Abstract
Background: Toxoplasma gondii and Neospora caninum are major protozoan parasites of worldwide distribution and significance in veterinary medicine and, for T. gondii, in public health. Cats and dogs, as final hosts for T. gondii and N. caninum, respectively, have a key function in environmental contamination with oocysts and, thus, in parasite transmission. Very little is known about the prevalence of T. gondii infections in dogs and cats in Egypt, and even less about the prevalence of N. caninum in the same hosts. Methods: In the current study, 223 serum samples of both dogs (n = 172) and cats (n = 51) were investigated for specific antibodies to T. gondii and N. caninum using commercially available ELISAs. A risk factor analysis was conducted to identify factors associated with seropositivity. Results & discussion: Exposure to T. gondii was reported in 23.3% of the dogs and in 9.8% of the cats, respectively. In addition, N. caninum-specific antibodies were recorded in 5.8% of dogs and in 3.4% of cats. A mixed infection was found in two dogs (1.2%) and in one cat (2%). Antibodies to T. gondii in dogs were significantly more frequent in dogs aged 3 years or more and in male German Shepherds. As this breed is often used as watchdogs and was the most sampled breed in Alexandria governorate, the purpose “watchdog” (compared to “stray” or “companion”), the male sex, and the governorate “Alexandria” also had a significantly higher seroprevalence for T. gondii. No factors associated with antibodies to N. caninum could be identified in dogs, and no significant factors were determined in cats for either T. gondii or N. caninum infection. Our study substantially adds to the knowledge of T. gondii infection in dogs and cats and presents data on N. caninum infection in cats for the first and in dogs in Egypt for the second time.
1. Introduction
Domestic dogs and cats as companion animals are important for the mental health of the owners by reducing stress and anxiety [1], and dogs are also kept as watchdogs to protect properties. In Egypt, however, stray ones heavily outnumber owned dogs and cats. Both dogs and cats, especially stray ones, play a significant role in the transmission and epidemiology of many infectious diseases, including Toxoplasma gondii and Neospora caninum [2,3,4]. Cats and dogs, as final hosts for T. gondii and N. caninum, respectively, have a key function in environmental contamination with oocysts and, thus, in parasite transmission.
Toxoplasma gondii and N. caninum are closely related intracellular protozoan parasites displaying high phenotypic and genotypic similarities [5]. Toxoplasmosis, caused by T. gondii, is a global disease affecting almost all endothermic animals, including humans [6]. In human medicine and in the veterinary industry, mainly in small ruminants, T. gondii causes great harm in the case of prenatal transmission resulting in abortions, stillbirths, and neonatal fatalities. Neosporosis is caused by N. caninum and affects a large number of warm-blooded animals. It causes enormous economic losses in the cattle industry by inducing abortion [7,8].
Both parasites, T. gondii and N. caninum undergo sexual as well as asexual reproduction. Sexual reproduction occurs only in the definitive hosts, i.e., in felids for T. gondii [9] and in canids such as dog [10], coyote [11], dingo [12], and grey wolf [13] for N. caninum, respectively, and results in the shedding of oocysts. Asexual multiplication occurs in many tissues and organs of intermediate hosts, including Felidae and Canidae, and results in the formation of tissue cysts and potentially in clinical signs [14]. Infection thus occurs either orally by ingestion of oocysts, raw or semi-cooked meat containing tissue cysts, or vertically from an infected mother to the fetus.
Several reviews indicate that T. gondii infections are common in cats worldwide [6,15,16]. While mostly asymptomatic in cats, fatal systemic infections, usually including pneumonitis, may occur [14,15,17]. In dogs, clinical toxoplasmosis is rare and usually linked to immunosuppression [14]. Neuromuscular disorders caused by T. gondii, in one case identified as clonal Type 1, were described in two dogs [18,19]. In addition, toxoplasmosis was reported as severe and life-threatening respiratory distress in an immunosuppressed dog [20].
Clinical neosporosis in dogs is an important disease; it most commonly manifests as paralytic neuromuscular signs or as reproductive problems in infected bitches [21]. In cats, natural clinical infections with N. caninum have not been reported [7].
Few studies have investigated the seroprevalence of either T. gondii or N. caninum in dogs and cats in Egypt. Three studies assessed seroprevalence for T. gondii in cats, and results varied between 38.7% and 97.4% [22,23,24]. For T. gondii in dogs, three studies reported seroprevalence rates between 28% and 98.0% [3,25,26]. Only one study so far investigated 29 Egyptian dogs for anti-N. caninum antibodies and found a prevalence of 27.6% [27]. No data are available for the seroprevalence of N. caninum in cats in Egypt. Moreover, information on the seroprevalence of both parasites in cats and dogs is still missing for many Egyptian regions, and risk factor analysis has not been attempted so far. Thus, our study investigated the seroprevalence of T. gondii and N. caninum in dogs and cats from different governorates representing various regions of Egypt. In addition, various variables were assessed to identify factors associated with both infections in dogs and cats.
2. Materials and Methods
2.1. Ethical Statement
This study was performed according to standard procedures identified by the “Research Board” of the Faculty of Veterinary Medicine, South Valley University, Qena, Egypt. The study was approved by the Research Code of Ethics at South Valley University number 36 (RCOE-36). Blood samples were collected by highly trained veterinarians and after the verbal consent of the animal owners to participate in the study.
2.2. Animal Population and Location
A total of 223 blood samples were collected from both dogs (n = 172) and cats (n = 51) from different governorates representing most Egyptian geographic and climatic regions (Table 1, Figure 1). In the case of dogs, 100 blood samples were collected between January 2021 and March 2022, among which 50 dogs were referred to the pet clinic in Kafr Elsheikh for routine vaccinations or treatment of different clinical disorders (companion dogs), and 50 dogs were sampled at the dog shelter in Giza (stray dogs). In addition, a total of 31 samples were obtained from animal care centers at Luxor governorate (n = 13) and Hurghada city of Red Sea governorate (n = 23) during the period from May 2019 to March 2020 (stray dogs). Moreover, 36 dogs used as watchdogs for private properties were sampled from July to September 2022 from Alexandria. The age of the sampled dogs ranged from 3 months to 11 years, with a mean of 2.9 years. The female-to-male ratio was 78:94. Regarding cats, a total of 51 samples were collected in a period between January 2021 and September 2022 from pet animal clinics and hospitals in Cairo (n = 24), Kafr Elsheikh (n = 15), Qena (n = 4), and Red Sea (n = 8) governorates (companion cats). Except for dogs and cats referred to the animal clinics, all other tested animals were apparently and clinically healthy. The age of the sampled cats ranged from 8 months to 5 years, with a mean of 2.5 years. The female-to-male ratio was 27:24.

Table 1.
Description of samples.
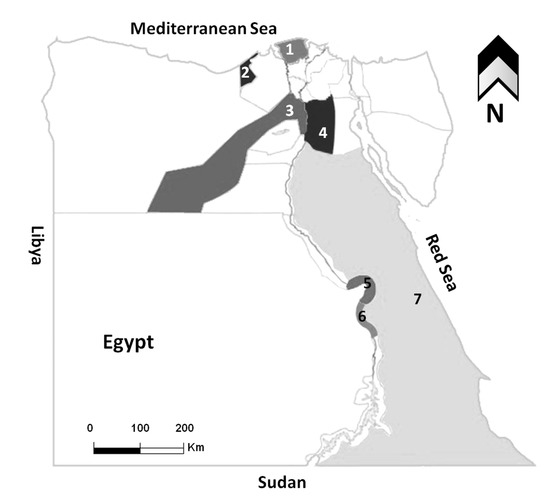
Figure 1.
Map of Egypt showing the governorates where samples were collected. Areas with different colors refer to the investigated governorates, 1; Kafr Elsheikh, 2; Alexandria, 3; Giza, 4; Cairo, 5; Qena, 6; Luxor, 7; Red Sea.
Variables such as age, sex, location, purpose (stray vs. companion vs. watchdog), and breeds were recorded for each sampled animal.
2.3. Sample Collection and Preparation
Blood samples were collected via puncture of the cephalic vein using glass tubes without anticoagulant, except for cats sampled in Cairo (n = 24), where EDTA blood was collected. All blood samples were kept in an icebox during transportation until separation, and serum and plasma samples, respectively, were stored at −20 °C until use.
2.4. ELISA Testing and Interpretation of Results
Serum samples from dogs and serum or plasma samples from cats were tested for anti-T. gondii and anti-N. caninum antibodies, respectively, using commercial Multi-species ELISA kits (ID Screen® Toxoplasmosis Indirect Multi-species and ID Screen® Neospora caninum Competition, both ID Vet, Grables, France). Positive and negative control sera were provided in the kits, and the tests were performed following the manufacturer’s instructions. The optical density (OD) of ELISA results was read at 450 nm and measured with an Infinite® F50/Robotic ELISA reader (Tecan Group Ltd., Männedorf, Switzerland).
The Toxoplasmosis kit detects specific immunoglobulin G (IgG) antibodies against the P30 T. gondii protein using a peroxidase-conjugated anti-multi-species secondary antibody. The percentage sample (S) to positive (P) ratio (S/P %) for each of the samples was calculated according to the following formula:
Samples with S/P% values greater than 50% were considered to be positive, those between 40 and 50% were classified as doubtful, and measurements less than or equal to 40% were considered to be negative as per the manufacturer.
The N. caninum kit detects specific antibodies against a purified N. caninum extract using an anti-N. caninumperoxidase-conjugated competing antibody. The percentage sample (S) to negative (N) ratio (S/N %) for each of the test samples was calculated according to the following formula:
Samples with S/N% values less than or equal to 50% were considered to be positive, those greater than 50% and less than or equal to 60% were classified as doubtful, and measurements greater than 60% were considered to be negative as per the manufacturer.
For both T. gondii and N. caninum test procedures, all samples were tested once except for the doubtful samples that were tested twice.
2.5. Statistical Analysis
The significance of the differences in the prevalence rates and risk factor analysis was assessed with Fisher exact probability test (two-tailed), 95% confidence intervals (including continuity correction), and odds ratios using an online statistical website www.vassarstats.net (accession dates; 15–17 April 2022) as described previously [28].
3. Results
Investigation of 172 dog sera revealed that 40 (23.3%) and 10 (5.8%) were seropositive for T. gondii and N. caninum, respectively (Table 2). Considering the different breeds investigated in the current study, the prevalence of T. gondii was 11/75 (14.7%) in native Baladi dogs, 21/47 (44.7%) in German Shepherds, 1/7 (14.3%) in Rottweiler, 1/5 (20%) in Doberman, 2/5 (40%) in a mixed breed, 1/7 (14.3%) in Pitbull, 1/4 (25%) in Belgian Malinois and Griffon Bruxellois, and 2/3 (66.7%) in Husky, respectively. In Boxer (n = 4), Golden Retriever (n = 7), mixed Pitbull (n = 1), Great Dane (n = 1), and Labrador (n = 3), no antibodies to T. gondii were detected (Table 3). For N. caninum antibodies, results were 6/75 (8%) seropositive animals in Baladi, 1/7 (14.3%) in Rottweiler, 1/3 (33.3%) in Husky, 1/7 (14.3%) in Golden Retrievers, and 1/1 (100%) in Great Dane, while all other tested breeds were seronegative (Table 3).

Table 2.
Seroprevalence of Toxoplasma gondii, Neospora caninum, and mixed infection.

Table 3.
Seroprevalence of Toxoplasma gondii and Neospora caninum in dogs and cats in relation to breeds.
In the case of the cat, an investigation of 51 samples revealed that 5 (9.8%) and 2 (3.9%) were seropositive for T. gondii and N. caninum, respectively (Table 2). Native Baladi cats, Persian, and Siamese cats were the most prevalent breeds in our study and among the cat population in Egypt. A seroprevalence of T. gondii of 0/12, 2/30 (6.7%), and 2/4 (50%) among Baladi, Persian, and Siamese cats, respectively, was demonstrated. Additionally, the only sampled British longhair tested positive for antibodies to T. gondii (100%). Antibodies to N. caninum were found in two cats only, one Baladi and one Siamese cat, respectively, resulting in a seroprevalence of 1/12 (8.3%) and 1/4 (25%) for each breed, respectively (Table 3). Regarding mixed infection, 2/172 dogs (1.2%) and 1/51 cats (2%) were demonstrated as seropositive for both T. gondii and N. caninum antibodies (Table 2).
Risk factor analysis was conducted to assess the influence of age, sex, purpose, geographical location, and breed on the seroprevalence of T. gondii in dogs and cats and N. caninum in dogs. All tested variables were identified as factors significantly associated with T. gondii infection in dogs (Table 4). Seroprevalence of T. gondii in dogs > 3 years old (40.4%) set as reference group was higher than that in 1–3 years old dogs (14.3%) (odds ratio [OR] = 0.2, p = 0.0009), and also than antibody level in younger dogs > 1 year old (20.7%) (OR = 0.4, p = 0.09). Moreover, the seropositive rate was significantly higher in male dogs (30.9%, OR = 2.7, p = 0.011) than in female ones (14.1%). Regarding breed, German Shepherd dogs exhibited a higher T. gondii seroprevalence (44.7%) than Baladi dogs (14.7%, OR = 0.2, p = 0.0006), or other breeds (16%, OR = 0.2, p = 0.0035) (Table 4).

Table 4.
Risk factors for Toxoplasma gondii antibodies in dogs.
When stray dogs as a reference (18.5%), a significantly higher seroprevalence was found in watchdogs (44.4%, OR = 3.5, p = 0.006), many of them German Shepherds (n = 30), but not for the companion dogs (16.4%, OR = 0.9, p = 0.821). Watchdogs were only sampled from Alexandria, and German Shepherds were overrepresented in the sample from this governorate. Thus, seroprevalence in Alexandria (44.4%, OR = 4.2, p = 0.007) was significantly higher than that in Giza (16%) set as reference. However, the differences were not significant in the case of Kafr Elsheikh (18%, OR = 1.2, p = 1), Luxor (30.8%, OR = 2.3, p = 0.249), or Red Sea (13%, OR = 0.8, p = 1), compared to Giza samples.
In order to analyze the effect of breed in more detail, three groups were created, namely native Baladi dogs (n = 75), German Shepherds (n = 47), and all other breeds (n = 50). In these smaller groups, age was no longer a significant risk factor for infection with T. gondii (Table 5). Male sex was retained as a risk factor in German Shepherds only (54.1%, OR = 10.6, p = 0.015) against female ones as a reference group (10%). Location and purpose were identified as associated factors in the “other breeds” group only, with companion dogs and dogs from Kafr Elsheikh having a lower seroprevalence (Table 5).

Table 5.
Risk factors assessment of Toxoplasma gondii infection in dogs in relation to breeds.
No risk factors were identified for N. caninum in dogs (Table S1) or for T. gondii in cats (Table S2). Risk factor analysis was not conducted for N. caninum in cats because of the low number of positive samples (2/43).
A summary of previous reports and their comparison with those in the current study on the seroprevalence of T. gondii and N. caninum in dogs and cats in Egypt is given in Table 6.

Table 6.
Previous reports on the prevalence of Toxoplasma gondii and Neospora caninum in dogs and cats in Egypt.
4. Discussion
In view of the paucity of available data for Egypt, the current study focused on the prevalence of T. gondii and N. caninum antibodies among dogs and cats. Very recent studies have demonstrated a high seroprevalence of T. gondii (46.1%) and a lower but still important seroprevalence of N. caninum (11.9%) in small ruminants in Egypt [29]. Moreover, cattle are affected by both parasites, with three in ten herds seropositive on bulk milk for N. caninum and one in ten seropositive for T. gondii [30]. Thus, the knowledge of the prevalence of these parasites in their final hosts is of utmost importance. In the present study, we used commercially available ELISAs to assess the seroprevalence in cats and dogs. The advantage of using commercial ELISAs in seroepidemiologic studies is that the results obtained should show little inter-laboratory variation and thus be widely comparable. However, this test also classified some samples as inconclusive, and it would have been nice to test them with a confirmatory test. Unfortunately, this was not possible in our study. Our study provides the first seroprevalence rate of N. caninum in cats in Egypt, and it was low at 3.4%. However, our study comprised companion cats only, and the sample size was quite small. Nevertheless, our study is one of the very few studies available on this topic worldwide and the first using the ID Screen Neospora ELISA in cats [7]. Therefore, we could not compare our results with other studies; neither could we shed light on the yet unknown risk factors for infection in cats [7]. Moreover, we were only the second group to investigate N. caninum antibodies in dogs in Egypt, after a lapse of about 20 years and on a considerably larger sample size. Compared with the earlier study, our seroprevalence of 5.8% was lower than the 27.6% obtained by El-Ghaysh et al. (2003) [27] using the direct agglutination test (DAT). However, our result was well in accordance with results obtained in other parts of the world using the same ID Screen Neospora ELISA: Dwinata et al. (2018) [31] obtained 3.4% in Indonesia, Villagra-Blanco et al. (2018) [32] 7.33% in Germany, and Lefkaditis et al. (2020) [33] 7.63% in Greece. It is interesting to see how little variation was detected in N. caninum seroprevalence in dogs when using the same method. A recent systematic review and meta-analysis of seroprevalence in the dog population worldwide established an overall seroprevalence of 23.31% in the Eastern Mediterranean region [34]. In this meta-analysis, the only significant associations with N. caninum infection in dogs were the continent, country, year, WHO regions, sample size, and diagnostic method used [34]. Consistently, no risk factors for N. caninum infection in dogs were observed in our study when analyzing the variables age, sex, breed, purpose, and governorate.
Few reports have investigated the seroprevalence of T. gondii in cats in Egypt, and information from Kafr Elsheikh and Cairo governorates has been entirely missing so far [22,23,24]. Our seropositive rate for T. gondii in cats (9.8%) was lower than that reported by Awad and Barakat (38.7%) (2019) [23] and Sherif et al. (54,2%) (2019) [24], both studies used rapid tests and Al-Kappany et al. (2010) [22] who reported 97.4% using a modified agglutination test (MAT) at a cut-off of 1:5, respectively. The ID Screen Toxoplasma ELISA we used was validated for use in cat serum and found to be equivalent to MAT with a cut-off of 1:40 [35]. We only found a few studies using the same ELISA in cats; these studies demonstrated a seroprevalence of 47% in owned cats in Romania [35] and of 21.93% in feral cats in Panama [36]. Our lower seroprevalence could thus be influenced by the test used, as well as by the studied population of sole companion cats. No risk factors were identified when analyzing age, sex, location, and breeds as predisposing factors for T. gondii infection in cats. This result was in concordance with Arruda et al. (2021) [37], who used IFAT, and referred to all tested variables. However, limited data on risk factors assessment of T. gondii infection in cats worldwide is available [15].
Arguably the most interesting results of our study concerned the seroprevalence of T. gondii in dogs in Egypt. Only three studies had investigated this topic, with a total of 176 dogs compared to the 172 included in our study, and focused on the greater Cairo region, namely Cairo and Giza governorates [3,25,26]. We now provided the first record for dogs of Kafr Elsheikh, Alexandria, and Luxor governorates. The seroprevalence of 23.3% for T. gondii in dogs obtained in our study was lower than the seroprevalences of 28%, 46.5%, and 98% reported by Khaled et al. (1982) [26], Rifaat et al. (1977) [25], and El Behairy et al. (2013) [3], respectively. These variations might be related to the different diagnostic tests used. The ID Screen Toxoplasma ELISA is also marketed for the use in dog sera and has been used for this purpose in studies from New Caledonia (Roqueplo et al., 2011; seroprevalence 32.8%) [38], from Grenada, West Indies (Sharma et al., 2014, seroprevalence 33.4%) [39], from the Philippines (Guy and Penuliar, 2016; seroprevalence 15.2%) [40], from Iran (Zarra-Nezhad et al., 2017; seroprevalence 46.67%) [41], from Panama (Fábrega et al., 2020; seroprevalence 25.70%) [36], and from Malaysia (Watanabe et al., 2020; seroprevalence 23.4%) [42]. Again, our results fit well within the rates obtained using the same diagnostic test.
In the risk factor analysis for T. gondii in dogs, we revealed that older and male dogs are more likely to be infected with T. gondii than younger and female dogs. In addition, location (Alexandria vs. other tested regions), purpose (watchdogs vs. stray or companion dogs), and breeds (German Shepherd vs. Baladi or other breeds) were identified as risk factors in our study. However, most of the dogs kept as watchdogs in our study were male German Shepherds, and they all originated from Alexandria, which might explain the high prevalence of T. gondii antibodies in these groups. Indeed, when the factors were analyzed within the German Shepherd group alone, only the male sex was retained as a risk factor for infection. However, our results were consistent with Raimundo et al. (2015) [43], who also found age and breed to be risk factors for infection, and with Arruda et al. (2021) [37], who reported breed as a risk factor. A study only including German Shepherds found a similar seroprevalence (46.3%) to ours in this breed [44]. The authors argued that this high seroprevalence might be related to the use of German Shepherd dogs as watchdogs in rural areas, guarding animal farms, or private properties, and thus have an increased risk of infection. This explanation also pertains to our study.
5. Conclusions
Our study provides valuable data on the prevalence of N. caninum and T. gondii among dogs and cats, adding substantially to the still scarce epidemiological knowledge on these important parasites in Egypt. We were able to include a large number of dogs in our study and to identify risk factors for their infection with T. gondii. As no successful and complete treatment or vaccine regimens are available to date to control both parasites, good animal husbandry practices, routine testing of the animals, and surveillance of the current epidemiological situation are recommended approaches for the prevention of infection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11121464/s1, Table S1: Risk factors for N. caninum antibodies in dogs. Table S2: Risk factors for T. gondii antibodies in cats.
Author Contributions
Conceptualization, R.M.F. and C.F.F.; Data curation, D.B.S., R.M.F., and C.F.F.; Formal analysis, D.B.S., R.M.F., H.H.A., and C.F.F.; Investigation, D.B.S., R.M.F., H.H.A., and C.F.F.; Methodology, D.B.S. and R.M.F.; Project administration, R.M.F. and C.F.F.; Resources, D.B.S., R.M.F., H.H.A., M.S.S., W.M.A., S.M.A., A.E.A.M., S.M., O.A., X.S., N.G., and C.F.F.; Software, R.M.F.; Supervision, R.M.F.; Validation, D.B.S., R.M.F., and C.F.F.; Visualization, R.M.F.; Writing—original draft, D.B.S., R.M.F., and S.M.A., X.S., N.G., and C.F.F.; Writing—review & editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to instructions established by the “Research Board” of the Faculty of Veterinary Medicine, South Valley University, Qena, Egypt. The protocols were approved by the Research Code of Ethics at South Valley University number 36 (RCOE-36).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated and analyzed during this study are included in this published article. Raw data supporting the findings of this study are available from the corresponding author at the request.
Acknowledgments
We thank all veterinarians and owners of animal clinics and hospitals who helped in collecting blood samples from dogs and cats and the animal owners for their cooperation in providing animals and the required data and information of each animal. We appreciate the great help of our colleagues at the Department of Animal Medicine, Faculty of Veterinary Medicine, South Valley University, Qena, and Department of Parasitology and Animal Disease, Veterinary Research Institute, National Research Centre, Giza, Egypt, for their cooperation and technical assistance. The visit of D.B.S to BITS-P (Hyderabad) was supported by Wellcome Trust – DBT India Alliance Senior Fellowship awarded to N.G (IA/S/19/1/504263).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martin, F.; Bachert, K.E.; Snow, L.; Tu, H.W.; Belahbib, J.; Lyn, S.A. Depression, anxiety, and happiness in dog owners and potential dog owners during the COVID-19 pandemic in the United States. PLoS ONE 2021, 16, e0260676. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, J.K.; Lindsay, D.S.; Parker, B.B.; Dobesh, M. Dogs as possible mechanical carriers of Toxoplasma, and their fur as a source of infection of young children. Int. J. Infect. Dis. 2003, 7, 292–293. [Google Scholar] [CrossRef] [PubMed]
- El Behairy, A.M.; Choudhary, S.; Ferreira, L.R.; Kwok, O.C.; Hilali, M.; Su, C.; Dubey, J.P. Genetic characterization of viable Toxoplasma gondii isolates from stray dogs from Giza, Egypt. Vet. Parasitol. 2013, 193, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaky, H.H.; Umeda, K.; Nguyen, T.; Mohamed, A.E.A.; Fereig, R.M. A review on current knowledge of major zoonotic protozoan diseases affecting farm and pet animals. Ger. J. Vet. Res. 2021, 2, 61–76. [Google Scholar] [CrossRef]
- Al-Bajalan, M.; Xia, D.; Armstrong, S.; Randle, N.; Wastling, J.M. Toxoplasma gondii and Neospora caninum induce different host cell responses at proteome-wide phosphorylation events; a step forward for uncovering the biological differences between these closely related parasites. Parasitol. Res. 2017, 116, 2707–2719. [Google Scholar] [CrossRef]
- Dubey, J.P.; Beattie, C.P. Toxoplasmosis of Animals and Man; CRC Press: Boca Raton, FL, USA, 1988; pp. 1–220. [Google Scholar]
- Dubey, J.P.; Hemphill, A.; Calero-Bernal, R.; Schares, G. Neosporosis in Animals, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 351–355. [Google Scholar]
- Fereig, R.M.; Nishikawa, Y. From signaling pathways to distinct immune responses: Key factors for establishing or combating Neospora caninum infection in different susceptible hosts. Pathogens 2020, 9, 384. [Google Scholar] [CrossRef]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef]
- McAllister, M.M.; Dubey, J.P.; Lindsay, D.S.; Jolley, W.R.; Wills, R.A.; McGuire, A.M. Dogs are definitive hosts of Neospora caninum. Int. J. Parasitol. 1998, 28, 1473–1479. [Google Scholar] [CrossRef]
- Gondim, L.F.P.; McAllister, M.M.; Pitt, W.C.; Zemlicka, D.E. Coyotes (Canis latrans) are definitive hosts of Neospora caninum. Int. J. Parasitol. 2004, 34, 159–161. [Google Scholar] [CrossRef]
- King, J.S.; Slapeta, J.; Jenkins, D.J.; Al-Qassab, S.E.; Ellis, J.T.; Windsor, P.A. Australian dingoes are definitive hosts of Neospora caninum. Int. J. Parasitol. 2010, 40, 945–950. [Google Scholar] [CrossRef]
- Dubey, J.P.; Jenkins, M.C.; Rajendran, C.; Miska, K.; Ferreira, L.R.; Martins, J.; Kwok, O.C.H.; Choudhary, S. Gray wolf (Canis lupus) is a natural definitive host for Neospora caninum. Vet. Parasitol. 2011, 181, 382–387. [Google Scholar] [CrossRef]
- Calero-Bernal, R.; Gennari, S.M. Clinical toxoplasmosis in dogs and cats: An Update. Front. Vet. Sci. 2019, 6, 54. [Google Scholar] [CrossRef]
- Dubey, J.P. Toxoplasmosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–313. [Google Scholar]
- Ding, H.; Gao, Y.M.; Deng, Y.; Lamberton, P.H.L.; Lu, D.B. A systematic review and meta-analysis of the seroprevalence of Toxoplasma gondii in cats in mainland China. Parasites Vectors 2017, 10, 27. [Google Scholar] [CrossRef]
- Spycher, A.; Geigy, C.; Howard, J.; Posthaus, H.; Gendron, K.; Gottstein, B.; Debache, K.; Herrmann, D.C.; Schares, G.; Frey, C.F. Isolation and genotyping of Toxoplasma gondii causing fatal systemic toxoplasmosis in an immunocompetent 10-year-old cat. J. Vet. Diagn. Investig. 2011, 23, 104–108. [Google Scholar] [CrossRef]
- Patitucci, A.N.; Alley, M.R.; Jones, B.R.; Charleston, W.A.G. Protozoa1 encephalomyelitis of dogs involving Neosporum caninum and Toxoplasma gondii in New Zealand. N. Z. Vet. J. 1997, 45, 231–235. [Google Scholar] [CrossRef]
- Migliore, S.; La Marca, S.; Stabile, C.; Di Marco Lo Presti, V.; Vitale, M. A rare case of acute toxoplasmosis in a stray dog due to infection of T. gondii clonal type I: Public health concern in urban settings with stray animals? BMC Vet. Res. 2017, 13, 249. [Google Scholar] [CrossRef]
- Pepper, A.; Mansfield, C.; Stent, A.; Johnstone, T. Toxoplasmosis as a cause of life-threatening respiratory distress in a dog receiving immunosuppressive therapy. Clin. Case Rep. 2019, 7, 942–948. [Google Scholar] [CrossRef]
- Barber, J.S.; Trees, A.J. Clinical aspects of 27 cases of neosporosis in dogs. Vet. Rec. 1996, 139, 439–443. [Google Scholar] [CrossRef]
- Al-Kappany, Y.M.; Rajendran, C.; Ferreira, L.R.; Kwok, O.C.; Abu-Elwafa, S.A.; Hilali, M.; Dubey, J.P. High prevalence of toxoplasmosis in cats from Egypt: Isolation of viable Toxoplasma gondii, tissue distribution, and isolate designation. J. Parasitol. 2010, 96, 1115–1118. [Google Scholar] [CrossRef]
- Awad, R.A.; Barakat, A.M.A. Serological diagnosis of toxoplasmosis in household cats in Egypt. Egypt. J. Vet. Sci. 2019, 50, 57–63. [Google Scholar] [CrossRef]
- Sherif, H.; Gerges, A.A.; Elsify, A.; Hadad, G.A. Molecular and serological survey for toxoplasmosis in cats from an urban populations in Egypt. Alex. J. Vet. Sci. 2019, 63, 26–30. [Google Scholar] [CrossRef]
- Rifaat, M.A.; Morsy, T.A.; Sadek, M.S.M.; Arafa, M.S.; Azab, M.E.; Abdel Ghaffar, F.M. Isolation of Toxoplasma parasite from animals in Egypt. J. Egypt. Soc. Parasitol. 1977, 7, 219–223. [Google Scholar]
- Khaled, M.L.; Morsy, T.A.; Sadek, M.S.; Salama, M.M. The presence of antibodies against toxoplasmosis, leishmaniasis and amoebiasis in stray dogs in Cairo, Egypt. J. Egypt. Soc. Parasitol. 1982, 12, 341–347. [Google Scholar] [PubMed]
- El-Ghaysh, A.; Khalil, F.; Hilali, M.; Nassar, A.M. Serological diagnosis of Neospora caninum infection in some domestic animals from Egypt. Vet. Med. J. Giza 2003, 51, 355–361. [Google Scholar]
- Fereig, R.M.; Mahmoud, H.; Mohamed, S.; AbouLaila, M.R.; Abdel-Wahab, A.; Osman, S.A.; Zidan, S.A.; El-Khodary, S.A.; Mohamed, A.; Nishikawa, Y. Seroprevalence and epidemiology of Toxoplasma gondii in farm animals in different regions of Egypt. Vet. Parasitol. Reg. Stud. Rep. 2016, 3–4, 1–6. [Google Scholar] [CrossRef]
- Fereig, R.M.; Wareth, G.; Abdelbaky, H.H.; Mazeed, A.M.; El-Diasty, M.; Abdelkhalek, A.; Mahmoud, H.Y.A.H.; Ali, O.A.; El-tayeb, A.; Alsayeqh, A.F.; et al. Seroprevalence of specific antibodies to Toxoplasma gondii, Neospora caninum, and Brucella spp. in sheep and goats in Egypt. Animals 2022, 12, 3327. [Google Scholar] [CrossRef]
- Fereig, R.M.; Abdelbaky, H.H.; Mazeed, A.M.; El-Alfy, E.S.; Saleh, S.; Omar, M.A.; Alsayeqh, A.F.; Frey, C.F. Prevalence of Neospora caninum and Toxoplasma gondii antibodies and DNA in raw milk of various ruminants in Egypt. Pathogens 2022, 11, 1305. [Google Scholar] [CrossRef]
- Dwinata, I.M.; Oka, I.; Agustina, K.K.; Damriyasa, I.M. Seroprevalence of Neospora caninum in local Bali dog. Vet. World 2018, 11, 926–929. [Google Scholar] [CrossRef]
- Villagra-Blanco, R.; Angelova, L.; Conze, T.; Schares, G.; Bärwald, A.; Taubert, A.; Hermosilla, C.; Wehrend, A. Seroprevalence of Neospora caninum-specific antibodies in German breeding bitches. Parasites Vectors 2018, 11, 96. [Google Scholar] [CrossRef]
- Lefkaditis, M.; Spanoudis, K.; Tsakiroglou, M.; Panorias, A.; Sossidou, A. Seroprevalence of Neospora caninum infection in stray dogs in Chalkidiki, Northern Greece. J. Hell. Vet. Med. Soc. 2021, 71, 2511–2514. [Google Scholar] [CrossRef]
- Anvari, D.; Saberi, R.; Sharif, M.; Sarvi, S.; Hosseini, S.A.; Moosazadeh, M.; Hosseininejad, Z.; Chegeni, T.N.; Daryani, A. Seroprevalence of Neospora caninum infection in dog population worldwide: A systematic review and Meta-analysis. Acta Parasitol. 2020, 65, 273–290. [Google Scholar] [CrossRef]
- Györke, A.; Opsteehg, M.; Mirceana, V.; Iovua, A.; Cozmaa, V. Toxoplasma gondii in Romanian household cats: Evaluation of serological tests, epidemiology and risk factors. Prev. Vet. Med. 2011, 102, 321–328. [Google Scholar] [CrossRef]
- Fábrega, L.; Restrepo, C.M.; Torres, A.; Smith, D.; Chan, P.; Pérez, D.; Cumbrera, A.; Caballero, E.Z. Frequency of Toxoplasma gondii and risk factors associated with the infection in stray dogs and cats of Panama. Microorganisms 2020, 8, 927. [Google Scholar] [CrossRef]
- Arruda, I.F.; Millar, P.R.; Barbosa, A.; Abboud, L.; Dos Reis, I.C.; Moreira, A.; Guimarães, M.; Amendoeira, M. Toxoplasma gondii in domiciled dogs and cats in urban areas of Brazil: Risk factors and spatial distribution. Toxoplasma gondii chez les chiens et les chats domiciliés dans des zones urbaines du Brésil: Facteurs de risque et répartition spatiale. Parasite 2021, 28, 56. [Google Scholar] [CrossRef]
- Roqueplo, C.; Halos, L.; Cabre, O.; Davoust, B. Toxoplasma gondii in wild and domestic animals from New Caledonia. Parasite 2011, 18, 345–348. [Google Scholar] [CrossRef][Green Version]
- Sharma, R.N.; Ordas, G.; Tiwari, K.; Chikweto, A.; Bhaiyat, M.I.; Allie, C.D.; Paterson, T. Prevalence of Toxoplasma gondii antibodies in stray and owned dogs of Grenada, West Indies. Vet. World 2014, 7, 661–664. [Google Scholar] [CrossRef][Green Version]
- Guy, L.R.M.; Penuliar, G.M. Seroprevalence and risk factor analysis of Toxoplasma gondii among stray and domesticated dogs (Canis familiaris) in Antipolo and Metro Manila. Philipp. J. Sci. 2016, 145, 49–55. [Google Scholar]
- Zarra-Nezhad, F.; Borujeni, M.P.; Mosallanejad, B.; Hamidinejat, H. A seroepidemiological survey of Toxoplasma gondii infection in referred dogs to Veterinary Hospital of Ahvaz, Iran. Int. J. Vet. Sci. Med. 2017, 5, 148–151. [Google Scholar] [CrossRef]
- Watanabe, M.; Sadiq, M.B.; Mulop, N.; Mohammed, K.; Rani, P.; Fong, L.S.; Aziz, N.A.; Kamaludeen, J.; Ramanoon, S.Z.; Mansor, R.; et al. Prevalence of Toxoplasma gondii antibodies in stray dogs from various locations in West and East Malaysia. Korean J. Parasitol. 2020, 58, 487–492. [Google Scholar] [CrossRef]
- Raimundo, J.M.; Guimaraes, A.; Moraes, L.M.D.B.; Santos, L.A.; Nepomuceno, L.L.; Barbosa, S.M.; Pires, M.S.; Santos, H.A.; Massard, C.L.; Machado, R.Z.; et al. Toxoplasma gondii and Neospora caninum in dogs from the state of Tocantins: Serology and associated factors. Braz. J. Vet. Parasitol. 2015, 24, 475–481. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, S.E.; Lee, E.G.; Song, K.H. Nested PCR-based detection of Toxoplasma gondii in German shepherd dogs and stray cats in South Korea. Res. Vet. Sci. 2008, 85, 125–127. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).