The Continuum of Microbial Ecosystems along the Female Reproductive Tract: Implications for Health and Fertility
Abstract
1. Introduction
2. The Vaginal Microbiome: An Influencer of Microbial Niches along the Female Reproductive Tract
3. Intrinsic Host & Extrinsic Environmental Factors That Contribute to Vaginal Dysbiosis
4. Bacterial Vaginosis
4.1. Bacterial Vaginosis and the Urinary Tract
4.2. Bacterial Vaginosis, Diseases of the Reproductive Tract, and Adverse Pregnancy Outcomes
4.3. Bacterial Vaginosis and the Pathogenesis of Tubal Infertility
5. The Microbiomes of the Fallopian Tubes, Endometrium, and Cervix
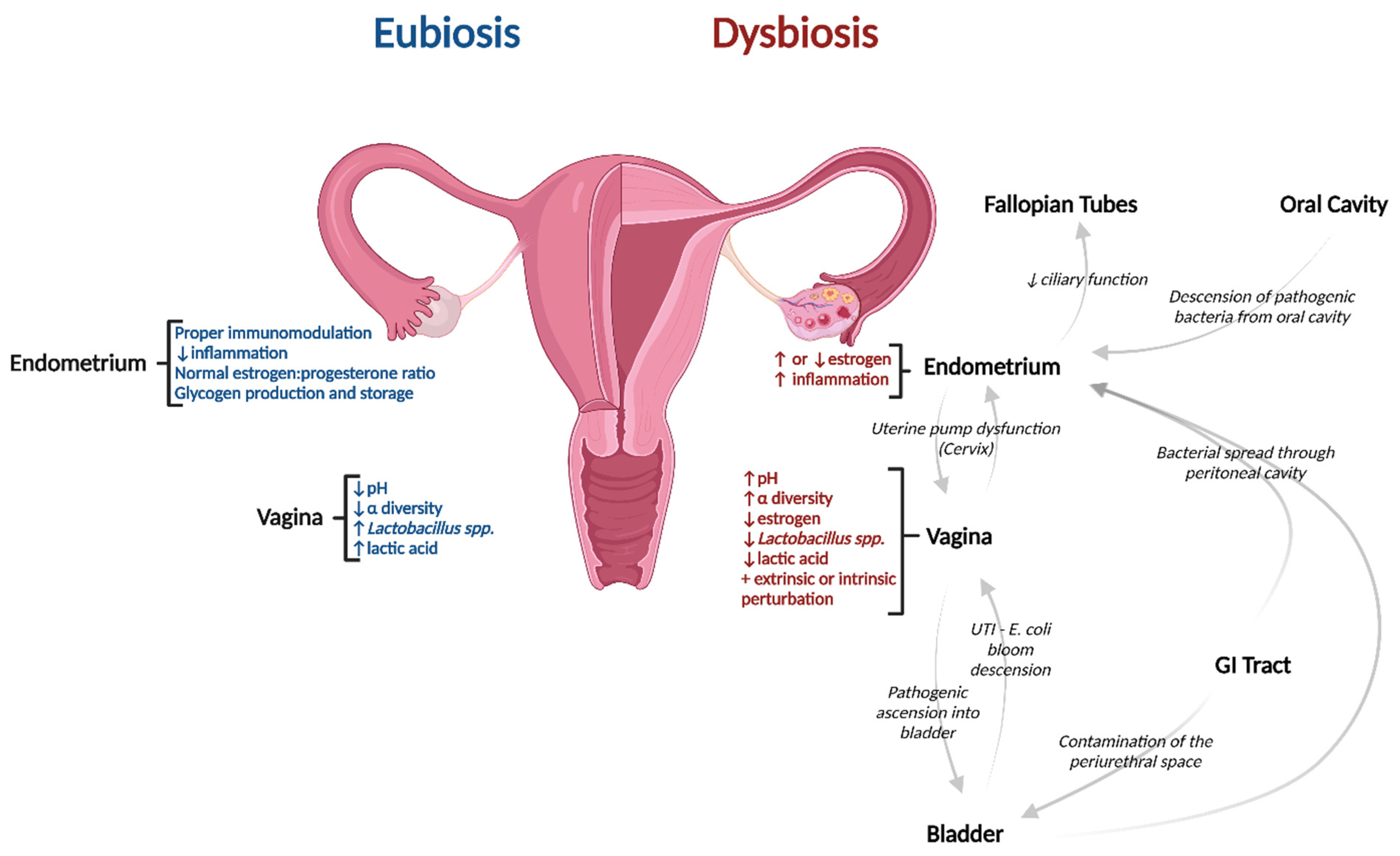
Pathogenesis of Dysbiotic Disorders along the Upper Reproductive Tract
6. Immunological Responses, Redox Potential & Metabolic Function
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Whiteside, S.A.; Razvi, H.; Dave, S.; Reid, G.; Burton, J.P. The Microbiome of the Urinary Tract—A Role beyond Infection. Nat. Rev. Urol. 2015, 12, 81–90. [Google Scholar] [CrossRef]
- Brubaker, L.; Wolfe, A.J. The Female Urinary Microbiota/Microbiome: Clinical and Research Implications. Rambam Maimonides Med. J. 2017, 8, 2. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Khor, B.; Snow, M.; Herrman, E.; Ray, N.; Mansukhani, K.; Patel, K.A.; Said-Al-Naief, N.; Maier, T.; Machida, C.A. Interconnections Between the Oral and Gut Microbiomes: Reversal of Microbial Dysbiosis and the Balance Between Systemic Health and Disease. Microorganisms 2021, 9, 496. [Google Scholar] [CrossRef]
- Magruder, M.; Sholi, A.N.; Gong, C.; Zhang, L.; Edusei, E.; Huang, J.; Albakry, S.; Satlin, M.J.; Westblade, L.F.; Crawford, C.; et al. Gut Uropathogen Abundance Is a Risk Factor for Development of Bacteriuria and Urinary Tract Infection. Nat. Commun. 2019, 10, 5521. [Google Scholar] [CrossRef]
- Garofalo, L.; Zwickey, H.; Bradley, R.; Hanes, D. Naturopathic Management of Urinary Tract Infections: A Retrospective Chart Review. J. Altern. Complement. Med. 2021, 27, 1116–1123. [Google Scholar] [CrossRef]
- Bautista, C.T.; Wurapa, E.; Sateren, W.B.; Morris, S.; Hollingsworth, B.; Sanchez, J.L. Bacterial Vaginosis: A Synthesis of the Literature on Etiology, Prevalence, Risk Factors, and Relationship with Chlamydia and Gonorrhea Infections. Mil. Med. Res. 2016, 3, 4. [Google Scholar] [CrossRef]
- Allsworth, J.E.; Peipert, J.F. Severity of bacterial vaginosis and the risk of sexually transmitted infection. Am. J. Obstet. Gynecol. 2011, 205, 113.e1–113.e6. [Google Scholar] [CrossRef]
- Peipert, J.F.; Montagno, A.B.; Cooper, A.S.; Sung, C.J. Bacterial vaginosis as a risk factor for upper genital tract infection. Am. J. Obstet. Gynecol. 1997, 177, 1184–1187. [Google Scholar] [CrossRef]
- Onderdonk, A.B.; Delaney, M.L.; Fichorova, R.N. The Human Microbiome during Bacterial Vaginosis. Clin. Microbiol. Rev. 2016, 29, 223–238. [Google Scholar] [CrossRef]
- Maarsingh, J.D.; Łaniewski, P.; Herbst-Kralovetz, M.M. Immunometabolic and potential tumor-promoting changes in 3D cervical cell models infected with bacterial vaginosis-associated bacteria. Commun. Biol. 2022, 5, 725. [Google Scholar] [CrossRef]
- Wilson, J.D.; Ralph, S.G.; Rutherford, A.J. Rates of bacterial vaginosis in women undergoing in vitro fertilisation for different types of infertility. BJOG 2002, 109, 714–717. [Google Scholar] [CrossRef]
- Lozano, F.M.; Bernabeu, A.; Lledo, B.; Morales, R.; Diaz, M.; Aranda, F.I.; Llacer, J.; Bernabeu, R. Characterization of the Vaginal and Endometrial Microbiome in Patients with Chronic Endometritis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 263, 25–32. [Google Scholar] [CrossRef]
- Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion. Infertility. Available online: https://www.cdc.gov/reproductivehealth/infertility/index.htm (accessed on 27 August 2022).
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The Vaginal Microbiome and Preterm Birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef]
- Grewal, K.; Lee, Y.S.; Smith, A.; Brosens, J.J.; Bourne, T.; Al-Memar, M.; Kundu, S.; MacIntyre, D.A.; Bennett, P.R. Chromosomally Normal Miscarriage Is Associated with Vaginal Dysbiosis and Local Inflammation. BMC Med. 2022, 20, 38. [Google Scholar] [CrossRef]
- Hu, J.; Benny, P.; Wang, M.; Ma, Y.; Lambertini, L.; Peter, I.; Xu, Y.; Lee, M.J. Intrauterine Growth Restriction Is Associated with Unique Features of the Reproductive Microbiome. Reprod. Sci. 2021, 28, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W.; Fardini, Y.; Chen, C.; Iacampo, K.G.; Peraino, V.A.; Shamonki, J.M.; Redline, R.W. Term Stillbirth Caused by Oral Fusobacterium Nucleatum. Obstet. Gynecol. 2010, 115, 442–445. [Google Scholar] [CrossRef]
- Lv, L.-J.; Li, S.-H.; Li, S.-C.; Zhong, Z.-C.; Duan, H.-L.; Tian, C.; Li, H.; He, W.; Chen, M.-C.; He, T.-W.; et al. Early-Onset Preeclampsia Is Associated With Gut Microbial Alterations in Antepartum and Postpartum Women. Front. Cell. Infect. Microbiol. 2019, 9, 224. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The Microbiota Continuum along the Female Reproductive Tract and Its Relation to Uterine-Related Diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Chen, C.; Fang, H.; Zhao, Y.; Fei, W.; Chen, F.; Zheng, C. The Role of Microbiomes in Pregnant Women and Offspring: Research Progress of Recent Years. Front. Pharmacol. 2020, 11, 643. [Google Scholar] [CrossRef]
- Al-Judaibi, A.A. Microbiota and their Influence in the Human Body. J. Pure Appl. Microbiol. 2021, 15, 42–52. [Google Scholar] [CrossRef]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal Microbiota and the Potential of Lactobacillus Derivatives in Maintaining Vaginal Health. Microb. Cell Fact. 2020, 19, 203. [Google Scholar] [CrossRef]
- Gong, Z.; Luna, Y.; Yu, P.; Fan, H. Lactobacilli Inactivate Chlamydia Trachomatis through Lactic Acid but Not H2O2. PLoS ONE 2014, 9, e107758. [Google Scholar] [CrossRef]
- Aroutcheva, A.A.; Simoes, J.A.; Faro, S. Antimicrobial Protein Produced by Vaginal Lactobacillus Acidophilus That Inhibits Gardnerella Vaginalis. Infect. Dis. Obstet. Gynecol. 2001, 9, 33–39. [Google Scholar] [CrossRef]
- Witkin, S.S.; Alvi, S.; Bongiovanni, A.M.; Linhares, I.M.; Ledger, W.J. Lactic Acid Stimulates Interleukin-23 Production by Peripheral Blood Mononuclear Cells Exposed to Bacterial Lipopolysaccharide. FEMS Immunol. Med. Microbiol. 2011, 61, 153–158. [Google Scholar] [CrossRef]
- Ma, Z.S.; Li, L. Quantifying the Human Vaginal Community State Types (CSTs) with the Species Specificity Index. PeerJ 2017, 5, e3366. [Google Scholar] [CrossRef]
- Dhumal, S.S.; Naik, P.; Dakshinamurthy, S.; Sullia, K. Semen pH and Its Correlation with Motility and Count—A Study in Subfertile Men. JBRA Assist. Reprod. 2021, 25, 172–175. [Google Scholar] [CrossRef]
- Brooks, J.P.; Buck, G.A.; Chen, G.; Diao, L.; Edwards, D.J.; Fettweis, J.M.; Huzurbazar, S.; Rakitin, A.; Satten, G.A.; Smirnova, E.; et al. Changes in Vaginal Community State Types Reflect Major Shifts in the Microbiome. Microb. Ecol. Health Dis. 2017, 28, 1303265. [Google Scholar] [CrossRef]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.E.; Zhong, X.; Koenig, S.S.K.; Fu, L.; Ma, Z.S.; Zhou, X.; et al. Temporal Dynamics of the Human Vaginal Microbiota. Sci. Transl. Med. 2012, 4, 132ra52. [Google Scholar] [CrossRef]
- Zhou, X.; Bent, S.J.; Schneider, M.G.; Davis, C.C.; Islam, M.R.; Forney, L.J. Characterization of Vaginal Microbial Communities in Adult Healthy Women Using Cultivation-Independent Methods. Microbiology 2004, 150, 2565–2573. [Google Scholar] [CrossRef]
- Pavlova, S.I.; Kilic, A.O.; Kilic, S.S.; So, J.-S.; Nader-Macias, M.E.; Simoes, J.A.; Tao, L. Genetic Diversity of Vaginal Lactobacilli from Women in Different Countries Based on 16S rRNA Gene Sequences. J. Appl. Microbiol. 2002, 92, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Elovitz, M.A.; Gajer, P.; Riis, V.; Brown, A.G.; Humphrys, M.S.; Holm, J.B.; Ravel, J. Cervicovaginal Microbiota and Local Immune Response Modulate the Risk of Spontaneous Preterm Delivery. Nat. Commun. 2019, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Zapata, H.J.; Quagliarello, V.J. The Microbiota and Microbiome in Aging: Potential Implications in Health and Age-Related Diseases. J. Am. Geriatr. Soc. 2015, 63, 776–781. [Google Scholar] [CrossRef]
- Borrow, A.P.; Cameron, N.M. The Role of Oxytocin in Mating and Pregnancy. Horm. Behav. 2012, 61, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Bardos, J.; Fiorentino, D.; Longman, R.E.; Paidas, M. Immunological Role of the Maternal Uterine Microbiome in Pregnancy: Pregnancies Pathologies and Alterated Microbiota. Front. Immunol. 2019, 10, 2823. [Google Scholar] [CrossRef] [PubMed]
- Achilles, S.L.; Austin, M.N.; Meyn, L.A.; Mhlanga, F.; Chirenje, Z.M.; Hillier, S.L. Impact of Contraceptive Initiation on Vaginal Microbiota. Am. J. Obstet. Gynecol. 2018, 218, 622.e1–622.e10. [Google Scholar] [CrossRef]
- Brotman, R.M.; Ghanem, K.G.; Klebanoff, M.A.; Taha, T.E.; Scharfstein, D.O.; Zenilman, J.M. The Effect of Vaginal Douching Cessation on Bacterial Vaginosis: A Pilot Study. Am. J. Obstet. Gynecol. 2008, 198, 628.e1–628.e7. [Google Scholar] [CrossRef]
- Carter, K.; Bassis, C.; McKee, K.; Bullock, K.; Eastman, A.; Young, V.; Bell, J. The Impact of Tampon Use on the Vaginal Microbiota across Four Menstrual Cycles. Am. J. Obstet. Gynecol. 2018, 219, 639. [Google Scholar] [CrossRef]
- Oh, J.E.; Kim, B.-C.; Chang, D.-H.; Kwon, M.; Lee, S.Y.; Kang, D.; Kim, J.Y.; Hwang, I.; Yu, J.-W.; Nakae, S.; et al. Dysbiosis-Induced IL-33 Contributes to Impaired Antiviral Immunity in the Genital Mucosa. Proc. Natl. Acad. Sci. USA 2016, 113, E762–E771. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.S.; Katoch, V.; Rajguru, S.A.; Rajpoot, N.; Singh, P.; Wakhle, S. Periodontal Disease: A Possible Risk-Factor for Adverse Pregnancy Outcome. J. Int. Oral Health 2015, 7, 137–142. [Google Scholar]
- Nicolò, S.; Tanturli, M.; Mattiuz, G.; Antonelli, A.; Baccani, I.; Bonaiuto, C.; Baldi, S.; Nannini, G.; Menicatti, M.; Bartolucci, G.; et al. Vaginal Lactobacilli and Vaginal Dysbiosis-Associated Bacteria Differently Affect Cervical Epithelial and Immune Homeostasis and Anti-Viral Defenses. IJMS 2021, 22, 6487. [Google Scholar] [CrossRef] [PubMed]
- Piyathilake, C.J.; Ollberding, N.J.; Kumar, R.; Macaluso, M.; Alvarez, R.D.; Morrow, C.D. Cervical Microbiota Associated with Higher Grade Cervical Intraepithelial Neoplasia in Women Infected with High-Risk Human Papillomaviruses. Cancer Prev. Res. 2016, 9, 357–366. [Google Scholar] [CrossRef] [PubMed]
- ns-1904637Mirmonsef, P.; Hotton, A.L.; Gilbert, D.; Burgad, D.; Landay, A.; Weber, K.M.; Cohen, M.; Ravel, J.; Spear, G.T. Free Glycogen in Vaginal Fluids Is Associated with Lactobacillus Colonization and Low Vaginal pH. PLoS ONE 2014, 9, e102467. [Google Scholar] [CrossRef]
- Witkin, S.S.; Linhares, I.M.; Giraldo, P. Bacterial Flora of the Female Genital Tract: Function and Immune Regulation. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Hickey, R.J.; Zhou, X.; Pierson, J.D.; Ravel, J.; Forney, L.J. Understanding Vaginal Microbiome Complexity from an Ecological Perspective. Transl. Res. 2012, 160, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Caretto, M.; Giannini, A.; Russo, E.; Simoncini, T. Preventing Urinary Tract Infections after Menopause without Antibiotics. Maturitas 2017, 99, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Park, Y.J. Probiotics in the Prevention and Treatment of Postmenopausal Vaginal Infections: Review Article. J. Menopausal Med. 2017, 23, 139–145. [Google Scholar] [CrossRef]
- Sharkey, D.J.; Macpherson, A.M.; Tremellen, K.P.; Robertson, S.A. Seminal Plasma Differentially Regulates Inflammatory Cytokine Gene Expression in Human Cervical and Vaginal Epithelial Cells. Mol. Hum. Reprod. 2007, 13, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Liang, J.; Zhai, X.; Li, G.; Wang, H.; Han, L.; Lin, L.; Ren, Y.; Liu, S.; Liu, C.; et al. Oxytocin Signalling in Dendritic Cells Regulates Immune Tolerance in the Intestine and Alleviates DSS-Induced Colitis. Clin. Sci. 2021, 135, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F. Center Role of the Oxytocin-Secreting System in Neuroendocrine-Immune Network Revisited. J. Clin. Exp. Neuroimmunol. 2016, 1, 102. [Google Scholar]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-Gut Microbiome Axis: Physiological and Clinical Implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Nugent, R.P.; Krohn, M.A.; Hillier, S.L. Reliability of diagnosing bacterial vaginosis is improved 431 by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991, 29, 297–301. [Google Scholar] [CrossRef]
- Amsel, R.; Totten, P.A.; Spiegel, C.A.; Chen, K.C.; Eschenbach, D.; Holmes, K.K. Nonspecific Vaginitis. Diagnostic Criteria and Microbial and Epidemiologic Associations. Am. J. Med. 1983, 74, 14–22. [Google Scholar] [CrossRef]
- Harmanli, O.H.; Cheng, G.Y.; Nyirjesy, P.; Chatwani, A.; Gaughan, J.P. Urinary Tract Infections in Women with Bacterial Vaginosis. Obstet. Gynecol. 2000, 95, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Gilbert, N.M. Roles of the Vagina and the Vaginal Microbiota in Urinary Tract Infection: Evidence from Clinical Correlations and Experimental Models. GMS Infect. Dis. 2020, 8, Doc02. [Google Scholar] [CrossRef] [PubMed]
- Gottschick, C.; Deng, Z.-L.; Vital, M.; Masur, C.; Abels, C.; Pieper, D.H.; Wagner-Döbler, I. The Urinary Microbiota of Men and Women and Its Changes in Women during Bacterial Vaginosis and Antibiotic Treatment. Microbiome 2017, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Soper, D.E.; Brockwell, N.J.; Dalton, H.P.; Johnson, D. Observations Concerning the Microbial Etiology of Acute Salpingitis. Am. J. Obstet. Gynecol. 1994, 170, 1008–1014. [Google Scholar] [CrossRef]
- Kunaseth, J.; Waiyaput, W.; Chanchaem, P.; Sawaswong, V.; Permpech, R.; Payungporn, S.; Sophonsritsuk, A. Vaginal Microbiome of Women with Adenomyosis: A Case-Control Study. PLoS ONE 2022, 17, e0263283. [Google Scholar] [CrossRef] [PubMed]
- Brunham, R.C.; Gottlieb, S.L.; Paavonen, J. Pelvic Inflammatory Disease. N. Engl. J. Med. 2015, 372, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- McQueen, D.B.; Bernardi, L.A.; Stephenson, M.D. Chronic Endometritis in Women with Recurrent Early Pregnancy Loss and/or Fetal Demise. Fertil. Steril. 2014, 101, 1026–1030. [Google Scholar] [CrossRef]
- Allsworth, J.E.; Lewis, V.A.; Peipert, J.F. Viral Sexually Transmitted Infections and Bacterial Vaginosis: 2001-2004 National Health and Nutrition Examination Survey Data. Sex. Transm. Dis. 2008, 35, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Moreno, I.; Simón, C. Bacterial Vaginosis and Its Association with Infertility, Endometritis, and Pelvic Inflammatory Disease. Am. J. Obstet. Gynecol. 2021, 224, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Fichorova, R.N.; Onderdonk, A.B.; Yamamoto, H.; Delaney, M.L.; DuBois, A.M.; Allred, E.; Leviton, A. Maternal microbe-specific modulation of inflammatory response in extremely low-gestational-age newborns. mBio 2011, 2, e00280–e00310. [Google Scholar] [CrossRef]
- Blencowe, H.; Krasevec, J.; de Onis, M.; Black, R.E.; An, X.; Stevens, G.A.; Borghi, E.; Hayashi, C.; Estevez, D.; Cegolon, L.; et al. National, Regional, and Worldwide Estimates of Low Birthweight in 2015, with Trends from 2000: A Systematic Analysis. Lancet Glob. Health 2019, 7, e849–e860. [Google Scholar] [CrossRef]
- Lyons, R.A.; Saridogan, E.; Djahanbakhch, O. The Reproductive Significance of Human Fallopian Tube Cilia. Hum. Reprod. Update 2006, 12, 363–372. [Google Scholar] [CrossRef]
- Gaudoin, M.; Rekha, P.; Morris, A.; Lynch, J.; Acharya, U. Bacterial Vaginosis and Past Chlamydial Infection Are Strongly and Independently Associated with Tubal Infertility but Do Not Affect in Vitro Fertilization Success Rates. Fertil. Steril. 1999, 72, 730–732. [Google Scholar] [CrossRef]
- Fontaine, E.A.; Clark, J.B.; Abeck, D.; Taylor-Robinson, D. The Effect of a Toxin from Bacteroides Ureolyticus on the Mucosal Epithelium of Human and Bovine Oviducts. Br. J. Exp. Pathol. 1988, 69, 631–638. [Google Scholar] [PubMed]
- Taylor-Robinson, D.; Boustouller, Y.L. Damage to Oviduct Organ Cultures by Gardnerella Vaginalis. Int. J. Exp. Pathol. 2011, 92, 260–265. [Google Scholar] [CrossRef]
- Koumans, E.H.; Markowitz, L.E.; Hogan, V.; CDC BV Working Group. Indications for Therapy and Treatment Recommendations for Bacterial Vaginosis in Nonpregnant and Pregnant Women: A Synthesis of Data. Clin. Infect. Dis. 2002, 35 (Suppl. 2), S152–S172. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.S.; Morton, A.N.; Hocking, J.; Garland, S.M.; Morris, M.B.; Moss, L.M.; Horvath, L.B.; Kuzevska, I.; Fairley, C.K. High Recurrence Rates of Bacterial Vaginosis over the Course of 12 Months after Oral Metronidazole Therapy and Factors Associated with Recurrence. J. Infect. Dis. 2006, 193, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Hickey, R.J.; Forney, L.J. Gardnerella vaginalis does not always cause Bacterial Vaginosis. J. Infect. Dis. 2014, 210, 1682–1683. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, E.S.; Willner, D.; Buttini, M.; Hafner, L.M.; Theodoropoulos, C.; Huygens, F. The Fallopian Tube Microbiome: Implications for Reproductive Health. Oncotarget 2018, 9, 21541–21551. [Google Scholar] [CrossRef]
- Koedooder, R.; Mackens, S.; Budding, A.; Fares, D.; Blockeel, C.; Laven, J.; Schoenmakers, S. Identification and Evaluation of the Microbiome in the Female and Male Reproductive Tracts. Hum. Reprod. Update 2019, 25, 298–325. [Google Scholar] [CrossRef]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence That the Endometrial Microbiota Has an Effect on Implantation Success or Failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Kunz, G.; Beil, D.; Deiniger, H.; Einspanier, A.; Mall, G.; Leyendecker, G. The Uterine Peristaltic Pump. In The Fate of the Male Germ Cell; Ivell, R., Holstein, A.-F., Eds.; Springer US: Boston, MA, USA, 1997; pp. 267–277. [Google Scholar] [CrossRef]
- Romero, R.; Gomez-Lopez, N.; Winters, A.D.; Jung, E.; Shaman, M.; Bieda, J.; Panaitescu, B.; Pacora, P.; Erez, O.; Greenberg, J.M.; et al. Evidence That Intra-Amniotic Infections Are Often the Result of an Ascending Invasion—A Molecular Microbiological Study. J. Perinat. Med. 2019, 47, 915–931. [Google Scholar] [CrossRef]
- Jiménez, E.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Olivares, M.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Is Meconium from Healthy Newborns Actually Sterile? Res. Microbiol. 2008, 159, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Curry, A.; Williams, T.; Penny, M.L. Pelvic Inflammatory Disease: Diagnosis, Management and Prevention. Am. Fam. Physician 2019, 100, 357–364. [Google Scholar]
- Haggerty, C.L.; Hillier, S.L.; Bass, D.C.; Ness, R.B.; PID Evaluation and Clinical Health study investigators. Bacterial Vaginosis and Anaerobic Bacteria Are Associated with Endometritis. Clin. Infect. Dis. 2004, 39, 990–995. [Google Scholar] [CrossRef]
- Den Heijer, C.D.J.; Hoebe, C.J.P.A.; Driessen, J.H.M.; Wolffs, P.; van den Broek, I.V.F.; Hoenderboom, B.M.; Williams, R.; de Vries, F.; Dukers-Muijrers, N.H.T.M. Chlamydia Trachomatis and the Risk of Pelvic Inflammatory Disease, Ectopic Pregnancy, and Female Infertility: A Retrospective Cohort Study Among Primary Care Patients. Clin. Infect. Dis. 2019, 69, 1517–1525. [Google Scholar] [CrossRef]
- Kairys, N.; Roepke, C. Tubo-Ovarian Abscess. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wiesenfeld, H.C.; Hillier, S.L.; Krohn, M.A.; Landers, D.V.; Sweet, R.L. Bacterial Vaginosis Is a Strong Predictor of Neisseria Gonorrhoeae and Chlamydia Trachomatis Infection. Clin. Infect. Dis. 2003, 36, 663–668. [Google Scholar] [CrossRef]
- Simms, I.; Eastick, K.; Mallinson, H.; Thomas, K.; Gokhale, R.; Hay, P.; Herring, A.; Rogers, P.A. Associations between Mycoplasma Genitalium, Chlamydia Trachomatis and Pelvic Inflammatory Disease. J. Clin. Pathol. 2003, 56, 616–618. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.Y.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef] [PubMed]
- Daliry, A.; Pereira, E.N.G.d.S. Role of Maternal Microbiota and Nutrition in Early-Life Neurodevelopmental Disorders. Nutrients 2021, 13, 3533. [Google Scholar] [CrossRef] [PubMed]
- Bordeleau, M.; Fernández de Cossío, L.; Chakravarty, M.M.; Tremblay, M.È. From Maternal Diet to Neurodevelopmental Disorders: A Story of Neuroinflammation. Front. Cell. Neurosci. 2021, 14, 612705. [Google Scholar] [CrossRef] [PubMed]
- Duluc, D.; Gannevat, J.; Anguiano, E.; Zurawski, S.; Carley, M.; Boreham, M.; Stecher, J.; Dullaers, M.; Banchereau, J.; Oh, S. Functional Diversity of Human Vaginal APC Subsets in Directing T-Cell Responses. Mucosal Immunol. 2013, 6, 626–638. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the Microbiota and the Immune System. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Guzeloglu-Kayisli, O.; Kayisli, U.A.; Taylor, H.S. The Role of Growth Factors and Cytokines during Implantation: Endocrine and Paracrine Interactions. Semin. Reprod. Med. 2009, 27, 62–79. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Ménard, C.; Terme, M.; Flament, C.; Taieb, J.; Chaput, N.; Puig, P.E.; Novault, S.; Escudier, B.; Vivier, E.; et al. CD4+CD25+ Regulatory T Cells Inhibit Natural Killer Cell Functions in a Transforming Growth Factor-Beta-Dependent Manner. J. Exp. Med. 2005, 202, 1075–1085. [Google Scholar] [CrossRef]
- Robinson, L.S.; Schwebke, J.; Lewis, W.G.; Lewis, A.L. Identification and Characterization of NanH2 and NanH3, Enzymes Responsible for Sialidase Activity in the Vaginal Bacterium Gardnerella Vaginalis. J. Biol. Chem. 2019, 294, 5230–5245. [Google Scholar] [CrossRef]
- Holmes, K.K.; Chen, K.C.; Lipinski, C.M.; Eschenbach, D.A. Vaginal Redox Potential in Bacterial Vaginosis (nonspecific Vaginitis). J. Infect. Dis. 1985, 152, 379–382. [Google Scholar] [CrossRef]
- Baldewijns, S.; Sillen, M.; Palmans, I.; Vandecruys, P.; Van Dijck, P.; Demuyser, L. The Role of Fatty Acid Metabolites in Vaginal Health and Disease: Application to Candidiasis. Front. Microbiol. 2021, 12, 705779. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.J.; Graham, C.H. The Role of Macrophages in Utero-Placental Interactions during Normal and Pathological Pregnancy. Immunol. Investig. 2008, 37, 535–564. [Google Scholar] [CrossRef] [PubMed]
- Plesniarski, A.; Siddik, A.B.; Su, R.-C. The Microbiome as a Key Regulator of Female Genital Tract Barrier Function. Front. Cell. Infect. Microbiol. 2021, 11, 790627. [Google Scholar] [CrossRef] [PubMed]
| Disruption | Mechanism | Result | Reference |
|---|---|---|---|
| Biological | |||
| Menstruation | Temporary dip in estrogen during the secretory phase | Estrogen promotes proliferation of Lactobacillus spp. Responsible for maintaining a low vaginal pH. A decrease in estrogen levels results in a decrease in Lactobacilli and a temporary rise in vaginal pH which increases susceptibility to opportunistic pathogens. | Bardos et al., 2020 [36] |
| Menopause | Decrease in overall estrogen levels | Chronic low estrogen levels decrease accumulation of glycogen in the vaginal epithelium thereby reducing colonization of lactobacilli dependent on epithelial glycogen levels. | Achilles et al., 2018 [37] |
| Pregnancy | Inflammatory responses are moderated | Towards the end of pregnancy, the lower reproductive tissues increase microbial diversity and decrease Lactobaccilus spp. | Bardos et al., 2020 [36] |
| Lifestyle | |||
| Hygiene products | Changes to pH levels | Vaginal douching has been linked to increased susceptibility to bacterial vaginosis. | Brotman et al., 2008 [38] |
| Tampons | Changes to lactic acid production and pH | Decreased vaginal microbiome stability is associated with tampon use. | Carter et al., 2018 [39] |
| Antibiotic use | Killing of commensal bacteria | Depletion of commensal bacteria due to antibiotic treatment was found to cause increased secretions of IL-33 in vaginal epithelium, suppressing antiviral immunity. | Oh et al., 2016 [40] |
| IUD-use | Changes in commensal microbial populations | Copper IUD use was associated with an increase in Gardnerella vaginalis and Atopobium vaginae possibly leading to increased bacterial vaginosis risk. LNG-releasing IUD use was associated with a decrease in Lactobacillus spp. Furthermore, an increase in Candida spp. | Achilles et al., 2018 [37] |
| Sexual activity | Changes in commensal microbial populations, shift in CST type | The number of sex partners and age when sexual activity started is associated with CST grouping and microbiome stability. Women with new sexual partners are at an increased risk for bacterial vaginosis. | Chen et al., 2017 [8] |
| Causative Pathogenesis | |||
| Leaky gut | Hematogenous spread of GI commensals into peritoneal cavity | Increased risk of placental infection, pre-term birth, and miscarriage | Bardos et al., 2020 [36] |
| Periodontal disease | Microbial transmission from oral cavity | Increased risk of intra-uterine infection and pre-eclampsia | Parihar et al., 2015 [41] |
| Resultant Pathogenesis | |||
| HPV infection | Opportunistic infection | Subjects with HPV infections have less Lactobacillus spp. Furthermore, more microbial diversity | Nicolò et al., 2021 [42] |
| Cervical cancer | Variable gene expression of L. iners-dependent community functionality and composition | L. Iners decreased amongst women with HPV infection, suggesting a protective effect. Alternatively, L. iners is associated with CIN 2+ and ICC | Piyathilake et al., 2016 [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakama, C.; Thompson, B.; Szybala, C.; McBeth, A.; Dobner, P.; Zwickey, H. The Continuum of Microbial Ecosystems along the Female Reproductive Tract: Implications for Health and Fertility. Pathogens 2022, 11, 1244. https://doi.org/10.3390/pathogens11111244
Nakama C, Thompson B, Szybala C, McBeth A, Dobner P, Zwickey H. The Continuum of Microbial Ecosystems along the Female Reproductive Tract: Implications for Health and Fertility. Pathogens. 2022; 11(11):1244. https://doi.org/10.3390/pathogens11111244
Chicago/Turabian StyleNakama, Claudia, Brice Thompson, Cory Szybala, Andrea McBeth, Piper Dobner, and Heather Zwickey. 2022. "The Continuum of Microbial Ecosystems along the Female Reproductive Tract: Implications for Health and Fertility" Pathogens 11, no. 11: 1244. https://doi.org/10.3390/pathogens11111244
APA StyleNakama, C., Thompson, B., Szybala, C., McBeth, A., Dobner, P., & Zwickey, H. (2022). The Continuum of Microbial Ecosystems along the Female Reproductive Tract: Implications for Health and Fertility. Pathogens, 11(11), 1244. https://doi.org/10.3390/pathogens11111244







