Sublingual Immunotherapy for Japanese Cedar Pollinosis: Current Clinical and Research Status
Abstract
:1. Introduction
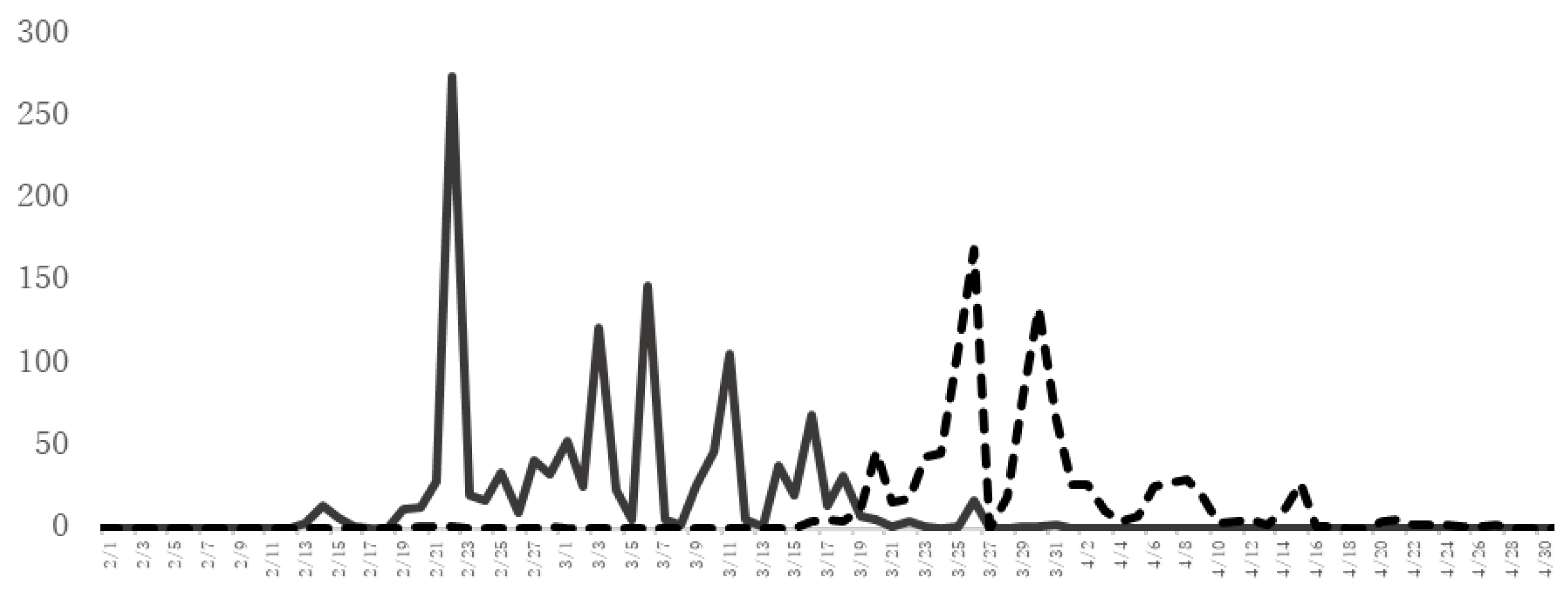
2. Characteristics of JC Pollinosis in Japan
3. General Drug Treatment for JC Pollinosis
4. AIT for JC Pollinosis
5. Clinical Effects of JC Pollen SLIT for JC Pollinosis
| During Treatment | After Treatment | ||||
|---|---|---|---|---|---|
| Year | 1st | 2nd | 3rd | 4th | 5th |
| JC pollen SLIT drop | 18% ** | 30% ** | |||
| JC pollen SLIT tablet | 32.1% * | 45.1% * | 46.3% * | 45.3% * | 34.0% ** |
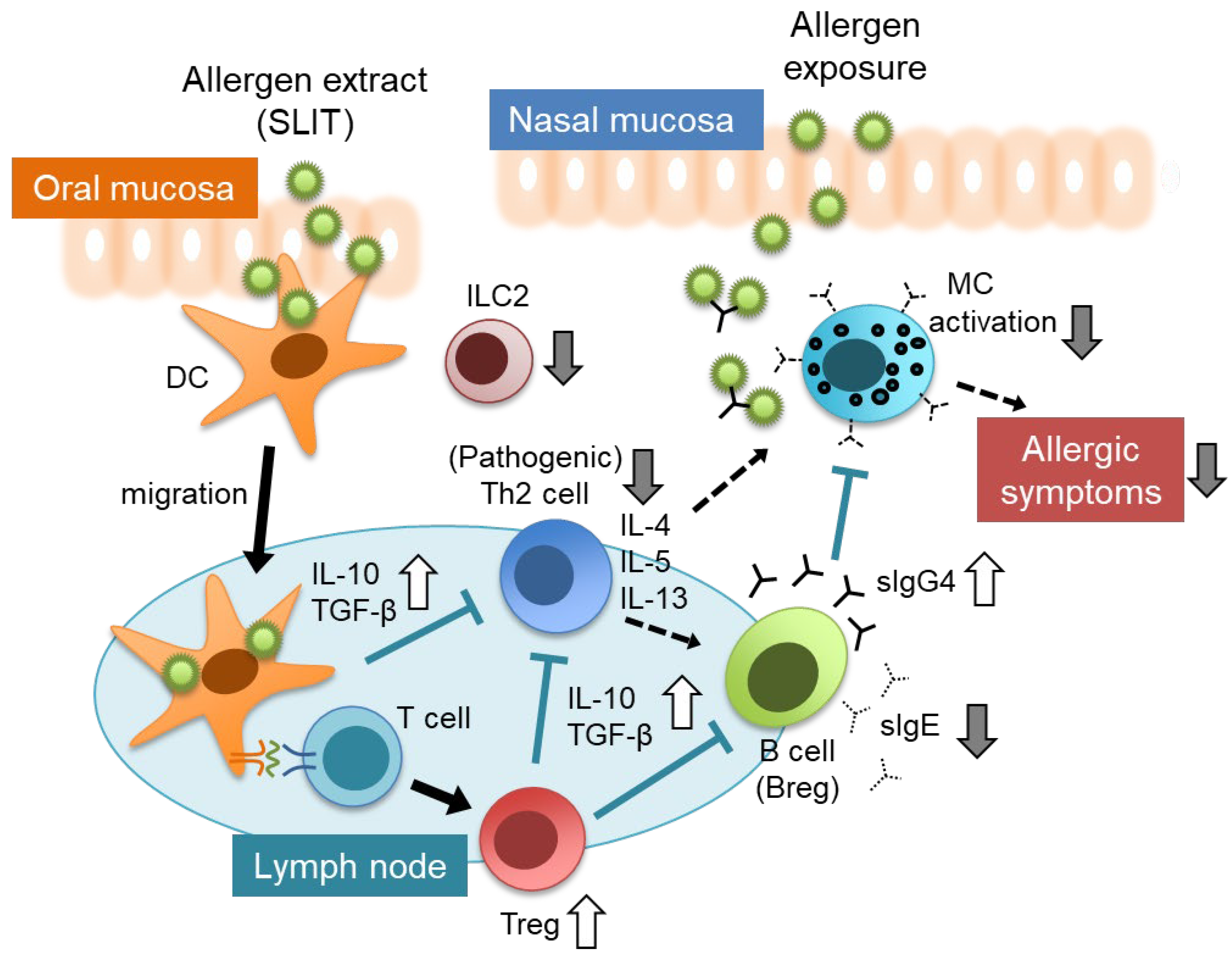
6. Mechanisms and Factors Related to the Clinical Efficacy of SLIT
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okubo, K.; Kurono, Y.; Ichimura, K.; Enomoto, T.; Okamoto, Y.; Kawauchi, H.; Suzaki, H.; Fujieda, S.; Masuyama, K.; Japanese Society of Allergology. Japanese guidelines for allergic rhinitis 2020. Allergol. Int. 2020, 69, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, A.; Sakashita, M.; Gotoh, M.; Kawashima, K.; Matsuoka, T.; Kondo, S.; Yamada, T.; Takeno, Y.; Takeuchi, K.; Urashima, M.; et al. Epidemiological survey of allergic rhinitis in Japan 2019. J. Otolaryngol. Jpn. 2020, 123, 485–490. [Google Scholar] [CrossRef]
- Baba, K.; Nakae, K. National epidemiological survey of nasal allergy 2008 (compared with 1998) in otolaryngologists and their family members. Prog. Med. 2008, 28, 2001–2012. [Google Scholar]
- Sakashita, M.; Tsutsumiuchi, T.; Kubo, S.; Tokunaga, T.; Takabayashi, T.; Imoto, Y.; Kato, Y.; Yoshida, K.; Kimura, Y.; Kato, Y.; et al. Comparison of sensitization and prevalence of Japanese cedar pollen and mite-induced perennial allergic rhinitis between 2006 and 2016 in hospital workers in Japan. Allergol. Int. 2021, 70, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, S.; Okamoto, Y.; Horiguchi, S.; Sakurai, D.; Chazono, H.; Hanazawa, T.; Okawa, T.; Aoki, S.; Konno, A. Effects of aging on the natural history of seasonal allergic rhinitis in middle-aged subjects in South chiba, Japan. Int. Arch. Allergy Immunol. 2012, 157, 73–80. [Google Scholar] [CrossRef]
- Saito, Y. Japanese cedar pollinosis: Discovery, nomenclature, and epidemiological trends. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2014, 90, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Hamasaki, S.; Okamoto, Y.; Yonekura, S.; Okuma, Y.; Sakurai, T.; Iinuma, T.; Yamamoto, H.; Sakurai, D.; Horiguchi, S.; Yokota, M. Characteristics of the Chiba environmental challenge chamber. Allergol. Int. 2014, 63, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Osada, T.; Okano, M. Japanese cedar and cypress pollinosis updated: New allergens, cross-reactivity, and treatment. Allergol. Int. 2021, 70, 281–290. [Google Scholar] [CrossRef]
- Yamada, T.; Saito, H.; Fujieda, S. Present state of Japanese cedar pollinosis: The national affliction. J. Allergy Clin. Immunol. 2014, 133, 632–639.e5. [Google Scholar] [CrossRef]
- Honda, K.; Saito, H.; Fukui, N.; Ito, E.; Ishikawa, K. The relationship between pollen count levels and prevalence of Japanese cedar pollinosis in Northeast Japan. Allergol. Int. 2013, 62, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Okubo, K.; Okano, M.; Sato, N.; Tamaki, Y.; Suzuki, H.; Uddin, A.; Fogel, R. Add-On Omalizumab for Inadequately Controlled Severe Pollinosis Despite Standard-of-Care: A Randomized Study. J. Allergy Clin. Immunol. Pract. 2020, 8, 3130–3140.e2. [Google Scholar] [CrossRef]
- Gotoh, M.; Okubo, K.; Yuta, A.; Ogawa, Y.; Nagakura, H.; Ueyama, S.; Ueyama, T.; Kawashima, K.; Yamamoto, M.; Fujieda, S.; et al. Safety profile and immunological response of dual sublingual immunotherapy with house dust mite tablet and Japanese cedar pollen tablet. Allergol. Int. 2020, 69, 104–110. [Google Scholar] [CrossRef]
- Matsuoka, T.; Igarashi, S.; Kuroda, Y.; Fukano, C.; Natsui, K.; Doi-Ohashi, K.; Masuyama, K. Dual sublingual immunotherapy with Japanese Cedar Pollen droplets and House Dust Mite tablets. Allergol. Int. 2019, 68, 533–535. [Google Scholar] [CrossRef]
- Okamoto, Y.; Okubo, K.; Yonekura, S.; Hashiguchi, K.; Goto, M.; Otsuka, T.; Murata, T.; Nakao, Y.; Kanazawa, C.; Nagakura, H.; et al. Efficacy and safety of sublingual immunotherapy for two seasons in patients with Japanese cedar pollinosis. Int. Arch. Allergy Immunol. 2015, 166, 177–188. [Google Scholar] [CrossRef]
- Gotoh, M.; Yonekura, S.; Imai, T.; Kaneko, S.; Horikawa, E.; Konno, A.; Okamoto, Y.; Okubo, K. Long-Term Efficacy and Dose-Finding Trial of Japanese Cedar Pollen Sublingual Immunotherapy Tablet. J. Allergy Clin. Immunol. Pract. 2019, 7, 1287–1297.e8. [Google Scholar] [CrossRef]
- Yonekura, S.; Gotoh, M.; Kaneko, S.; Kanazawa, K.; Takeuji, Y.; Okubo, K.; Okamoto, Y. Treatment duration-dependent efficacy of Japanese cedar pollen sublingual immunotherapy: Evaluation of a phase II/III trial over three pollen dispersal seasons. Allergol. Int. 2019, 68, 494–505. [Google Scholar] [CrossRef]
- Yonekura, S.; Gotoh, M.; Kaneko, S.; Maekawa, Y.; Okubo, K.; Okamoto, Y. Disease-Modifying Effect of Japanese Cedar Pollen Sublingual Immunotherapy Tablets. J. Allergy Clin. Immunol. Pract. 2021, 9, 4103–4116.e14. [Google Scholar] [CrossRef]
- Durham, S.R.; Emminger, W.; Kapp, A.; de Monchy, J.G.; Rak, S.; Scadding, G.K.; Wurtzen, P.A.; Andersen, J.S.; Tholstrup, B.; Riis, B.; et al. SQ-standardized sublingual grass immunotherapy: Confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J. Allergy Clin. Immunol. 2012, 129, 717–725.e5. [Google Scholar] [CrossRef] [Green Version]
- Didier, A.; Malling, H.J.; Worm, M.; Horak, F.; Sussman, G.L. Prolonged efficacy of the 300IR 5-grass pollen tablet up to 2 years after treatment cessation, as measured by a recommended daily combined score. Clin. Transl. Allergy 2015, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Bozek, A.; Foks, A.; Trzaska, K.; Canonica, G.W. Long-term effects of allergen sublingual immunotherapy. Postepy Dermatol. Alergol. 2020, 37, 943–947. [Google Scholar] [CrossRef] [Green Version]
- Yonekura, S.; Gotoh, M.; Okano, M.; Kurokawa, T.; Maekawa, Y.; Okubo, K.; Okamoto, Y. Japanese cedar pollen sublingual immunotherapy is effective in treating seasonal allergic rhinitis during the pollen dispersal period for Japanese cedar and Japanese cypress. Allergol. Int. 2022, 71, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Komiyama, N.; Itoh, M.; Itoh, H.; Sone, T.; Kino, K.; Takagi, I.; Ohta, N. Purification, characterization and molecular cloning of Cha o 1, a major allergen of Chamaecyparis obtusa (Japanese cypress) pollen. Mol. Immunol. 1996, 33, 451–460. [Google Scholar] [CrossRef]
- Mori, T.; Yokoyama, M.; Komiyama, N.; Okano, M.; Kino, K. Purification, identification, and cDNA cloning of Cha o 2, the second major allergen of Japanese cypress pollen. Biochem. Biophys. Res. Commun. 1999, 263, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M. A long-term follow-up study after discontinuation of immunotherapy for Japanese cedar pollinosis. Jpn. J. Allergol. 2006, 55, 655–661. [Google Scholar]
- Tofukuji, S.; Katayama, K.; Nakano, Y.; Ishida, S.; Tsuchida, J.; Tajiri, M.; Shimo, Y.; Tanaka, H.; Shichijo, M. Allergen-specific sublingual immunotherapy is dose and duration dependent in a murine allergic rhinitis model. J. Allergy Clin. Immunol. 2018, 142, 1977–1979.e9. [Google Scholar] [CrossRef] [Green Version]
- Alvaro-Lozano, M.; Akdis, C.A.; Akdis, M.; Alviani, C.; Angier, E.; Arasi, S.; Arzt-Gradwohl, L.; Barber, D.; Bazire, R.; Cavkaytar, O.; et al. EAACI Allergen Immunotherapy User’s Guide. Pediatr. Allergy Immunol. 2020, 31 (Suppl S25), 1–101. [Google Scholar] [CrossRef]
- Tan, T.J.; Layhadi, J.A.; Shamji, M.H. Mechanisms and biomarkers of subcutaneous immunotherapy and sublingual immunotherapy in allergen immunotherapy. Allergy Asthma Proc. 2022, 43, 254–259. [Google Scholar] [CrossRef]
- Mitthamsiri, W.; Pradubpongsa, P.; Sangasapaviliya, A.; Boonpiyathad, T. Decreased CRTH2 Expression and Response to Allergen Re-stimulation on Innate Lymphoid Cells in Patients with Allergen-Specific Immunotherapy. Allergy Asthma Immunol. Res. 2018, 10, 662–674. [Google Scholar] [CrossRef]
- Eljaszewicz, A.; Ruchti, F.; Radzikowska, U.; Globinska, A.; Boonpiyathad, T.; Gschwend, A.; Morita, H.; Helbling, A.; Arasi, S.; Kahlert, H.; et al. Trained immunity and tolerance in innate lymphoid cells, monocytes, and dendritic cells during allergen-specific immunotherapy. J. Allergy Clin. Immunol. 2021, 147, 1865–1877. [Google Scholar] [CrossRef]
- Golebski, K.; Layhadi, J.A.; Sahiner, U.; Steveling-Klein, E.H.; Lenormand, M.M.; Li, R.C.Y.; Bal, S.M.; Heesters, B.A.; Vilà-Nadal, G.; Hunewald, O.; et al. Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity 2021, 54, 291–307.e7. [Google Scholar] [CrossRef]
- Yonekura, S.; Okamoto, Y.; Sakurai, D.; Okubo, K.; Gotoh, M.; Kaneko, S.; Konno, A. An analysis of factors related to the effect of sublingual immunotherapy on Japanese cedar pollen induced allergic rhinitis. Allergol. Int. 2018, 67, 201–208. [Google Scholar] [CrossRef]
- Sakurai, D.; Yonekura, S.; Iinuma, T.; Sakurai, T.; Morimoto, Y.; Mita, Y.; Arai, T.; Suzuki, S.; Okuma, Y.; Kaneko, S.; et al. Sublingual immunotherapy for allergic rhinitis: Subjective versus objective tools to evaluate its success. Rhinology 2016, 54, 221–230. [Google Scholar] [CrossRef]
- Nakayama, T.; Hirahara, K.; Onodera, A.; Endo, Y.; Hosokawa, H.; Shinoda, K.; Tumes, D.J.; Okamoto, Y. Th2 Cells in Health and Disease. Annu. Rev. Immunol. 2017, 35, 53–84. [Google Scholar] [CrossRef]
- Morimoto, Y.; Hirahara, K.; Kiuchi, M.; Wada, T.; Ichikawa, T.; Kanno, T.; Okano, M.; Kokubo, K.; Onodera, A.; Sakurai, D.; et al. Amphiregulin-Producing Pathogenic Memory T Helper 2 Cells Instruct Eosinophils to Secrete Osteopontin and Facilitate Airway Fibrosis. Immunity 2018, 49, 134–150.e6. [Google Scholar] [CrossRef] [Green Version]
- Iinuma, T.; Okamoto, Y.; Morimoto, Y.; Arai, T.; Sakurai, T.; Yonekura, S.; Sakurai, D.; Hirahara, K.; Nakayama, T. Pathogenicity of memory Th2 cells is linked to stage of allergic rhinitis. Allergy 2018, 73, 479–489. [Google Scholar] [CrossRef]
- Ihara, F.; Sakurai, D.; Yonekura, S.; Iinuma, T.; Yagi, R.; Sakurai, T.; Ito, T.; Matsuura, A.; Morimoto, Y.; Arai, T.; et al. Identification of specifically reduced Th2 cell subsets in allergic rhinitis patients after sublingual immunotherapy. Allergy 2018, 73, 1823–1832. [Google Scholar] [CrossRef]
- Iinuma, T.; Kiuchi, M.; Hirahara, K.; Kurita, J.; Kokubo, K.; Yagyu, H.; Yoneda, R.; Arai, T.; Sonobe, Y.; Fukuyo, M.; et al. Single-cell immunoprofiling after immunotherapy for allergic rhinitis reveals functional suppression of pathogenic TH2 cells and clonal conversion. J. Allergy Clin. Immunol. 2022, 150, 850–860.e5. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakurai, D.; Ishii, H.; Shimamura, A.; Watanabe, D.; Yonaga, T.; Matsuoka, T. Sublingual Immunotherapy for Japanese Cedar Pollinosis: Current Clinical and Research Status. Pathogens 2022, 11, 1313. https://doi.org/10.3390/pathogens11111313
Sakurai D, Ishii H, Shimamura A, Watanabe D, Yonaga T, Matsuoka T. Sublingual Immunotherapy for Japanese Cedar Pollinosis: Current Clinical and Research Status. Pathogens. 2022; 11(11):1313. https://doi.org/10.3390/pathogens11111313
Chicago/Turabian StyleSakurai, Daiju, Hiroki Ishii, Ayumi Shimamura, Daisuke Watanabe, Takaaki Yonaga, and Tomokazu Matsuoka. 2022. "Sublingual Immunotherapy for Japanese Cedar Pollinosis: Current Clinical and Research Status" Pathogens 11, no. 11: 1313. https://doi.org/10.3390/pathogens11111313
APA StyleSakurai, D., Ishii, H., Shimamura, A., Watanabe, D., Yonaga, T., & Matsuoka, T. (2022). Sublingual Immunotherapy for Japanese Cedar Pollinosis: Current Clinical and Research Status. Pathogens, 11(11), 1313. https://doi.org/10.3390/pathogens11111313






