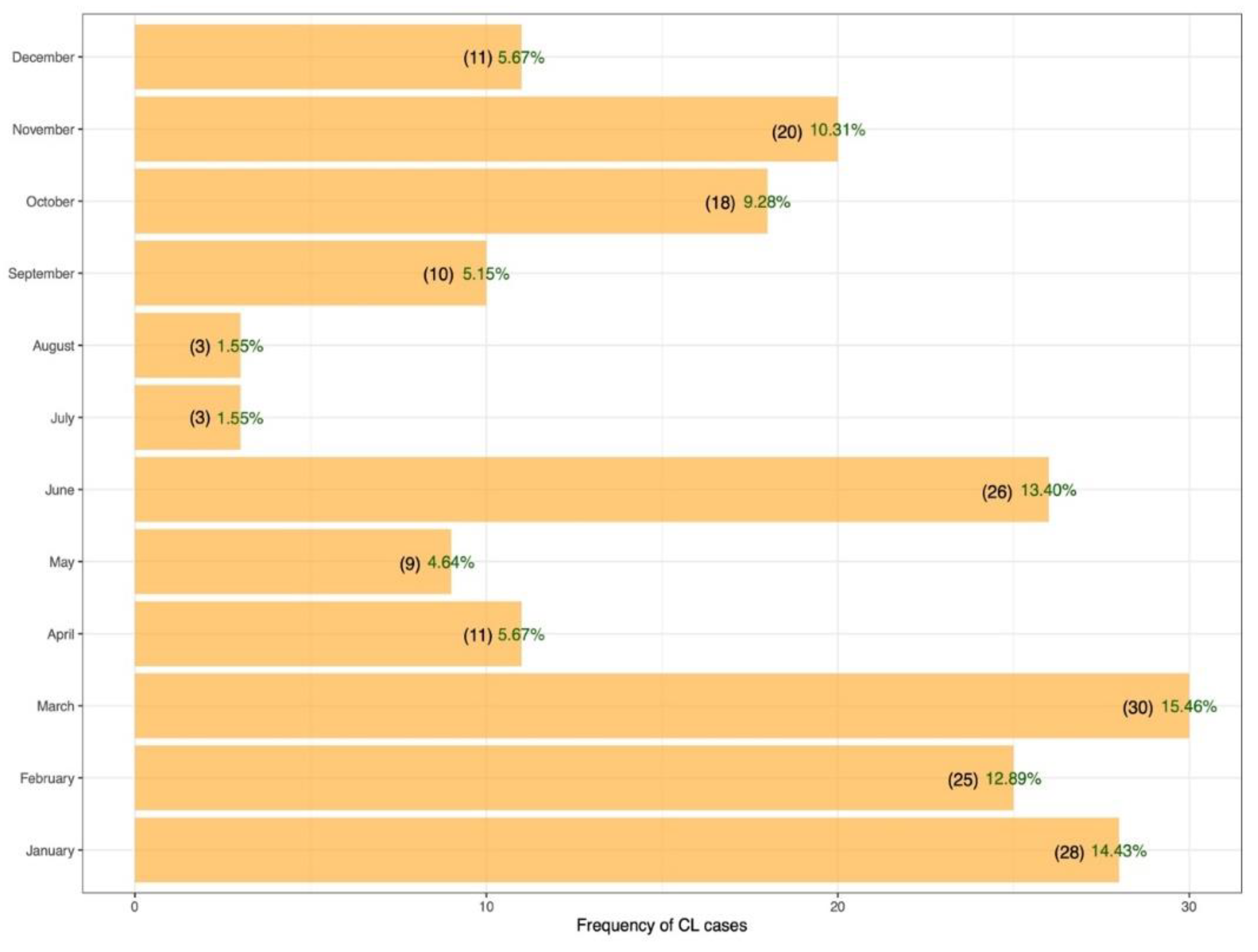
3.2. Clinical Characteristics of Cutaneous Leishmaniasis
3.3. Molecular Characterization of Cutaneous Leishmaniasis in Asir Region
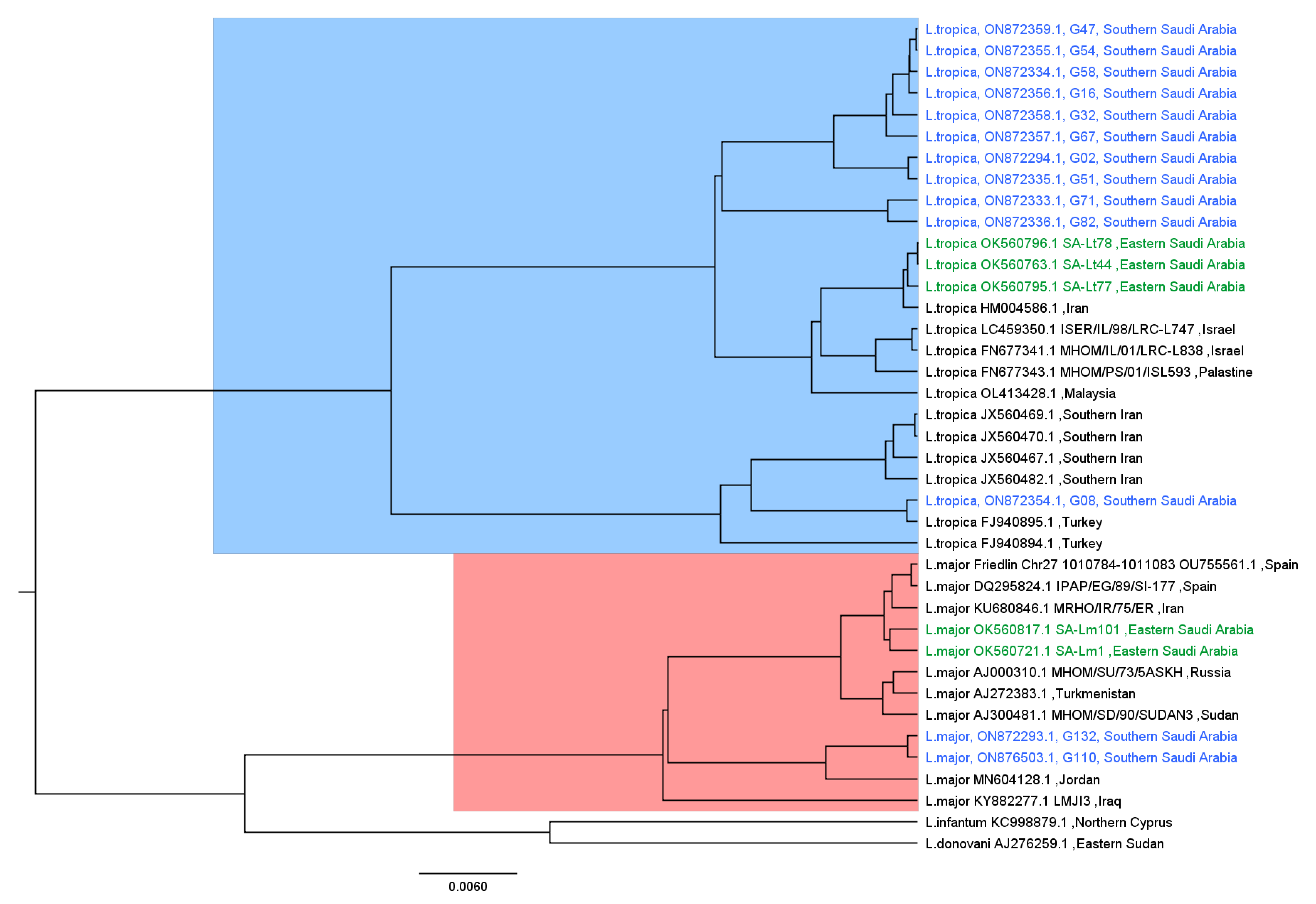
3.4. Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben Hadj Ahmed, S.; Kaabi, B.; Chelbi, I.; Cherni, S.; Derbali, M.; Laouini, D.; Zhioua, E. Colonization of Phlebotomus papatasi changes the effect of pre-immunization with saliva from lack of protection towards protection against experimental challenge with Leishmania major and saliva. Parasit. Vectors 2011, 4, 126. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; de Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2022. Leishmaniasis. Available online: https://www.who.int/News-Room/Fact-Sheets/Detail/Leishmaniasis (accessed on 23 June 2022).
- Monge-Maillo, B.; Norman, F.F.; Cruz, I.; Alvar, J.; López-Vé lez, R.; López-Vélez, R. Visceral leishmaniasis and HIV coinfection in the Mediterranean Region. PLoS Negl. Trop. Dis. 2014, 8, e3021. [Google Scholar] [CrossRef] [PubMed]
- Hirve, S.; Boelaert, M.; Matlashewski, G.; Mondal, D.; Arana, B.; Kroeger, A.; Olliaro, P. Transmission dynamics of visceral leishmaniasis in the Indian Subcontinent—A systematic literature review. PLoS Negl. Trop. Dis. 2016, 10, e0004896. [Google Scholar] [CrossRef] [PubMed]
- Herrador, Z.; Gherasim, A.; Jimenez, B.C.; Granados, M.; San Martín, J.V.; Aparicio, P. Epidemiological changes in leishmaniasis in Spain according to hospitalization-based records, 1997-2011: Raising awareness towards leishmaniasis in non-HIV patients. PLoS Negl. Trop. Dis. 2015, 9, e0003594. [Google Scholar] [CrossRef]
- Zoghlami, Z.; Chouihi, E.; Barhoumi, W.; Dachraoui, K.; Massoudi, N.; Helel, K.B.; Habboul, Z.; Hadhri, M.H.; Limam, S.; Mhadhbi, M.; et al. Interaction between canine and human visceral leishmaniases in a holoendemic focus of Central Tunisia. Acta Trop. 2014, 139, 32–38. [Google Scholar] [CrossRef]
- Ghawar, W.; Toumi, A.; Snoussi, M.A.; Chlif, S.; Zâatour, A.; Boukthir, A.; Bel Haj Hamida, N.; Chemkhi, J.; Diouani, M.F.; Ben Salah, A. Leishmania major infection among Psammomys obesus and Meriones shawi: Reservoirs of zoonotic cutaneous leishmaniasis in Sidi Bouzid (Central Tunisia). Vector-Borne Zoonotic Dis. 2011, 11, 1561–1568. [Google Scholar] [CrossRef]
- Assimina, Z.; Koutis, C.; Babatsikou, F. Leishmaniasis: An overlooked public health concern. Health Sci. J. 2008, 2, 196–205. [Google Scholar]
- Gadisa, E.; Tsegaw, T.; Abera, A.; Elnaiem, D.-E.; den Boer, M.; Aseffa, A.; Jorge, A. Eco-epidemiology of visceral leishmaniasis in Ethiopia. Parasit. Vectors 2015, 8, 381. [Google Scholar] [CrossRef]
- Barhoumi, W.; Fares, W.; Cherni, S.; Derbali, M.; Dachraoui, K.; Chelbi, I.; Ramalho-Ortigao, M.; Beier, J.C.; Zhioua, E. Changes of sand fly populations and Leishmania infantum infection rates in an irrigated village located in arid Central Tunisia. Int. J. Environ. Res. Public Health 2016, 13, 329. [Google Scholar] [CrossRef]
- Chelbi, I.; Mathlouthi, O.; Zhioua, S.; Fares, W.; Boujaama, A.; Cherni, S.; Barhoumi, W.; Dachraoui, K.; Derbali, M.; Abbass, M.; et al. The Impact of illegal waste sites on the transmission of zoonotic cutaneous leishmaniasis in Central Tunisia. Int. J. Environ. Res. Public Health 2021, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Aoun, K.; Bouratbine, A. Cutaneous leishmaniasis in North Africa: A Review. Parasite 2014, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Chahed, M.K.; Bellali, H.; Ben Jemaa, S.; Bellaj, T. Psychological and psychosocial consequences of zoonotic cutaneous leishmaniasis among women in Tunisia: Preliminary findings from an exploratory Study. PLoS Negl. Trop. Dis. 2016, 10, e0005090. [Google Scholar] [CrossRef] [PubMed]
- Bousslimi, N.; Aoun, K.; Ben-Abda, I.; Ben-Alaya-Bouafif, N.; Raouane, M.; Bouratbine, A. Epidemiologic and clinical features of cutaneous leishmaniasis in Southeastern Tunisia. Am. J. Trop. Med. Hyg. 2010, 83, 1034. [Google Scholar] [CrossRef]
- Tayeh, A.; Jalouk, L.; Cairncross, S. Twenty years of cutaneous leishmaniasis in Aleppo, Syria. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 657–659. [Google Scholar] [CrossRef]
- Reithinger, R.; Mohsen, M.; Aadil, K.; Sidiqi, M.; Erasmus, P.; Coleman, P.G. Anthroponotic cutaneous leishmaniasis, Kabul, Afghanistan. Emerg. Infect. Dis. 2003, 9, 727–729. [Google Scholar] [CrossRef]
- Bsrat, A.; Berhe, N.; Balkew, M.; Yohannes, M.; Teklu, T.; Gadisa, E.; Medhin, G.; Abera, A. Epidemiological study of cutaneous leishmaniasis in Saesie Tsaeda-Emba District, Eastern Tigray, Northern Ethiopia. Parasit. Vectors. 2015, 8, 149. [Google Scholar] [CrossRef]
- Montalvo, A.M.; Fraga, J.; El Safi, S.; Gramiccia, M.; Jaffe, C.L.; Dujardin, J.-C.; Van der Auwera, G. Direct Leishmania species typing in Old World clinical samples: Evaluation of 3 sensitive methods based on the heat-shock protein 70 gene. Diagn. Microbiol. Infect. Dis. 2014, 80, 35–39. [Google Scholar] [CrossRef]
- Karunaweera, N.D.; Pratlong, F.; Siriwardane, H.V.Y.D.; Ihalamulla, R.L.; Dedet, J.P. Sri Lankan cutaneous leishmaniasis is caused by Leishmania donovani zymodeme MON-37. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 380–381. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A.; AbuKhamsin, A. Cutaneous Leishmaniasis: A 46-year study of the epidemiology and clinical features in Saudi Arabia (1956-2002). Int. J. Infect. Dis. 2004, 8, 244–250. [Google Scholar] [CrossRef]
- Amin, T.T.; Al-Mohammed, H.I.; Kaliyadan, F.; Mohammed, B.S. Cutaneous leishmaniasis in Al Hassa, Saudi Arabia: Epidemiological trends from 2000 to 2010. Asian Pac. J. Trop. Med. 2013, 6, 667–672. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Leishmaniasis Resource: Saudi Arabia. 2014. Available online: www.who.int/Leishmaniasis/Resources/SAUDI_ARABIA.Pdf (accessed on 29 August 2014).
- Alraey, Y. Distribution and epidemiological features of cutaneous leishmaniasis in Asir Province, Saudi Arabia, from 2011 to 2020. J. Infect. Public Health 2022, 15, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Salam, N.; Al-Shaqha, W.M.; Azzi, A. Leishmaniasis in the Middle East: Incidence and epidemiology. PLoS Negl. Trop. Dis. 2014, 8, e3208. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, W.S.; Solórzano, C.; Weedall, G.D.; Dyer, N.A.; Kelly-Hope, L.; Casas-Sánchez, A.; Alraey, Y.; Alyamani, E.J.; Halliday, A.; Balghonaim, S.M.; et al. Old World cutaneous leishmaniasis treatment response varies depending on parasite species, geographical location and development of secondary infection. Parasit. Vectors. 2019, 12, 195. [Google Scholar] [CrossRef]
- Schönian, G.; Nasereddin, A.; Dinse, N.; Schweynoch, C.; Schallig, H.D.F.H.; Presber, W.; Jaffe, C.L. PCR Diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn. Microbiol. Infect. Dis. 2003, 47, 349–358. [Google Scholar] [CrossRef]
- Owino, B.O.; Matoke-Muhia, D.; Alraey, Y.; Mwangi, J.M.; Ingonga, J.M.; Ngumbi, P.M.; Casas-Sanchez, A.; Acosta-Serrano, A.; Masiga, D.K. Association of Phlebotomus guggisbergi with Leishmania major and Leishmania tropica in a complex transmission setting for cutaneous leishmaniasis in Gilgil, Nakuru County, Kenya. PLoS Negl. Trop. Dis. 2019, 13, e0007712. [Google Scholar] [CrossRef]
- Al-Rashed, A.S.; Al Jindan, R.; Al Jaroodi, S.; Al Mohanna, A.; Abdelhady, A.; El-Badry, A.A. Genotypic and phylogenic analyses of cutaneous leishmaniasis in Al Ahsa, Eastern Saudi Arabia during the coronavirus disease 2019 pandemic: First cases of Leishmania tropica with the predominance of Leishmania major. Sci. Rep. 2022, 12, 10753. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; Mcginnis, S.; Madden, T.L. NCBI BLAST: A Better Web Interface. Nucleic Acids Res. 2008, 36, 5–9. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple sequence alignment software persion 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian Evolutionary Analysis by Sampling Trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Jukes, T.H.; Cantor, C.R. CHAPTER 24—Evolution of Protein Molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: New York, NY, USA, 1969; pp. 21–132. ISBN 978-1-4832-3211-9. [Google Scholar] [CrossRef]
- Rambaut, A. (2010) FigTree v1.3.1. Institute of Evolutionary Biology, University of Edinburgh, Edinburgh. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 4 October 2016).
- Haouas, N.; Amer, O.; Ishankyty, A.; Alazmi, A.; Ishankyty, I. Profile and geographical distribution of reported cutaneous leishmaniasis cases in Northwestern Saudi Arabia, from 2010 to 2013. Asian Pac. J. Trop. Med. 2015, 8, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Haouas, N.; Amer, O.; Alshammri, F.F.; Al-Shammari, S.; Remadi, L.; Ashankyty, I. Cutaneous leishmaniasis in Northwestern Saudi Arabia: Identification of sand fly fauna and parasites. Parasit.Vectors. 2017, 10, 544. [Google Scholar] [CrossRef] [PubMed]
- Elmekki, M.A.; Elhassan, M.M.; Ozbak, H.A.; Qattan, I.T.; Saleh, S.M.; Alharbi, A.H. Epidemiological trends of cutaneous leishmaniasis in Al-Madinah Al-Munawarah Province, Western Region of Saudi Arabia. J. Glob. Infect. Dis. 2017, 9, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Karami, M.; Doudi, M.; Setorki, M. Assessing the epidemiology of cutaneous leishmaniasis in Isfahan, Iran. J. Vector Borne Dis. 2013, 50, 30–37. [Google Scholar]
- Morrone, A.; Pitidis, A.; Pajno, M.C.; Dassoni, F.; Latini, O.; Barnabas, G.A.; Padovese, V. Epidemiological and geographical aspects of leishmaniasis in Tigray, Northern Ethiopia: A retrospective analysis of medical records, 2005–2008. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 273–280. [Google Scholar] [CrossRef]
- Al-Zahrani, M.A.; Peters, W.; Evans, D.A.; Chin, C.; Smith, V.; Lane, R.P. Phlebotomus sergenti, a vector of Leishmania tropica in Saudi Arabia. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 416. [Google Scholar] [CrossRef]
- Ben Salah, A.; Kamarianakis, Y.; Chlif, S.; Alaya, N.B.; Prastacos, P. Zoonotic cutaneous leishmaniasis in Central Tunisia: Spatio-temporal dynamics. Int. J. Epidemiol. 2007, 36, 991–1000. [Google Scholar] [CrossRef]
- Chraiet-Rezgani, K.; Bouafif-Ben Alaya, N.; Habboul, Z.; Hajjej, Y.; Aoun, K. Epidemiological and clinical features of cutaneous leishmaniasis in Kairouan-Tunisia and characteristics in children. Bull. Soc. Pathol. Exot. 2016, 109, 80–83. [Google Scholar] [CrossRef]
- Kubeyinje, E.P.; Belagavi, C.S.; Jamil, Y.A. Cutaneous leishmaniasis in expatriates in Northern Saudi Arabia. East Afr. Med. J. 1997, 74, 249–251. [Google Scholar]
- Doha, S.A.; Samy, A.M. Bionomics of phlebotomine sand flies (Diptera: Psychodidae) in the Province of Al-Baha, Saudi Arabia. Mem. Inst. Oswaldo Cruz 2010, 105, 850–856. [Google Scholar] [CrossRef] [PubMed]
- El-Beshbishy, H.A.; Al-Ali, K.H.; El-Badry, A.A. Molecular characterization of Leishmania infection in sand flies from Al-Madinah Al-Munawarah Province, Western Saudi Arabia. Exp. Parasitol. 2013, 134, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Elbihar, S.; Kawasmeh, Z.A.; Al-Naiem, A.; Al-Atiya, S. Leishmania infecting man and wild animals in Saudi Arabia. 3. Leishmaniasis in Psammomys obesus Crestzschmar in Al Ahsa oasis. Trop. Med. Parasitol. 1987, 38, 89–92. [Google Scholar]
- Talmit-Frank, D.; Jaffe, C.L.; Naserddin, A.; Warburg, A.; King, R.; Svobodova, M.; Peleg, O.; Baneth, G. Leishmania tropica in the rocky hyraxes (Procavia capensis) in a focus of human cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 2010, 82, 814–818. [Google Scholar] [CrossRef]
- Kingdon, J. Arabian Mammals: A Natural History; Academic Press: London, UK, 1991. [Google Scholar]
- Chelbi, I.; Derbali, M.; Al-Ahmadi, Z.; Zaafouri, B.; El Fahem, A.; Zhioua, E. Phenology of Phlebotomus papatasi (Diptera: Psychodidae) relative to the seasonal prevalence of zoonotic cutaneous leishmaniasis in Central Tunisia. J. Med. Entomol. 2007, 44, 385–388. [Google Scholar] [CrossRef][Green Version]
- Kenawy, M.A.; Al Ashry, H.A.; Shobrak, M. Distribution and periodicity of sandflies (Diptera: Phlebotominae) along different altitudes in Asir region, southwest of Saudi Arabia. J. Entomol. Acarol. Res. 2015, 47, 5016. [Google Scholar] [CrossRef]
- Alanazi, A.D.; Alouffi, A.S.; Alyousif, M.S.; Rahi, A.A.; Ali, M.A.; Abdullah, H.H.A.M.; Brayner, F.A.; Mendoza-Roldan, J.A.; Bezerra-Santos, M.A.; Otranto, D. Molecular characterization of Leishmania species from stray dogs and human patients in Saudi Arabia. Parasitol. Res. 2021, 120, 4241–4246. [Google Scholar] [CrossRef]
- Abbas, M.A.S.; Lachheb, J.; Chelbi, I.; Louati, D.; Dachraoui, K.; Ben Miled, S.; Zhioua, E. Independent circulation of Leishmania major and Leishmania tropica in their respective sandfly vectors for transmission of zoonotic and chronic cutaneous leishmaniasis co-existing in a mixed focus of Central Tunisia. Pathogens 2022, 11, 855. [Google Scholar] [CrossRef]
- Shahbazi, F.; Shahabi, S.; Kazemi, B.; Mohebali, M.; Abadi, A.R.; Zare, Z. Evaluation of PCR assay in diagnosis and identification of cutaneous leishmaniasis: A comparison with the parasitological methods. Parasitol. Res. 2008, 103, 1159–1162. [Google Scholar] [CrossRef]
- Odiwuor, S.O.C.; Saad, A.A.; De Doncker, S.; Maes, I.; Laurent, T.; El Safi, S.; Mbuchi, M.; Büscher, P.; Dujardin, J.-C.; Van der Auwera, G. Universal PCR Assays for the differential detection of all Old World Leishmania species. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 209–218. [Google Scholar] [CrossRef]

| Species | Strain Name/WHO Code | Country | GenBank Accession |
|---|---|---|---|
| L. donovani | Eastern Sudan | AJ276259.1 | |
| L. infantum | Northern Cyprus | KC998879.1 | |
| L. major | MHOM/SU/73/5ASKH | Russia | AJ000310.1 |
| L. major | Turkmenistan | AJ272383.1 | |
| L. major | MHOM/SD/90/SUDAN3 | Sudan | AJ300481.1 |
| L. major | IPAP/EG/89/SI-177 | Spain | DQ295824.1 |
| L. major | Friedlin Chr27:1010784-1011083 | Spain | OU755561.1 |
| L. major | MRHO/IR/75/ER | Iran | KU680846.1 |
| L. major | LMJI3 | Iraq | KY882277.1 |
| L. major | Jordan | MN604128.1 | |
| L. major | SA-Lm1 | Eastern Saudi Arabia | OK560721.1 |
| L. major | SA-Lm101 | Eastern Saudi Arabia | OK560817.1 |
| L. major | MHOM/SA/2021/G132 | Southern Saudi Arabia | ON872293.1 |
| L. major | MHOM/SA/2021/G110 | Southern Saudi Arabia | ON876503.1 |
| L. tropica | Turkey | FJ940894.1 | |
| L. tropica | Turkey | FJ940895.1 | |
| L. tropica | MHOM/IL/01/LRC-L838 | Israel | FN677341.1 |
| L. tropica | MHOM/PS/01/ISL593 | Palestine | FN677343.1 |
| L. tropica | Iran | HM004586.1 | |
| L. tropica | Southern Iran | JX560467.1 | |
| L. tropica | Southern Iran | JX560469.1 | |
| L. tropica | Southern Iran | JX560470.1 | |
| L. tropica | Southern Iran | JX560482.1 | |
| L. tropica | ISER/IL/98/LRC-L747 | Israel | LC459350.1 |
| L. tropica | SA-Lt44 | Eastern Saudi Arabia | OK560763.1 |
| L. tropica | SA-Lt77 | Eastern Saudi Arabia | OK560795.1 |
| L. tropica | SA-Lt78 | Eastern Saudi Arabia | OK560796.1 |
| L. tropica | Malaysia | OL413428.1 | |
| L. tropica | MHOM/SA/2021/G02 | Southern Saudi Arabia | ON872294.1 |
| L. tropica | MHOM/SA/2021/G71 | Southern Saudi Arabia | ON872333.1 |
| L. tropica | MHOM/SA/2021/G58 | Southern Saudi Arabia | ON872334.1 |
| L. tropica | MHOM/SA/2021/G51 | Southern Saudi Arabia | ON872335.1 |
| L. tropica | MHOM/SA/2021/G82 | Southern Saudi Arabia | ON872336.1 |
| L. tropica | MHOM/SA/2021/G08 | Southern Saudi Arabia | ON872354.1 |
| L. tropica | MHOM/SA/2021/G54 | Southern Saudi Arabia | ON872355.1 |
| L. tropica | MHOM/SA/2021/G16 | Southern Saudi Arabia | ON872356.1 |
| L. tropica | MHOM/SA/2021/G67 | Southern Saudi Arabia | ON872357.1 |
| L. tropica | MHOM/SA/2021/G32 | Southern Saudi Arabia | ON872358.1 |
| L. tropica | MHOM/SA/2021/G47 | Southern Saudi Arabia | ON872359.1 |
| Characteristic | Female, N = 73 1 | Male, N = 121 1 | p-Value 2 | Characteristic | Female, N = 73 1 | Male, N = 121 1 | p-Value 2 |
|---|---|---|---|---|---|---|---|
| Nationality | <0.001 | Age_group | <0.001 | ||||
| Non-Saudi | 0 (0%) | 18 (15%) | 0–5 | 15 (21%) | 18 (15%) | ||
| Saudi | 73 (100%) | 103 (85%) | 6–12 | 18 (25%) | 23 (19%) | ||
| Lesion Number | 0.041 | 13–15 | 5 (6.8%) | 4 (3.3%) | |||
| Multiple | 24 (33%) | 24 (20%) | 16–18 | 1 (1.4%) | 4 (3.3%) | ||
| Single | 49 (67%) | 97 (80%) | 19–24 | 9 (12%) | 9 (7.4%) | ||
| Lesion Location | 0.8 | 25–29 | 4 (5.5%) | 16 (13%) | |||
| Abdomen | 2 (2.7%) | 3 (2.5%) | 30–39 | 6 (8.2%) | 14 (12%) | ||
| Face | 42 (58%) | 77 (64%) | 40–49 | 4 (5.5%) | 12 (9.9%) | ||
| Feet-Leg | 8 (11%) | 11 (9.1%) | 50–59 | 5 (6.8%) | 10 (8.3%) | ||
| Hand-Arm | 21 (29%) | 30 (25%) | 60+ | 6 (8.2%) | 11 (9.1%) | ||
| Lesion Appearance | >0.9 | Lesion size | 0.8 | ||||
| Dry | 71 (97%) | 118 (98%) | <1 cm | 6 (8.2%) | 10 (8.3%) | ||
| Wet | 2 (2.7%) | 3 (2.5%) | 1–3 cm | 56 (77%) | 97 (80%) | ||
| 4–5 cm | 11 (15%) | 14 (12%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alraey, Y.; Alhweti, R.; Almutairi, H.; Abdullah Al-Qahtani, A.; Alshahrani, M.I.; Asiri, M.H.; Alhammas, A.M.; Alwagdi, S.J.; Alshahrani, A.; Alouffi, A.; et al. Molecular Characterization of Leishmania Species among Patients with Cutaneous Leishmaniasis in Asir Province, Saudi Arabia. Pathogens 2022, 11, 1472. https://doi.org/10.3390/pathogens11121472
Alraey Y, Alhweti R, Almutairi H, Abdullah Al-Qahtani A, Alshahrani MI, Asiri MH, Alhammas AM, Alwagdi SJ, Alshahrani A, Alouffi A, et al. Molecular Characterization of Leishmania Species among Patients with Cutaneous Leishmaniasis in Asir Province, Saudi Arabia. Pathogens. 2022; 11(12):1472. https://doi.org/10.3390/pathogens11121472
Chicago/Turabian StyleAlraey, Yasser, Rasha Alhweti, Hatim Almutairi, Abdulrahman Abdullah Al-Qahtani, Mohammed Ibrahim Alshahrani, Mohammed Hussin Asiri, Abdulrhman Mousa Alhammas, Saeed Jubran Alwagdi, Abdulaziz Alshahrani, Abdulaziz Alouffi, and et al. 2022. "Molecular Characterization of Leishmania Species among Patients with Cutaneous Leishmaniasis in Asir Province, Saudi Arabia" Pathogens 11, no. 12: 1472. https://doi.org/10.3390/pathogens11121472
APA StyleAlraey, Y., Alhweti, R., Almutairi, H., Abdullah Al-Qahtani, A., Alshahrani, M. I., Asiri, M. H., Alhammas, A. M., Alwagdi, S. J., Alshahrani, A., Alouffi, A., Madkhali, A. M., Al-Salem, W. S., Al-Qahtani, A. A., Saif, A., Ben Hadj Ahmed, S., & Zhioua, E. (2022). Molecular Characterization of Leishmania Species among Patients with Cutaneous Leishmaniasis in Asir Province, Saudi Arabia. Pathogens, 11(12), 1472. https://doi.org/10.3390/pathogens11121472







