Abstract
Toxoplasma gondii, a major zoonotic pathogen distributed worldwide, causes severe infections in humans, animals, and birds. However, limited information is available regarding T. gondii infection in livestock in the Republic of Korea (ROK). Herein, we determined the prevalence of T. gondii infection in livestock in the ROK and identified animal species that can potentially transmit T. gondii to humans. B1 gene-targeting nested polymerase chain reaction detected T. gondii DNA in 3.3% (2/61), 2.9% (3/105), 14.1% (11/78), and 15.4% (14/91) of dairy cattle, beef cattle, Boer goats, and Korean native goats, respectively. The prevalence of T. gondii was significantly higher (p = 0.002) in goats than in cattle. The risk of contracting T. gondii infection was significantly higher by 6.18-fold in Korean native goats (95% confidence interval [CI]: 1.72–22.27%, p = 0.005) and by 5.58-fold in Boer goats (95% CI: 1.50–20.76%, p = 0.010) than in beef cattle. Our T. gondii DNA sequences exhibited 97.1–100% homology with those obtained from various hosts in other countries. To the best of our knowledge, this is the first study to report T. gondii infection using the blood samples of domestic ruminants in the ROK. The results revealed that the prevalence of T. gondii infection is higher in goats than in cattle as determined by molecular detection. Thus, these findings suggest that T. gondii can be transmitted from ruminants to humans via meat consumption.
1. Introduction
Toxoplasma gondii is an obligate intracellular parasite that can infect a wide range of host species [1]. According to the Food and Agriculture Organization of the United Nations, T. gondii constitutes the fourth leading cause of foodborne diseases and is a significant global public health concern [2]. Cats and wild felids comprise the only definitive hosts that excrete oocysts in their feces, whereas humans and other animals serve as intermediate hosts [3]. Toxoplasma gondii infection is primarily transmitted to intermediate hosts via the ingestion of tissue cysts from undercooked or raw meat, consumption of food and water contaminated with oocysts, or transplacental transmission [1,3,4,5].
The manifestations of T. gondii infection in humans range from asymptomatic conditions to chronic diseases, and include: abortion; severe congenital abnormalities, such as cerebral calcification, blindness, microcephaly, and seizure disorders; severe neuromuscular complications; pneumonia; and even death, in immunocompromised individuals and newborns [4,6,7,8]. Sheep and goats are more susceptible to T. gondii infection than cattle [9]. In small ruminants, T. gondii infection causes reproductive failures, such as stillbirths, abortion, fetal abnormalities, and postnatal mortality [8,10,11,12], consequently leading to economic losses in the livestock industry. Conversely, natural infection of cattle with T. gondii does not usually result in clinical symptoms or abortion [13]. However, cattle play important roles in the transmission of T. gondii infection to humans via the consumption of raw milk or of raw or undercooked meat, as these parasites leave the bloodstream and develop cysts in the muscle [13,14].
Serological tests and molecular-based methods have been primarily used to detect T. gondii infection. Serological assays include a modified agglutination test, indirect fluorescent antibody test, and enzyme-linked immunosorbent assay [15,16,17]. However, it is difficult to ascertain whether seropositivity indicates the presence of infective tissue cysts in various animal species and thus the risk of human infection. Conversely, among molecular-based techniques, polymerase chain reaction (PCR) targeting different genetic markers, including the glycerol-3-phosphate dehydrogenase (B1), internal transcribed spacer (ITS-1), and 18S rDNA sequences, has been employed to detect T. gondii infection [18,19,20]. This method enables the early diagnosis of toxoplasmosis; however, the disadvantage is that it amplifies the DNA of both live and dead parasites [21].
Toxoplasma gondii has been classified into three genetic lineages based on its virulence: types I, II, and III [22]. The distribution of T. gondii varies with geographical region and host. Type I is more virulent in mice than types II and III [22,23,24,25]. Types II and III are more common in animal samples than type I, whereas type I and type I-like atypical isolates are more likely to cause severe retinochoroiditis [26] and disseminated toxoplasmosis, respectively, in immunocompetent patients [27].
Studies in other countries have been mainly conducted with serum samples of small ruminants and the prevalence of T. gondii has been reported to be relatively high among the ovine population [3,6,28,29,30,31,32]. However, compared with other Asian countries, scarce information is available regarding T. gondii infection in livestock in the Republic of Korea (ROK). Considering that meat-producing animals constitute an important source of human infection, there is an urgent need to monitor the infection rate of T. gondii in various livestock species. Therefore, this study aimed to determine the prevalence of T. gondii in the blood of livestock in the ROK using the B1 gene and to identify the animal species that can potentially transmit this infection to humans.
2. Materials and Methods
2.1. Blood Collection
From October 2014 to June 2017, 335 blood samples were collected from healthy dairy cattle (n = 61), beef cattle (n = 105), Korean native goats (n = 91), and Boer goats (n = 78) on randomly selected farms in five different regions (Kangwon, Chungbuk, Jeonbuk, and Jeonnam provinces and Jeju Island) of the ROK (Figure 1). Each blood sample was collected in a collection tube with anticoagulant (BD Vacutainer®, Franklin Lakes, NJ, USA) and immediately delivered to the laboratory.
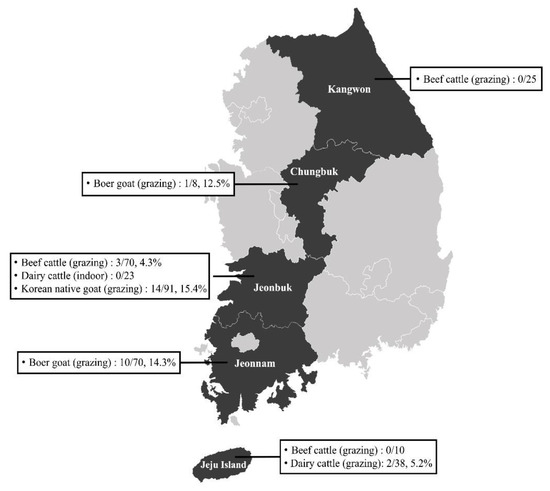
Figure 1.
Map of the farms selected for sample collection in the Republic of Korea.
2.2. DNA Extraction, Genomic Detection, and Sequencing
DNA was extracted from 200 μL of each blood sample using DNeasy Blood Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. The extracted DNA was stored at −80 °C until use. Toxoplasma gondii was screened via nested PCR targeting the highly conserved B1 gene under the following conditions: 94 °C for 5 min; followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 52 °C for 1 min, and extension at 72 °C for 1 min; and a final extension step at 72 °C for 10 min [33]. Toxoplasma gondii DNA was provided by Dr. Young-Ha Lee (School of Medicine, Chungnam National University) and was used as positive DNA when the T. gondii DNA was amplified from the samples. For all the reactions, negative and positive controls were included. The amplified PCR products (530 bp) were separated via electrophoresis on a 1.5% agarose gel and visualized after staining with ethidium bromide.
2.3. Sequencing and Phylogenetic Tree
All secondary PCR products were purified using AccuPower PCR Purification Kit (Bioneer, Daejeon, ROK) and used for direct sequencing (Macrogen, Daejeon, ROK). PCR amplicons were sequenced using BigDye Terminator 3.1 Cycle Sequencing Kit on a 3500 xL Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions with the same primer set that was used for conventional PCR. The nucleotide sequences obtained in this study were aligned with those in the GenBank database using ClustalX (v.2.0) and compared with global references for T. gondii. Phylogenetic analysis was performed via the maximum-likelihood method implemented in MEGA11 using the best substitution model. Bootstrap values were calculated by analyzing 1000 replicates to evaluate the reliability of the clusters. The model used in this study was Jukes Cantor (JC) + G + I. The nucleotide sequences obtained herein were deposited in the GenBank database under the accession numbers ON648716−ON648733.
2.4. Statistical Analysis
Exact confidence intervals (CIs) for prevalence at the 95% level were calculated. Data were analyzed using SPSS Statistics 25 software package for Windows (SPSS Inc., Chicago, IL, USA). Chi-square test (χ2) was used to determine any association between the prevalence of T. gondii and risk of infection in animal species. Univariate binary logistic regression analysis was performed to evaluate the association of T. gondii infection and species. The odds ratio and 95% CI were calculated to determine the probability of association detection. A p-value of ≤0.05 was considered statistically significant.
3. Results
3.1. Prevalence of T. gondii in Ruminants
Among the 335 ruminant blood samples examined, 30 were positive for T. gondii, with an overall prevalence of 9.0% (95% CI: 5.9–12.0%), based on PCR analysis targeting the B1 gene. The prevalence of T. gondii was the highest in Korean native goats (15.4%) and the lowest in beef cattle (2.9%; Table 1). Furthermore, the prevalence of T. gondii was significantly higher (p = 0.002; Table 1) in goats (14.8%, 25/169) than in cattle (3.0%, 5/166). The risk factor associated with T. gondii infection in animal species was determined using univariate binary logistic regression analysis (Table 1). Korean native goats and Boer goats were at higher risk of T. gondii infection than cattle. The risk of contracting T. gondii infection was significantly higher by 6.18-fold in Korean native goats (95% CI: 1.72–22.27%, p = 0.005) and by 5.58-fold in Boer goats (95% CI: 1.50–20.76%, p = 0.010) than in beef cattle. Statistical analysis revealed that dairy cattle were not at higher risk of T. gondii infection than beef cattle.

Table 1.
Prevalence of T. gondii infection among ruminants.
3.2. Sequence and Phylogenetic Analysis
From the 30 positive samples, 18 good amplicons were obtained. The amplicons sequenced from beef cattle, dairy cattle, Boer goats, and Korean native goats were 3 (100%), 1 (50%), 3 (27.3%), and 11 (78.6%), respectively (Table 1). The T. gondii sequences in this study exhibited 96.5–99.8% homology with each other (Table S1). The 18 sequences were compared with those from various hosts reported in other countries, including sheep, goats, cows, humans, ticks, cats, birds, water, and mussels, showing a relatively high nucleotide sequence homology of 97.1–100% (Table S1). The sequences obtained in this study exhibited 97.6–99.8% and 97.4–99.4% homology with those obtained from cat feces and rabbits, respectively, which were previously reported in Korea (Table S1). Phylogenetic analysis revealed that these 18 sequences formed one cluster with other T. gondii strains (Figure 2), exhibiting no discrimination according to types. The sequences of the reference strains RH (type I), PRU (type II), and VEG (type III) were extremely similar, with differences in only two nucleotides (data not shown).

Figure 2.
Phylogenetic tree based on the B1 gene of T. gondii constructed via the maximum-likelihood method using the JC + G + I model. The numbers over the branches indicate bootstrap values as a percentage of 1000 replicates that support each phylogenetic branch. Only bootstrap values exceeding 70% are presented. The sequences marked with circles and red boldface type were determined in this study.
4. Discussion
To the best of our knowledge, this is the first study to detect T. gondii infection in blood samples collected from domestic livestock in the ROK. Based on our findings, the infection rate of T. gondii was higher in goats (14.8%) than in cattle (3.0%). Our prevalence results in goats were consistent with those reported in Iran (11.7%), Israel (11.6%), and Algeria (18.68%) [16,29,34], whereas the prevalence of T. gondii in cattle in this study was relatively lower than that reported in Egypt (13.46%), Iran (56%), Israel (7.5%) Pakistan (12.2%), Poland (10.2%), and Tunisia (19.3%) [4,6,13,29,35,36]. The reason for this difference in cattle remains unclear. However, it could be due to the difference in the sample sources used to detect T. gondii. Although blood is generally used for serological analysis, other studies frequently use tissue samples, such as those of the brain, diaphragm, heart, liver, muscle, and tongue for T. gondii detection in cattle [4,29,35,36]. A previous study reported that T. gondii diagnosis using blood samples can yield different results, explaining that infection of host with tachyzoites, bradyzoites, and sporozoites considerably affects the time of blood infection and its persistence [37]. Moreover, the presence of the parasite at the tachyzoite stage represents acute phase infection [37]. Considering that the vast majority of T. gondii infections in numerous host animals, including the animals investigated in this study, were asymptomatic, these animals were considered a potential source of T. gondii infection to humans. Therefore, these results highlight the importance of the early detection of T. gondii and the need for a surveillance program to prevent human infections.
In this study, the prevalence of T. gondii in cattle in the ROK was lower than that reported in Egypt (13.5%) [6], Iran (56%) [4], and Pakistan (12.2%) [13] based on molecular analysis. The low prevalence may be attributed to different geographical locations and management systems (breeding, feeding, and water supply source) for animal production. The cattle used in this study were primarily grazing in a pasture. Previous studies have reported that grazing increases the risk of exposure to T. gondii in cattle [38,39]. Pasture contaminated with oocysts due to cats constitutes the primary transmission route of T. gondii for cattle [38]. However, our findings were inconsistent with those reported in previous studies [38,39]. At this point, we cannot explain the exact reason for this low prevalence. It is speculated that cats would not have had much access to the pastures, thereby leading to less oocyst contamination of the pastures. Moreover, according to our results, there was no significant difference in the prevalence of T. gondii between beef and dairy cattle (Table 1). Although no precise conclusions can be drawn due to the limited number of samples, the breeding system in the current study did not appear to significantly affect T. gondii infection. Furthermore, it is uncertain whether the prevalence of T. gondii in cattle was low only in the areas examined or if the infection rate was generally low in the ROK. Therefore, large-scale epidemiological studies are warranted to determine the association between the prevalence of T. gondii and management or cattle breeding systems.
In the ROK, goat meat is considered a health supplement, and thus, the number of goats raised has been increasing [40]. Small ruminants are intermediate hosts of T. gondii and are highly susceptible [41]; even adult goats die of toxoplasmosis [42]. Unlike cattle, goats often graze on grass close to the soil and feed on shrub leaves [34], which increase their vulnerability to T. gondii infection. The high prevalence of T. gondii in goats reported herein is closely related to management systems (contact with other animals or contamination of soil). Thus, this raises the possibility that there are more cats in goat farms than in cattle farms. The presence of cats in the barn increases the risk of exposure to T. gondii through the dispersal of millions of oocysts into the environment, especially soil; this results in oocysts contaminating the soil, which increases T. gondii infection [43,44]. Toxoplasma gondii is a major pathogen that causes abortion and neonatal mortality in goats [45], leading to significant economic loss. Nevertheless, its importance has been neglected in the ROK. Consequently, our results suggest that goats may serve as a major source of T. gondii transmission to humans owing to the steady increase in meat consumption. Therefore, it is necessary to understand the significance of this infection in goats, its impact on public health, and its control.
In this study, we detected T. gondii infection by amplifying and sequencing the B1 gene. The B1 is a multicopy, highly conserved gene and is used for detecting T. gondii DNA from various samples [26,46]. The advantage of this gene is its high specificity and sensitivity, facilitating the detection of 50 fg T. gondii DNA [20,26]. Although the number of parasites was not estimated in this study as qPCR was not performed, the results are indicative of the infection status in animals, suggesting that the PCR procedure targeting the B1 gene is suitable for the routine detection of T. gondii. In addition, compared with the serological test results, the PCR results indicate active infection, which can help establish effective control strategies for T. gondii.
Currently, to the best of our knowledge, there is no information available for the genetic characterization of T. gondii in livestock in the ROK. Based on the phylogenetic analysis of the B1 gene, the sequences detected in this study belonged to the same group, with samples including types I and III. A limitation of this study is that a more accurate genotype of T. gondii could not be revealed because genotype analysis was not performed. Further studies are warranted to identify the sources (cat, rodents, food, water, or wildlife access to farms) of T. gondii infection, specific genotypes of T. gondii circulating in the ROK, and association between pathogenicity and genotype.
5. Conclusions
This study revealed the genomic detection of T. gondii in domestic ruminants in the ROK. Our findings demonstrated that the prevalence of T. gondii infection is higher in goats than in cattle, indicating that T. gondii infection may be affected by the rearing system of the animals. The detection of T. gondii in the blood suggests that the meat of domestic ruminants is a potential source of T. gondii infection transmission to humans. The results of this study will aid in establishing a strategy to control and manage T. gondii infection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens12040547/s1, Table S1: Comparison of nucleotide sequence homology between T. gondii from other animals and the sequences obtained in this study.
Author Contributions
Conceptualization, K.-S.C.; methodology, K.-S.C.; formal analysis, M.-J.J. and D.-H.J.; investigation, M.-J.J., H.-C.C. and Y.-J.P.; sampling, J.P.; data curation, K.-S.C.; writing, H.-C.C. and K.-S.C.; visualization, H.-C.C. and K.-S.C.; funding acquisition, K.-S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF), which is funded by the Mid-Career Research Program (grant No. NRF-2021R1A2C1011579). The funders had no role in any aspect of study design, data collection and analysis, or decisions with respect to publication.
Institutional Review Board Statement
All animal procedures were performed according to the ethics guidelines for the use of animal samples as approved by the Jeonbuk National University (Institutional Animal Care and Use Committee decision No. CBU 2014-00026). All procedures and possible consequences were explained to the surveyed farm owners or/managers. All procedures in this study were conducted in accordance with the ARRIVE guidelines.
Informed Consent Statement
Informed written consent to collect blood samples was obtained from cattle owners.
Data Availability Statement
The datasets generated during the current study are available in the GenBank repository at Banklt ID 2588636 [https://https://www.ncbi.nlm.nih.gov/WebSub/?form=history&tool=genbank, accessed on 22 February 2023], accession numbers ON648716–ON648733. These accession numbers will be released after 6 months.
Acknowledgments
We thank Young-Ha Lee (School of Medicine, Chungnam National University) for providing us with T. gondii DNA.
Conflicts of Interest
The authors declare that they have no competing interests.
Abbreviations
| CI | confidence interval |
| PCR | polymerase chain reaction |
| ROK | Republic of Korea |
| T. gondii | Toxoplasma gondii |
References
- Dubey, J.P. Toxoplasmosis–A waterborne zoonosis. Vet. Parasitol. 2004, 126, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, P.R.; Devleesschauwer, B.; Praet, N.; Speybroeck, N.; Willingham, A.L.; Kasuga, F.; Rokni, M.B.; Zhou, X.N.; Fevre, E.M.; Sripa, B.; et al. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: A data synthesis. PLoS Med. 2015, 12, e1001920. [Google Scholar] [CrossRef]
- Jilo, K.; Tegegne, D.; Kasim, S.; Dabasa, G.; Zewdei, W. Seroprevalence and public health significance of toxoplasmosis in small ruminants of pastoral community in Yabello district, Borana zone, southern Ethiopia. Vet. Med. Int. 2021, 2021, 6683797. [Google Scholar] [CrossRef] [PubMed]
- Fazel, R.; Rezanezhad, H.; Solhjoo, K.; Kalantari, M.; Erfanian, S.; Armand, B.; Jahromi, M.E. PCR-based detection of Toxoplasma gondii from cattle in southern Iran. Comp. Immunol. Microbiol. Infect. Dis. 2021, 77, 101677. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.E.; Dubey, J.P.; Baroch, J.A.; Swafford, S.R.; Fournet, V.F.; Hawkins-Cooper, D.; Pyburn, D.G.; Schmit, B.S.; Gamble, H.R.; Pedersen, K.; et al. Surveillance of feral swine for Trichinella spp. and Toxoplasma gondii in the USA and host-related factors associated with infection. Vet. Parasitol. 2014, 205, 653–665. [Google Scholar] [CrossRef]
- Khattab, R.A.; Barghash, S.M.; Mostafa, O.M.S.; Allam, S.A.; Taha, H.A.; Ashour, A.A.E. Seroprevalence and molecular characterization of Toxoplasma gondii infecting ruminants in the north-west of Egypt. Acta Trop. 2022, 225, 106139. [Google Scholar] [CrossRef]
- Robert-Gangneux, F.; Darde, M.L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef]
- Weiss, L.M.; Dubey, J.P. Toxoplasmosis: A history of clinical observations. Int. J. Parasitol. 2009, 39, 895–901. [Google Scholar] [CrossRef]
- Dubey, J.P. Toxoplasmosis in sheep--the last 20 years. Vet. Parasitol. 2009, 163, 1–14. [Google Scholar] [CrossRef]
- Alemayehu, G.; Mamo, G.; Alemu, B.; Desta, H.; Tadesse, B.; Benti, T.; Bahiru, A.; Yimana, M.; Wieland, B. Causes and flock level risk factors of sheep and goat abortion in three agroecology zones in Ethiopia. Front. Vet. Sci. 2021, 8, 615310. [Google Scholar] [CrossRef]
- Engeland, I.V.; Waldeland, H.; Kindahl, H.; Ropstad, E.; Andresen, O. Effect of Toxoplasma gondii infection on the development of pregnancy and on endocrine foetal-placental function in the goat. Vet. Parasitol. 1996, 67, 61–74. [Google Scholar] [CrossRef]
- Suazo-Cortez, R.; Martinez-Herrera, D.I.; Pardio-Sedas, V.T.; Cruz-Vazquez, C.R.; Morales-Alvarez, J.F.; Sanchez-Viveros, G.; Galindo-Tovar, M.E. Seroprevalence and risk factors associated with Toxoplasma gondii infection in sheep of Veracruz state, southeast Mexico. Vet. Res. Forum 2020, 11, 77–81. [Google Scholar] [CrossRef]
- Taalay, I.; Iqbal, R.K.; Asif, M.; Ahmad, A.; Amjad, M.; Anwar, F.N.; Aktas, M.; Ben Said, M.; Iqbal, F. Molecular survey of Toxoplasma gondii in cattle and buffaloes and phylogenetic position of Pakistani isolates based on ITS-1 gene. Comp. Immunol. Microbiol. Infect. Dis. 2022, 84, 101782. [Google Scholar] [CrossRef]
- Opsteegh, M.; Dam-Deisz, C.; de Boer, P.; DeCraeye, S.; Fare, A.; Hengeveld, P.; Luiten, R.; Schares, G.; van Solt-Smits, C.; Verhaegen, B.; et al. Methods to assess the effect of meat processing on viability of Toxoplasma gondii: Towards replacement of mouse bioassay by in vitro testing. Int. J. Parasitol. 2020, 50, 357–369. [Google Scholar] [CrossRef]
- Sun, T.; Rahman, S.U.; Cai, J.; Zeng, J.; Mi, R.; Zhang, Y.; Gong, H.; Ma, H.; Huang, Y.; Han, X. Seroprevalence and associated risk factors of Toxoplasma gondii infection in yaks (Bos grunniens) on the Qinghai–Tibetan Plateau of China. Parasite 2021, 28, 43. [Google Scholar] [CrossRef]
- Bahreh, M.; Hajimohammadi, B.; Eslami, G. Toxoplasma gondii in sheep and goats from central Iran. BMC Res. Notes 2021, 14, 46. [Google Scholar] [CrossRef]
- Aziz, M.N.; Iqbal, R.K.; Irfan, M.; Parveen, A.; Asif, M.; Ozubek, S.; Aktas, M.; Said, M.B.; Iqbal, F. First report on molecular epidemiology, seasonality and phylogeny of Toxoplasma gondii infecting goats from Khanewal district in Punjab, Pakistan. Acta Trop. 2022, 228, 106304. [Google Scholar] [CrossRef]
- Costa, J.M.; Cabaret, O.; Moukoury, S.; Bretagne, S. Genotyping of the protozoan pathogen Toxoplasma gondii using high-resolution melting analysis of the repeated B1 gene. J. Microbiol. Methods 2011, 86, 357–363. [Google Scholar] [CrossRef]
- Homan, W.L.; Limper, L.; Verlaan, M.; Borst, A.; Vercammen, M.; van Knapen, F. Comparison of the internal transcribed spacer, ITS 1, from Toxoplasma gondii isolates and Neospora caninum. Parasitol. Res. 1997, 83, 285–289. [Google Scholar] [CrossRef]
- Jones, C.D.; Okhravi, N.; Adamson, P.; Tasker, S.; Lightman, S. Comparison of PCR detection methods for B1, P30, and 18S rDNA genes of T. gondii in aqueous humor. Investig. Ophthalmol. Vis. Sci. 2000, 41, 634–644. [Google Scholar]
- Edvinsson, B.; Lappalainen, M.; Evengard, B.; Toxoplasmosis, E.S.G.f. Real-time PCR targeting a 529-bp repeat element for diagnosis of toxoplasmosis. Clin. Microbiol. Infect. 2006, 12, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Howe, D.K.; Sibley, L.D. Toxoplasma gondii comprises three clonal lineages: Correlation of parasite genotype with human disease. J. Infect. Dis. 1995, 172, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.G. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J. Infect. Dis. 2002, 185, S73–S82. [Google Scholar] [CrossRef] [PubMed]
- Mammari, N.; Halabi, M.A.; Yaacoub, S.; Chlala, H.; Darde, M.L.; Courtioux, B. Toxoplasma gondii modulates the host cell responses: An overview of apoptosis pathways. Biomed. Res. Int. 2019, 2019, 6152489. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Escobar, M.; Calero-Bernal, R.; Regidor-Cerrillo, J.; Vallejo, R.; Benavides, J.; Collantes-Fernandez, E.; Ortega-Mora, L.M. Isolation, genotyping, and mouse virulence characterization of Toxoplasma gondii from free ranging Iberian pigs. Front. Vet. Sci. 2020, 7, 604782. [Google Scholar] [CrossRef]
- Grigg, M.E.; Boothroyd, J.C. Rapid identification of virulent type I strains of the protozoan pathogen Toxoplasma gondii by PCR-restriction fragment length polymorphism analysis at the B1 gene. J. Clin. Microbiol. 2001, 39, 398–400. [Google Scholar] [CrossRef]
- Bossi, P.; Bricaire, F. Severe acute disseminated toxoplasmosis. Lancet 2004, 364, 579. [Google Scholar] [CrossRef]
- Cenci-Goga, B.T.; Ciampelli, A.; Sechi, P.; Veronesi, F.; Moretta, I.; Cambiotti, V.; Thompson, P.N. Seroprevalence and risk factors for Toxoplasma gondii in sheep in Grosseto district, Tuscany, Italy. BMC Vet. Res. 2013, 9, 25. [Google Scholar] [CrossRef]
- Mazuz, M.L.; Weiss, A.; Beer, O.; Tirosh-Levy, S.; Riklis, I.; Dveyrin, Z.; Rorman, E.; Cohen, N.Z.; Markovich, M.P.; Baneth, G. High infection rates of Toxoplasma gondii in cattle, sheep and pigs from Israel. Comp. Immunol. Microbiol. Infect. Dis. 2023, 92, 101928. [Google Scholar] [CrossRef]
- Dessi, G.; Tamponi, C.; Pasini, C.; Porcu, F.; Meloni, L.; Cavallo, L.; Sini, M.F.; Knoll, S.; Scala, A.; Varcasia, A. A survey on Apicomplexa protozoa in sheep slaughtered for human consumption. Parasitol. Res. 2022, 121, 1437–1445. [Google Scholar] [CrossRef]
- Mancianti, F.; Nardoni, S.; Mugnaini, L.; Poli, A. Toxoplasma gondii in waterfowl: The first detection of this parasite in Anas crecca and Anas clypeata from Italy. J. Parasitol. 2013, 99, 561–563. [Google Scholar] [CrossRef]
- Sharif, M.; Sarvi, S.; Shokri, A.; Hosseini Teshnizi, S.; Rahimi, M.T.; Mizani, A.; Ahmadpour, E.; Daryani, A. Toxoplasma gondii infection among sheep and goats in Iran: A systematic review and meta-analysis. Parasitol. Res. 2015, 114, 1–16. [Google Scholar] [CrossRef]
- Alfonso, Y.; Fraga, J.; Jimenez, N.; Fonseca, C.; Dorta-Contreras, A.J.; Cox, R.; Capo, V.; Bandera, F.; Pomier, O.; Ginorio, D. Detection of Toxoplasma gondii in cerebrospinal fluid from AIDS patients by nested PCR and rapid identification of type I allele at B1 gene by RFLP analysis. Exp. Parasitol. 2009, 122, 203–207. [Google Scholar] [CrossRef]
- Ait Issad, N.; Abdelouahed, K.; Bekhouche, S.; Boubeuker, R.; Hamoudi Adjmi, H.; Ouchene-Khelifi, N.A.; Ouchene, N.; Ait Oudhia, K.; Khelef, D. Molecular detection of the B1 gene of Toxoplasma gondii in blood samples of female sheep and goats in Tebessa, northeastern Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2020, 72, 101530. [Google Scholar] [CrossRef]
- Amdouni, Y.; Rjeibi, M.R.; Rouatbi, M.; Amairia, S.; Awadi, S.; Gharbi, M. Molecular detection of Toxoplasma gondii infection in slaughtered ruminants (sheep, goats and cattle) in northwest Tunisia. Meat Sci. 2017, 133, 180–184. [Google Scholar] [CrossRef]
- Sroka, J.; Karamon, J.; Wojcik-Fatla, A.; Piotrowska, W.; Dutkiewicz, J.; Bilska-Zajac, E.; Zajac, V.; Kochanowski, M.; Dabrowska, J.; Cencek, T. Toxoplasma gondii infection in slaughtered pigs and cattle in Poland: Seroprevalence, molecular detection and characterization of parasites in meat. Parasit. Vectors 2020, 13, 223. [Google Scholar] [CrossRef]
- Tavassoli, M.; Ghorbanzadehghan, M.; Esmaeilnejad, B. Detection of Toxoplasma gondii in sheep and goats blood samples by PCR-RFLP in Urmia. Vet. Res. Forum 2013, 4, 43–47. [Google Scholar]
- Fajardo, H.V.; D’ávila, S.; Bastos, R.R.; Cyrino, C.D.; de Lima Detoni, M.; Garcia, J.L.; das Neves, L.B.; Nicolau, J.L.; Amendoeira, M.R. Seroprevalence and risk factors of toxoplasmosis in cattle from extensive and semi-intensive rearing systems at Zona da Mata, Minas Gerais state, southern Brazil. Parasit. Vectors 2013, 6, 191. [Google Scholar] [CrossRef]
- Magalhaes, F.J.; Ribeiro-Andrade, M.; Alcantara, A.M.; Pinheiro, J.W.J.; Sena, M.J.; Porto, W.J.; Vieira, R.F.; Mota, R.A. Risk factors for Toxoplasma gondii infection in sheep and cattle from Fernando de Noronha island, Brazil. Rev. Bras. Parasitol. Vet. 2016, 25, 511–515. [Google Scholar] [CrossRef]
- Seo, M.G.; Kwon, O.D.; Kwak, D. Molecular and phylogenetic analysis of tick-borne pathogens in ticks parasitizing native Korean goats (Capra hircus coreanae) in South Korea. Pathogens 2020, 9, 71. [Google Scholar] [CrossRef]
- Su, Y.J.; Ma, Z.D.; Qiao, X.; Wang, P.T.; Kang, Y.T.; Yang, N.A.; Jia, W.; Zhao, Z.J. Geospatial epidemiology of Toxoplasma gondii infection in livestock, pets, and humans in China, 1984–2020. Parasitol. Res. 2022, 121, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cezar, C.K.; Kwok, O.C.H. Public health and economic importance of Toxoplasma gondii infections in goats: The last decade. Res. Vet. Sci. 2020, 132, 292–307. [Google Scholar] [CrossRef]
- Silva, B.M.; Queiroz, W.C.C.; Maia, M.O.; Pacheco, R.C.; Aguiar, D.M.; Campos, M.S.; Bresciani, K.D.S.; Costa, A.J.; Gomes, A.A.D.; Santos-Doni, T.R. Seroprevalence and risk factors of Toxoplasma gondii in cattle from Unai, Minas Gerais state, Brazil. Vet. Parasitol. Reg. Stud. Rep. 2021, 25, 100610. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodriguez, L.C.; Tafur-Gómez, G.A.; Guzman-Barragan, B.L. Toxoplasma gondii in small ruminants in northeastern areas of Colombia: Seroprevalence and risk factors. Parasite Epidemiol. Control. 2020, 10, e00147. [Google Scholar] [CrossRef] [PubMed]
- Tzanidakis, N.; Maksimov, P.; Conraths, F.J.; Kiossis, E.; Brozos, C.; Sotiraki, S.; Schares, G. Toxoplasma gondii in sheep and goats: Seroprevalence and potential risk factors under dairy husbandry practices. Vet. Parasitol. 2012, 190, 340–348. [Google Scholar] [CrossRef]
- Switaj, K.; Master, A.; Skrzypczak, M.; Zaborowski, P. Recent trends in molecular diagnostics for Toxoplasma gondii infections. Clin. Microbiol. Infect. 2005, 11, 170–176. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).