Complement Factor H Is an Early Predictive Biomarker of the Therapeutic Efficacy of Sublingual Immunotherapy for Japanese Cedar Pollinosis
Abstract
:1. Introduction
2. Materials and Methods
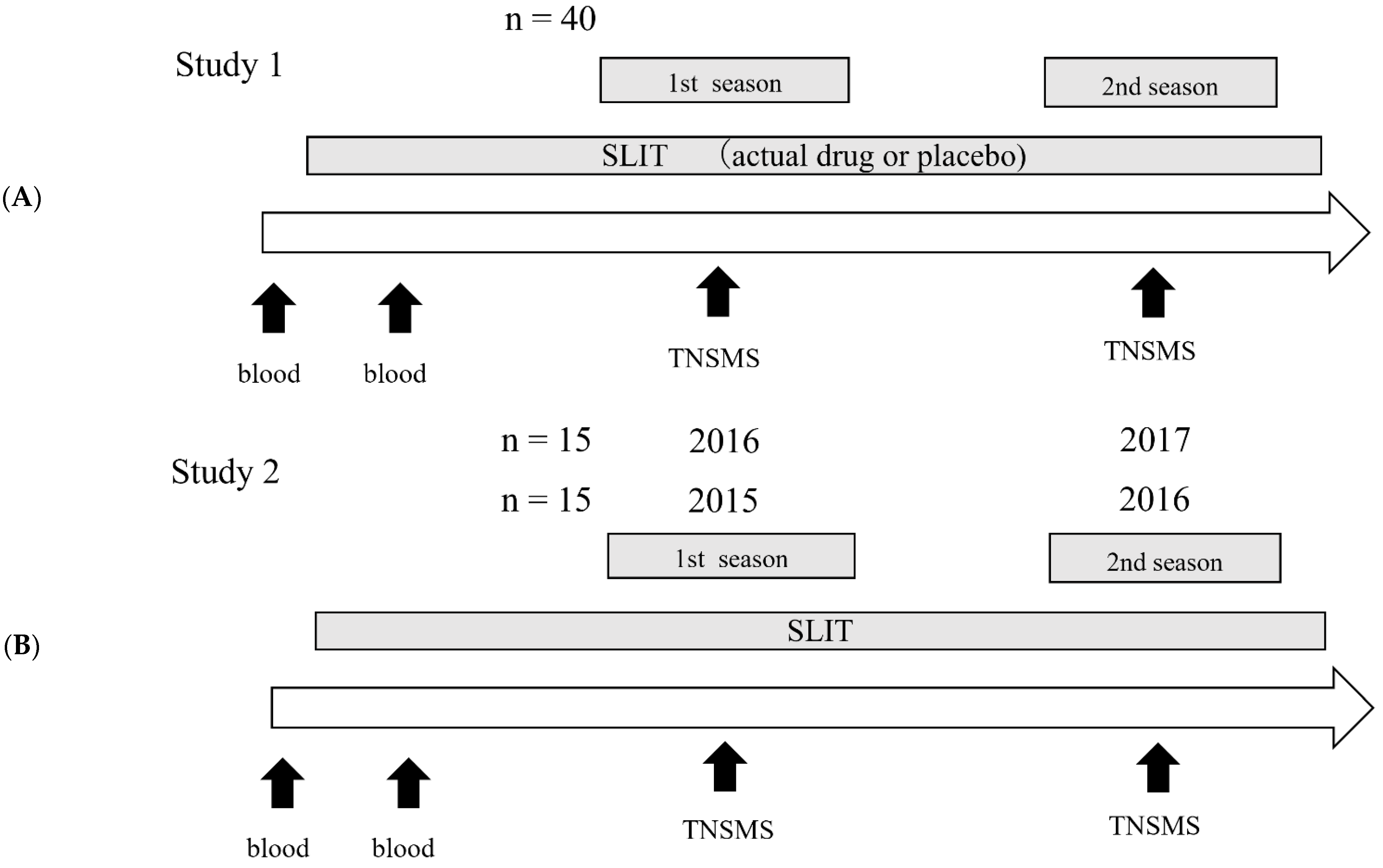
2.1. Study Design
2.2. Determination of Treatment Efficacy
2.2.1. Study 1
2.2.2. Study 2
2.3. Microarray
2.4. Real-Time PCR
2.5. Statistical Analysis
3. Results
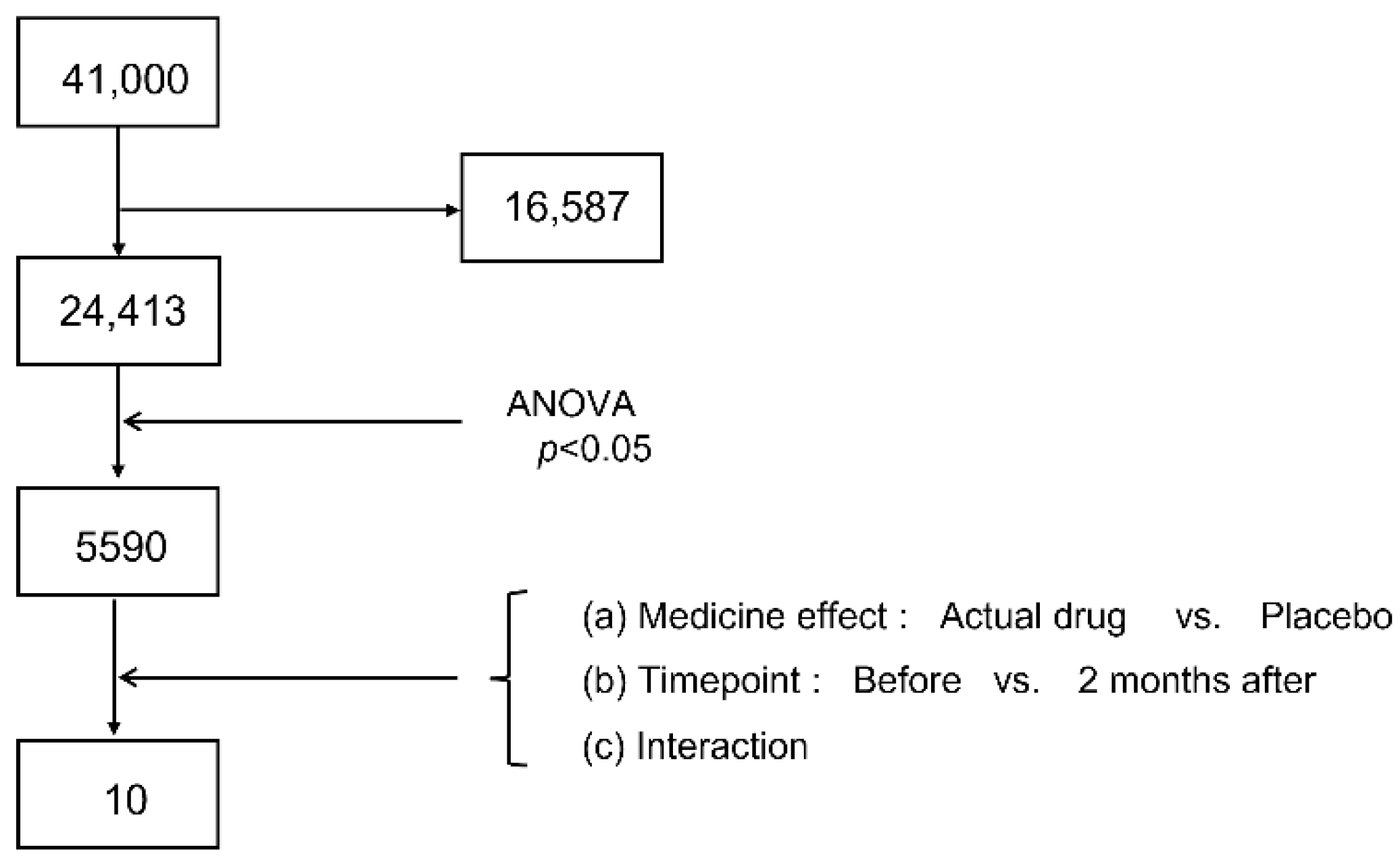
3.1. Candidate Narrowing by Microarray
3.2. Candidate Narrowing by Real-Time PCR
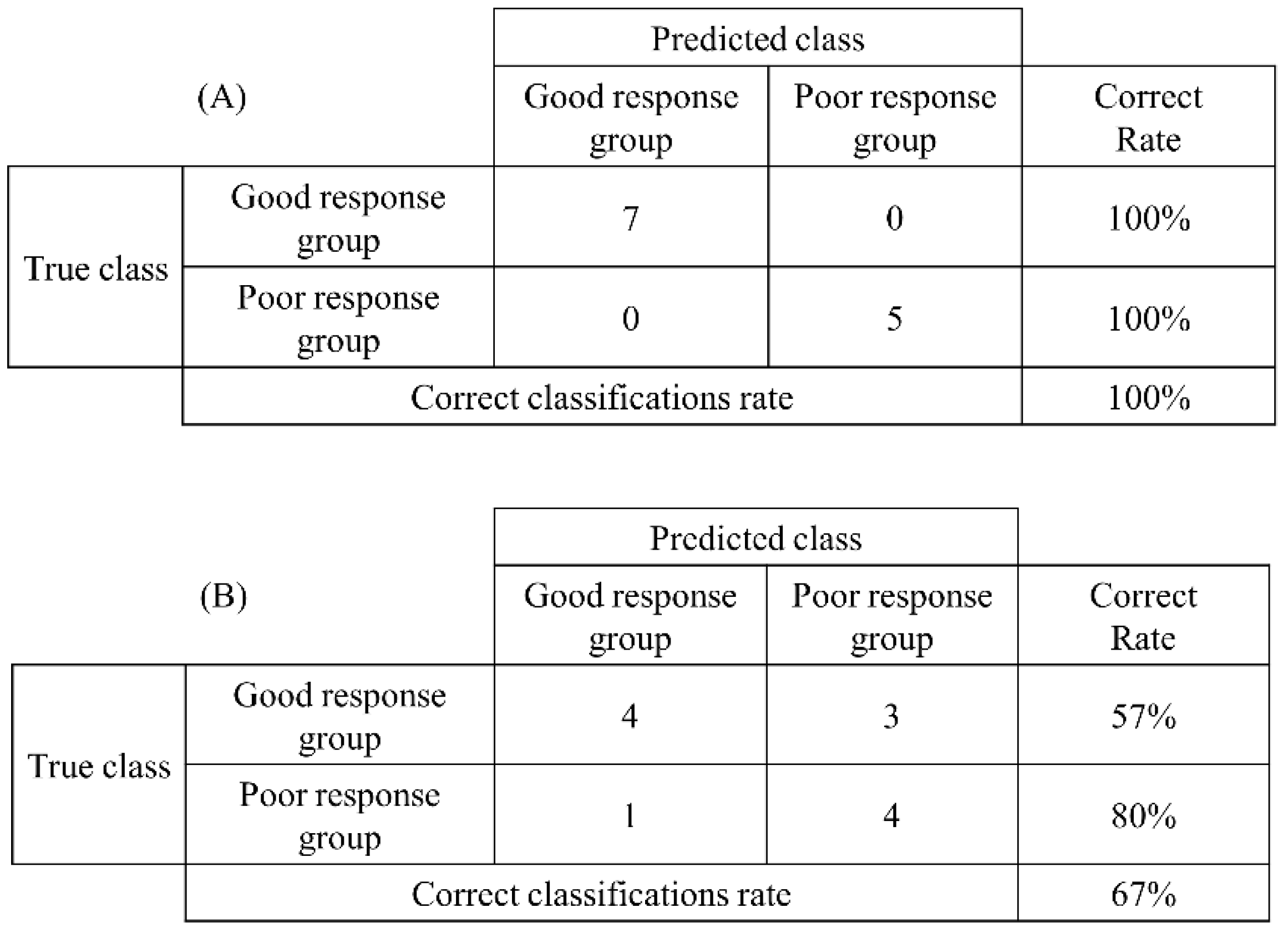
3.3. Evaluation of Prediction Accuracy by Discriminant Analysis
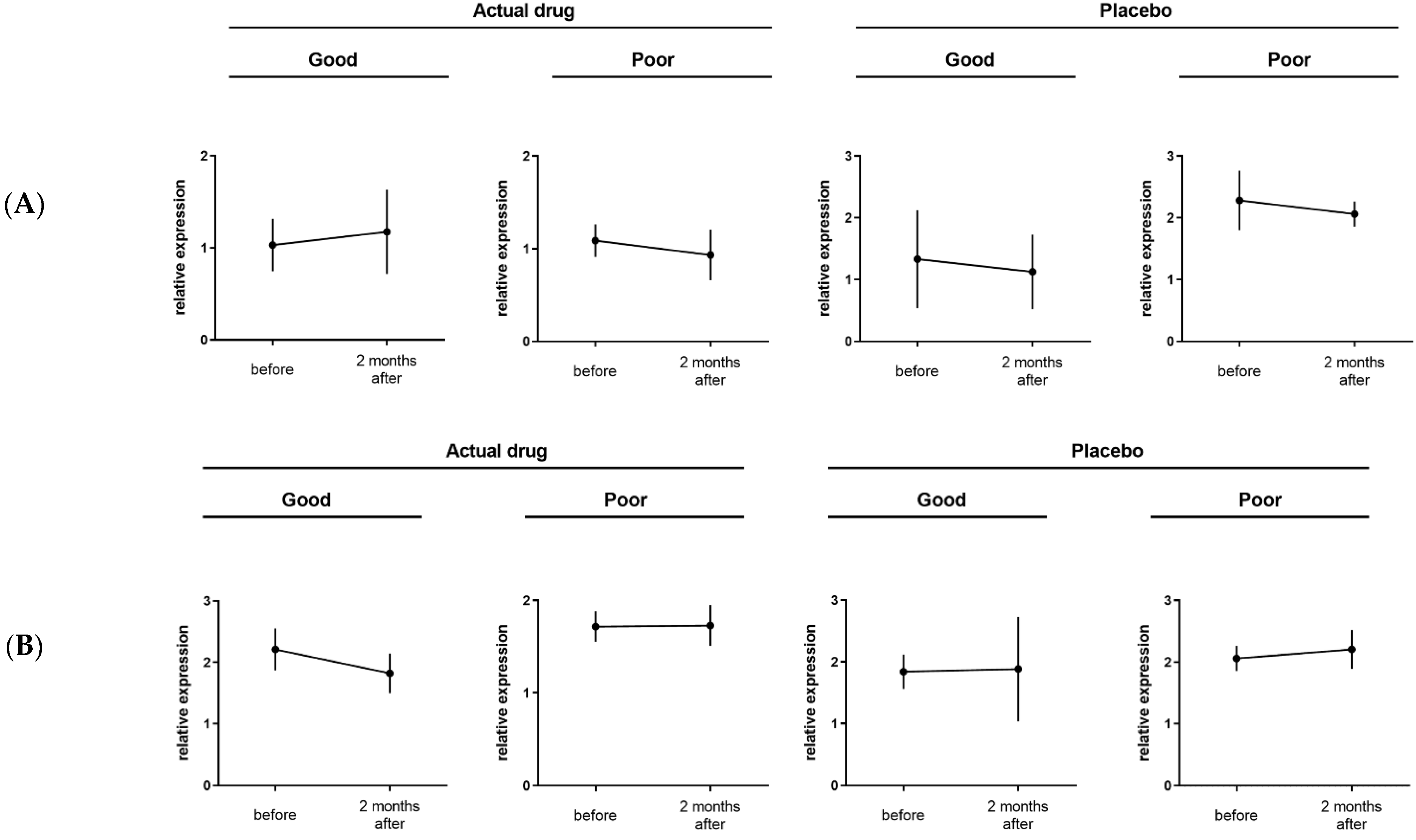
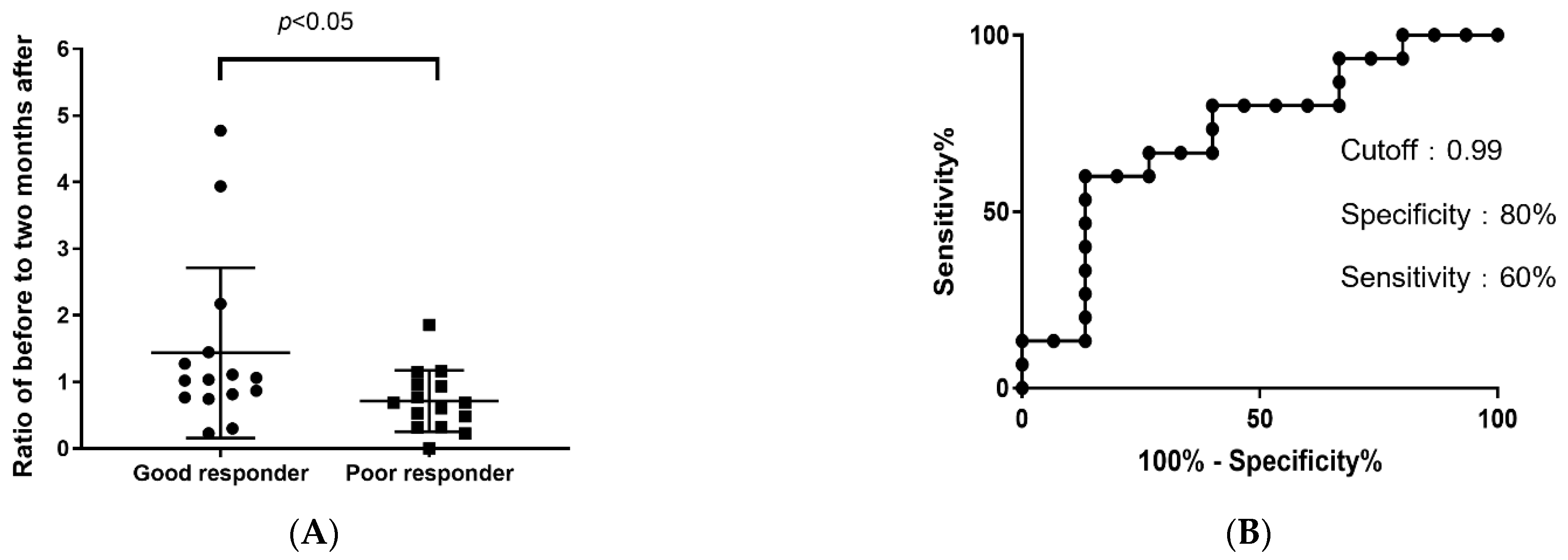
3.4. Efficacy of CFH as a Biomarker
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okubo, K.; Kurono, Y.; Ichimura, K.; Enomoto, T.; Okamoto, Y.; Kawauchi, H.; Suzaki, H.; Fujieda, S.; Masuyama, K. Japanese Society of Allergology. Japanese guidelines for allergic rhinitis 2020. Allergol. Int. 2020, 69, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Saito, H.; Fujieda, S. Present state of Japanese cedar pollinosis: The national affliction. J. Allergy Clin. Immunol. 2014, 133, 632–639.e5. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, S.; Okamoto, Y.; Yonekura, S.; Okawa, T.; Yamamoto, H.; Kunii, N.; Sakurai, D.; Fujimura, T.; Nakazawa, K.; Yasueda, H. A randomized controlled trial of sublingual immunotherapy for Japanese cedar pollinosis. Int. Arch. Allergy Immunol. 2008, 146, 76–84. [Google Scholar] [CrossRef]
- Okubo, K.; Gotoh, M.; Fujieda, S.; Okano, M.; Yoshida, H.; Morikawa, H.; Masuyama, K.; Okamoto, Y.; Kobayashi, M. A randomized double-blind comparative study of sublingual immunotherapy for cedar pollinosis. Allergol. Int. 2008, 57, 265–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimura, T.; Yonekura, S.; Horiguchi, S.; Taniguchi, Y.; Saito, A.; Yasueda, H.; Inamine, A.; Nakayama, T.; Takemori, T.; Taniguchi, M.; et al. Increase of regulatory T cells and the ratio of specific IgE to total IgE are candidates for response monitoring or prognostic biomarkers in 2-year sublingual immunotherapy (SLIT) for Japanese cedar pollinosis. Clin. Immunol. 2011, 139, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Okubo, K.; Yonekura, S.; Hashiguchi, K.; Goto, M.; Otsuka, T.; Murata, T.; Nakao, Y.; Kanazawa, C.; Nagakura, H.; et al. Efficacy and safety of sublingual immunotherapy for two seasons in patients with Japanese cedar pollinosis. Int. Arch. Allergy Immunol. 2015, 166, 177–188. [Google Scholar] [CrossRef]
- Yonekura, S.; Gotoh, M.; Kaneko, S.; Kanazawa, K.; Takeuji, Y.; Okubo, K.; Okamoto, Y. Treatment duration-dependent efficacy of Japanese cedar pollen sublingual immunotherapy: Evaluation of a phase II/III trial over three pollen dispersal seasons. Allergol. Int. 2019, 68, 494–505. [Google Scholar] [CrossRef]
- Sakurai, D.; Yonekura, S.; Iinuma, T.; Sakurai, T.; Morimoto, Y.; Mita, Y.; Arai, T.; Suzuki, S.; Okuma, Y.; Kaneko, S.; et al. Sublingual immunotherapy for allergic rhinitis: Subjective versus objective tools to evaluate its success. Rhinology 2016, 54, 221–230. [Google Scholar] [CrossRef]
- Antico, A. Long-term adherence to sublingual therapy: Literature review and suggestions for management strategies based on patients’ needs and preferences. Clin. Exp. Allergy 2014, 44, 1314–1326. [Google Scholar] [CrossRef]
- Shamji, M.H.; Kappen, J.H.; Akdis, M.; Jensen-Jarolim, E.; Knol, E.F.; Kleine-Tebbe, J.; Bohle, B.; Chaker, A.M.; Till, S.J.; Valenta, R.; et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: An EAACI Position Paper. Allergy 2017, 72, 1156–1173. [Google Scholar] [CrossRef]
- Piconi, S.; Trabattoni, D.; Rainone, V.; Borgonovo, L.; Passerini, S.; Rizzardini, G.; Frati, F.; Iemoli, E.; Clerici, M. Immunological effects of sublingual immunotherapy: Clinical efficacy is associated with modulation of programmed cell death ligand 1, IL-10, and IgG4. J. Immunol. 2010, 185, 7723–7730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scadding, G.W.; Shamji, M.H.; Jacobson, M.R.; Lee, D.I.; Wilson, D.; Lima, M.T.; Pitkin, L.; Pilette, C.; Nouri-Aria, K.; Durham, S.R. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin. Exp. Allergy 2010, 40, 598–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimabukuro, T.T.; Cole, M.; Su, J.R. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020–January 18, 2021. JAMA 2021, 325, 1101–1102. [Google Scholar] [CrossRef]
- Shamji, M.H.; Durham, S.R. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J. Allergy Clin. Immunol. 2017, 140, 1485–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimura, T.; Yonekura, S.; Taniguchi, Y.; Horiguchi, S.; Saito, A.; Yasueda, H.; Nakayama, T.; Takemori, T.; Taniguchi, M.; Sakaguchi, M.; et al. The induced regulatory T cell level, defined as the proportion of IL-10+Foxp3+ cells among CD25+CD4+ leukocytes, is a potential therapeutic biomarker for sublingual immunotherapy: A preliminary report. Int. Arch. Allergy Immunol. 2010, 153, 378–387. [Google Scholar] [CrossRef]
- Makou, E.; Herbert, A.P.; Barlow, P.N. Functional anatomy of complement factor H. Biochemistry 2013, 52, 3949–3962. [Google Scholar] [CrossRef] [PubMed]
- Klos, A.; Tenner, A.J.; Johswich, K.O.; Ager, R.R.; Reis, E.S.; Köhl, J. The role of the anaphylatoxins in health and disease. Mol. Immunol. 2009, 46, 2753–2766. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Köhl, J. A complex role for complement in allergic asthma. Expert Rev. Clin. Immunol. 2010, 6, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Sakashita, M.; Yamada, T.; Imoto, Y.; Hirota, T.; Tamari, M.; Ito, Y.; Kubo, S.; Osawa, Y.; Takahashi, N.; Fujieda, S. Long-term sublingual immunotherapy for Japanese cedar pollinosis and the levels of IL-17A and complement components 3a and 5a. Cytokine 2015, 75, 181–185. [Google Scholar] [CrossRef]
- Li, H.; Xu, E.; He, M. Cytokine responses to specific immunotherapy in house dust mite-induced allergic rhinitis patients. Inflammation 2015, 38, 2216–2223. [Google Scholar] [CrossRef]
- Yoshida, K.; Takabayashi, T.; Imoto, Y.; Sakashita, M.; Kato, Y.; Narita, N.; Fujieda, S. Increased thrombin-activatable fibrinolysis inhibitor in response to sublingual immunotherapy for allergic rhinitis. Laryngoscope 2021, 131, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
| Act/Good | Sex | Age | sIgE | First Season Severity | Second Season Severity |
|---|---|---|---|---|---|
| F | 60 | 50.9 | moderate | mild | |
| M | 61 | 31.4 | very severe | moderate | |
| M | 52 | 20.4 | very severe | moderate | |
| F | 43 | 16.0 | severe | mild | |
| F | 47 | 8.7 | moderate | mild | |
| F | 29 | 8.5 | moderate | mild | |
| F | 48 | 6.7 | severe | mild | |
| Act/Poor | Sex | Age | sIgE | First Season Severity | Second Season Severity |
| F | 55 | 100.0 | moderate | moderate | |
| F | 42 | 10.5 | moderate | moderate | |
| M | 48 | 8.9 | moderate | moderate | |
| F | 51 | 5.2 | moderate | severe | |
| Plc/Good | Sex | Age | sIgE | First Season Severity | Second Season Severity |
| F | 49 | 52.8 | very severe | moderate | |
| M | 43 | 19.5 | very severe | moderate | |
| F | 37 | 8.8 | severe | mild | |
| F | 48 | 8.8 | severe | mild | |
| F | 41 | 5.8 | moderate | mild | |
| Plc/Poor | Sex | Age | sIgE | First Season Severity | Second Season Severity |
| M | 58 | 38.1 | moderate | severe | |
| F | 52 | 27.1 | moderate | moderate | |
| M | 43 | 16.5 | severe | severe | |
| M | 58 | 15.6 | moderate | moderate | |
| F | 49 | 8.2 | moderate | moderate | |
| F | 28 | 5.2 | moderate | moderate | |
| F | 26 | 3.8 | moderate | moderate |
| Good | Sex | Age | sIgE | First Season TNSMS | Second Season TNSMS |
|---|---|---|---|---|---|
| F | 35 | 96.9 | 7.58 | 5.10 | |
| M | 43 | 85.7 | 5.94 | 4.35 | |
| F | 41 | 65.9 | 7.68 | 5.00 | |
| F | 62 | 58.4 | 5.71 | 4.32 | |
| M | 34 | 39.3 | 8.32 | 6.48 | |
| F | 18 | 37.8 | 8.43 | 5.05 | |
| F | 41 | 34.3 | 6.84 | 2.76 | |
| M | 21 | 32.6 | 3.00 | 1.43 | |
| F | 25 | 20.7 | 8.89 | 3.00 | |
| F | 63 | 19.2 | 4.43 | 3.26 | |
| F | 49 | 17.8 | 5.10 | 3.11 | |
| F | 61 | 10.1 | 7.16 | 5.67 | |
| M | 61 | 7.3 | 3.32 | 2.86 | |
| M | 27 | 5.2 | 5.05 | 3.71 | |
| F | 55 | 3.9 | 2.38 | 1.53 | |
| Poor | Sex | Age | sIgE | First Season TNSMS | Second Season TNSMS |
| F | 50 | 39.3 | 3.67 | 3.21 | |
| M | 24 | 21.3 | 3.11 | 3.38 | |
| M | 28 | 15.3 | 4.19 | 5.84 | |
| F | 30 | 11.1 | 3.16 | 3.00 | |
| F | 54 | 10.5 | 6.05 | 7.53 | |
| F | 42 | 8.6 | 8.29 | 8.16 | |
| F | 52 | 8.0 | 5.00 | 8.05 | |
| M | 49 | 7.2 | 2.22 | 2.53 | |
| F | 57 | 7.1 | 4.16 | 3.95 | |
| F | 56 | 6.8 | 6.20 | 6.17 | |
| F | 45 | 5.9 | 2.81 | 3.42 | |
| F | 45 | 4.8 | 4.19 | 5.16 | |
| M | 48 | 3.5 | 2.70 | 3.26 | |
| F | 60 | 1.8 | 1.74 | 2.62 | |
| M | 31 | 1.1 | 3.21 | 3.10 |
| ANOVA | Lower Rank Test | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medication | Time | Interaction | Act/Good | Act/Poor | Plc/Good | Plc/Poor | ||||||
| Gene Symbol | ANOVA Category | p-Value | p-Value | p-Value | FC | Reg. | FC | Reg. | FC | Reg. | FC | Reg. |
| CFH | a | 0.0239 | 0.7449 | 0.4867 | 1.23 | up | 1.08 | down | 1.10 | down | 1.02 | down |
| PRKCB | a | 0.0321 | 0.6997 | 0.1785 | 1.14 | down | 1.06 | up | 1.04 | up | 1.02 | up |
| SEPT7P2 | a | 0.0237 | 0.8003 | 0.2965 | 1.14 | down | 1.06 | up | 1.03 | up | 1.04 | up |
| GOLIM4 | b | 0.5854 | 0.0151 | 0.2764 | 1.50 | down | 1.03 | up | 1.14 | up | 1.24 | up |
| EIF2D | c | 0.5891 | 0.4864 | 0.0091 | 1.08 | up | 1.00 | down | 1.03 | down | 1.10 | down |
| FSCN1 | c | 0.5431 | 0.8282 | 0.0415 | 1.83 | up | 1.70 | down | 1.06 | down | 1.10 | down |
| DTX3 | c | 0.4475 | 0.0793 | 0.0097 | 1.13 | up | 1.32 | down | 1.01 | down | 1.17 | down |
| TTC39C | c | 0.2651 | 0.2308 | 0.0278 | 1.12 | up | 1.18 | down | 1.03 | down | 1.12 | down |
| TUBB3 | c | 0.5786 | 0.2574 | 0.0058 | 1.30 | down | 1.14 | up | 1.00 | up | 1.52 | up |
| ATP8B3 | c | 0.3515 | 0.1176 | 0.0114 | 1.18 | down | 1.13 | up | 1.17 | up | 1.23 | up |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoneda, R.; Iinuma, T.; Sakurai, D.; Kurita, J.; Arai, T.; Sonobe, Y.; Yonekura, S.; Okamoto, Y.; Hanazawa, T. Complement Factor H Is an Early Predictive Biomarker of the Therapeutic Efficacy of Sublingual Immunotherapy for Japanese Cedar Pollinosis. Pathogens 2022, 11, 1280. https://doi.org/10.3390/pathogens11111280
Yoneda R, Iinuma T, Sakurai D, Kurita J, Arai T, Sonobe Y, Yonekura S, Okamoto Y, Hanazawa T. Complement Factor H Is an Early Predictive Biomarker of the Therapeutic Efficacy of Sublingual Immunotherapy for Japanese Cedar Pollinosis. Pathogens. 2022; 11(11):1280. https://doi.org/10.3390/pathogens11111280
Chicago/Turabian StyleYoneda, Riyo, Tomohisa Iinuma, Daiju Sakurai, Junya Kurita, Tomoyuki Arai, Yuri Sonobe, Syuji Yonekura, Yoshitaka Okamoto, and Toyoyuki Hanazawa. 2022. "Complement Factor H Is an Early Predictive Biomarker of the Therapeutic Efficacy of Sublingual Immunotherapy for Japanese Cedar Pollinosis" Pathogens 11, no. 11: 1280. https://doi.org/10.3390/pathogens11111280
APA StyleYoneda, R., Iinuma, T., Sakurai, D., Kurita, J., Arai, T., Sonobe, Y., Yonekura, S., Okamoto, Y., & Hanazawa, T. (2022). Complement Factor H Is an Early Predictive Biomarker of the Therapeutic Efficacy of Sublingual Immunotherapy for Japanese Cedar Pollinosis. Pathogens, 11(11), 1280. https://doi.org/10.3390/pathogens11111280






