Molecular Epidemiological Investigation of Piroplasms and Anaplasmataceae Bacteria in Egyptian Domestic Animals and Associated Ticks
Abstract
1. Introduction
2. Results
2.1. Prevalence of Pathogens
2.2. Analysis of Epidemiological Factors
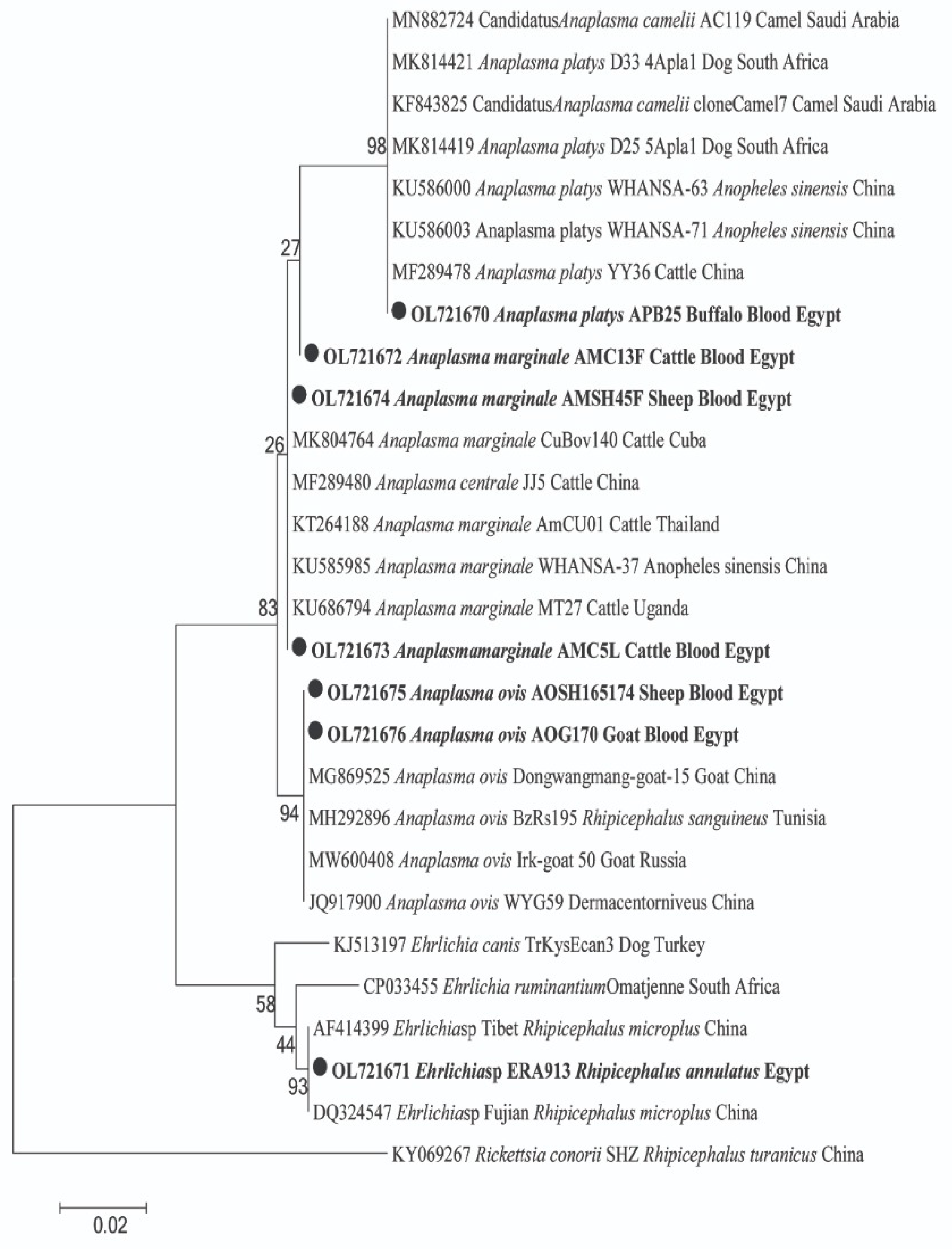
2.3. Sequencing and Phylogenetic Analyses of Pathogen in Animal Hosts
2.4. Ticks and Associated Pathogens
3. Discussion
4. Materials and Methods
4.1. Animals and Blood Sampling
4.2. Ticks
4.3. Molecular Investigation
4.3.1. DNA Extraction
4.3.2. Screening of Pathogens DNA by Standard PCR
4.3.3. Sequencing and Phylogenetic Analyses
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AL-Hosary, A.; Răileanu, C.; Tauchmann, O.; Fischer, S.; Nijhof, M.A.; Silaghi, C. Epidemiology and genotyping of An aplasma marginale and co-infection with piroplasms and other Anaplasmataceae in cattle and buffaloes from Egypt. Parasit. Vectors 2020, 13, 495. [Google Scholar] [CrossRef] [PubMed]
- Dahmana, H.; Amanzougaghene, N.; Davoust, B.; Carette, O.; Normand, T.; Demoncheaux, J.P.; Mulot, B.; Fabrizy, B.; Scandola, P.; Chik, M.; et al. Great diversity of Piroplasmida in Equidae in Africa and Europe, including potential new species. Vet. Parasitol. Reg. Stud. Rep. 2019, 18, e100332. [Google Scholar] [CrossRef]
- AL-Hosary, A.; Ahmed, L.; Ahmed, J.; Nijhof, A.; Clausen, P. Epidemiological study on tropical theileriosis (Theileria annulata infection) in the Egyptian Oases with special reference to the molecular characterization of Theileria spp. Ticks Tick Borne Dis. 2018, 9, 1489–1493. [Google Scholar] [CrossRef]
- Abdullah, H.H.; Amanzougaghene, N.; Dahmana, H.; Louni, M.; Raoult, D.; Mediannikov, O. Multiple vector-borne pathogens of domestic animals in Egypt. PLoS Neg. Trop. Dis. 2021, 15, e0009767. [Google Scholar] [CrossRef]
- Brown, C. Tropical theileriosis. In Foreign Animal Diseases, 7th ed.; Brown, C., Torres, A., Eds.; Boca Publications: Boca Raton, FL, USA, 2008; pp. 401–404. [Google Scholar]
- Bishop, R.P.; Odongo, D.O.; Mann, D.J.; Pearson, T.W.; Sugimoto, C.; Haines, L.R.; Glass, E.; Jensen, K.; Seitzer, U.; Ahmed, J.S.; et al. Theileria. In Genome Mapping and Genomics in Animal–Associated Microbes; Nene, V., Kole, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 191–231. [Google Scholar]
- Bilgic, H.B.; Karagenc, T.; Shiels, B.; Tait, A.; Eren, H.; Weir, W. Evaluation of cytochrome b as a sensitive target for PCR based detection of T. annulata carrier animals. Vet. Parasitol. 2010, 174, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Zeb, J.; Shams, S.; Din, I.U.; Ayaz, S.; Khan, A.; Nasreen, N.; Khan, H.; Khan, M.A.; Senbill, H. Molecular epidemiology and associated risk factors of Anaplasma marginale and Theileria annulata in cattle from North-western Pakistan. Vet. Parasitol. 2020, 279, 109044. [Google Scholar] [CrossRef]
- Pupin, R.C.; Guizelini, C.C.; Lemos, R.A.A.; Martins, T.B.; Borges, F.A.; Borges, F.G.L.; Gomes, D.C. Retrospective study of epidemiological, clinical and pathological findings of bovine babesiosis in Mato Grosso do Sul, Brazil (1995–2017). Ticks Tick Borne Dis. 2019, 10, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Ola-Fadunsin, S.D.; Sharma, R.S.K.; Abdullah, D.A.; Gimba, F.I.; Abdullah, F.F.J.; Sani, R.A. The molecular prevalence, distribution and risk factors associated with Babesia bigemina infection in Peninsular Malaysia. Ticks Tick Borne Dis. 2021, 12, 101653. [Google Scholar] [CrossRef] [PubMed]
- Tembue, A.A.M.; Silva, F.J.M.; Silva, J.B.; Santos, T.M.; Santos, H.A.; Soares, C.O.; Fonseca, A.H. Risk factors associated with the frequency of antibodies against Babesia bovis and Babesia bigemina in cattle in southern Mozambique. Pesq. Vet. Bras. 2011, 31, 663–666. [Google Scholar] [CrossRef]
- Elhariri, M.D.; Elhelw, R.A.; Hamza, D.A.; Soliman, D.E. Molecular detection of Anaplasma marginale in the Egyptian water bufaloes (Bubuloes bubalis) based on major surface protein 1α. J. Egyp. Soc. Parasitol. 2017, 47, 247–252. [Google Scholar] [CrossRef]
- Kocan, K.M.; de la Fuente, J.; Blouin, E.F. Characterization of the tick–pathogen– host interface of the tick–borne rickettsia Anaplasma marginale. In Ticks: Biology, Diseases and Control; Bowman, A.S., Nuttall, P.A., Eds.; Cambridge Univesity Press: Cambridge, UK, 2008; pp. 325–343. [Google Scholar]
- Aubry, P.; Geale, D.W. A review of bovine anaplasmosis. Transbound. Emerg. Dis. 2011, 58, 1–30. [Google Scholar] [CrossRef]
- Berthelsson, J.; Ramabu, S.S.; Lysholm, S.; Aspán, A.; Wensman, J.J. Anaplasma ovis infection in goat flocks around Gaborone, Botswana. Comp. Clin. Pathol. 2020, 29, 167–172. [Google Scholar] [CrossRef]
- Medley, G.F.; Perry, B.D.; Young, A.S. Preliminary analysis of the transmission dynamics of Theileria parva in eastern Africa. Parasitology 1993, 106, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Calder, J.A.; Reddy, G.R.; Chieves, L.; Courtney, C.H.; Littell, R.; Livengood, J.R. Monitoring Babesia bovis infections in cattle by using PCR-based tests. J. Clin. Microbiol. 1996, 34, 2748–2755. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.A.E.; AbouLaila, M.; ElKhatam, A.; Abdel-Wahab, A.; Rizk, M.A. An epidemiological survey of Theileria equi parasite in donkeys (Equus asinus) in Egypt. Vet. Parasitol. Reg. Stud. Rep. 2020, 21, 100449. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, J.; Cesar, E.; Campbell, M.D.; Samlal, M.; Ammons, D. A field PCR for the routine detection of Babesia equi in horses. J. Vet. Parasitol. 2003, 114, 81–87. [Google Scholar] [CrossRef]
- Rosales, R.; Rangel-Rivas, A.; Escalona, A.; Jordan, L.S.; Gonzatti, M.I.; Aso, P.M.; Perrone, T.; Silva-Iturriza, A.; Mijares, A. Detection of Theileria equi and Babesia caballi infections in Venezuelan horses using competitive-inhibition ELISA and PCR. Vet. Parasitol. 2013, 196, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Buling, A.; Criado-Fornelio, A.; Asenzo, G.; Benitez, D.; Barba-Carretero, J.C.; Florin-Christensen, M. A quantitative PCR assay for the detection and quantification of Babesia bovis and B. bigemina. Vet. Parasitol. 2007, 147, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Elsify, A.; Sivakumar, T.; Nayel, M.; Salama, A.; Elkhtam, A.; Rizk, M.; Mosaab, O.; Sultan, K.; Elsayed, S.; Igarashi, I.; et al. An epidemiological survey of bovine Babesia and Theileria parasites in cattle, buffaloes, and sheep in Egypt. Parasitol. Int. 2014, 64, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Sadeddine, R.; Diarra, A.Z.; Laroche, M.; Mediannikov, O.; Righi, S.; Benakhla, A.; Dahmana, H.; Raoult, D.; Parola, P. Molecular identification of protozoal and bacterial organisms in domestic animals and their infesting ticks from northeastern Algeria. Ticks Tick Borne Dis. 2020, 11, 101330. [Google Scholar] [CrossRef] [PubMed]
- El-Ashker, M.; Hotzel, H.; Gwida, M.; El-Beskawy, M.; Silaghi, C.; Tomaso, H. Molecular biological identification of Babesia, Theileria, and Anaplasma species in cattle in Egypt using PCR assays, gene sequence analysis and a novel DNA microarray. Vet. Parasitol. 2015, 207, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Soosaraei, M.; Haghi, M.M.; Etemadifar, F.; Fakhar, M.; Teshnizi, S.H.; Asfaram, S.; Esboei, B.R. Status of Anaplasma spp. infection in domestic ruminants from Iran: A systematic review with meta-analysis. Parasit. Epidemiol. Control. 2020, 11, e00173. [Google Scholar] [CrossRef] [PubMed]
- Kocan, K.M.; de la Fuente, J.; Blouin, E.F.; Coetzee, J.F.; Ewing, S.A. The natural history of Anaplasma marginale. Vet. Parasitol. 2010, 167, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.A.; Salama, A.; El-Sayed, S.A.; Elsify, A.; El-Ashkar, M.; Ibrahim, H.; Youssef, M.; El-Khodery, S. Animal level risk factors associated with Babesia and Theileria infections in cattle in Egypt. Acta Parasitol. 2017, 62, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, O.; El-Adawy, H.; Melzer, F.; Roesler, U.; Neubauer, H.; Mertens-Scholz, K. Seroprevalence and Molecular Detection of Bovine Anaplasmosis in Egypt. Pathogens 2020, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- CAPMAS. Animal Diseases. Available online: https://www.capmas.gov.eg/ (accessed on 17 June 2019).
- Ibrahim, H.M.; Adjou Moumouni, P.F.; Mohamed-Geba, K.; Sheir, S.K.; Hashem, I.S.; Cao, S.; Terkawi, M.A.; Kamyingkird, K.; Nishikawa, Y.; Suzuki, H.; et al. Molecular and serological prevalence of Babesia bigemina and Babesia bovis in cattle and water buffalos under small-scale dairy farming in Beheira and Faiyum Provinces, Egypt. Vet. Parasitol. 2013, 198, 187–192. [Google Scholar] [CrossRef]
- Nayel, M.; El-Dakhly, K.M.; Aboulaila, M.; Elsify, A.; Hassan, H.; Ibrahim, E.; Salama, A.; Yanai, T. The use of different diagnostic tools for Babesia and Theileria parasites in cattle in Menofia, Egypt. Parasitol. Res. 2012, 111, 1019–1024. [Google Scholar] [CrossRef]
- Adel, E.M. Studies on Some Blood Parasites Infecting Farm Animals in Gharbia Governorate. Ph.D. Thesis, Cairo University, Cairo, Egypt, 2007. [Google Scholar]
- El-Fayomy, A.O.; Ghoneim, A.M.; Abu-Samak, O.A.; Khidr, A.A. Contribution of Babesia to the illness of cows in Port Said Governorate, Egypt. Glob. Vet. 2013, 11, 118–222. [Google Scholar]
- AL-Hosary, A.; Răileanu, C.; Tauchmann, O.; Fischer, S.; Nijhof, M.A.; Silaghi, C. Tick species identification and molecular detection of tick-borne pathogens in blood and ticks collected from cattle in Egypt. Ticks Tick Borne Dis. 2021, 12, 101676. [Google Scholar] [CrossRef]
- Tumwebaze, M.A.; Lee, S.H.; Adjou Moumouni, P.F.; Mohammed-Geba, K.; Sheir, S.K.; Galal-Khallaf, A.; Abd El Latif, H.M.; Morsi, D.S.; Bishr, N.M.; Galon, E.M.; et al. First detection of Anaplasma ovis in sheep and Anaplasma platys-like variants from cattle in Menoufia governorate, Egypt. Parasitol. Int. 2020, 78, 102150. [Google Scholar] [CrossRef]
- Younis, E.E.; Hegazy, N.A.M.; El-Deeb, W.; El-Khatib, R.M. Epidemiological and biochemical studies on bovine anaplamosis in Dakahliaand Demiatta Governorates in Egypt. Bull. Anim. Health Prod. Afr. 2009, 57, 4. [Google Scholar]
- Abo El Fadl, E.A.; El-Ashker, M.; Suganuma, K.; Kayano, M. Discriminant analysis for the prediction and classification of tick-borne infections in some dairy cattle herds at Dakahlia Governorate, Egypt. Jpn. J. Vet. Res. 2017, 65, 127–133. [Google Scholar]
- Fereig, R.M.; Mohamed, S.G.A.; Mahmoud, H.; AbouLaila, M.R.; Guswanto, A.; Nguyen, T.T.; Mohamed, A.A.; Inoue, N.; Igarashi, I.; Nishikawa, Y. Seroprevalence of Babesia bovis, B. bigemina, Trypanosoma evansi, and Anaplasma marginale antibodies in cattle in southern Egypt. Ticks Tick Borne Dis. 2017, 8, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.E.I.; Ali, A.F.; el Hamied, O.A. Epidemiological Studies, Molecular Diagnosis of Anaplasma marginale in Cattle and Biochemical Changes Associated with it in Kaliobia Governorate. Am. J. Infect. Dis. Microbiol. 2013, 1, 46–49. [Google Scholar]
- Abdullah, H.H.A.M.; Aboelsoued, D.; Farag, K.T.; Abdel Megeed, N.K.; Abdel-Shafy, S.; Parola, P.; Raoult, D.; Mediannikov, O. Molecular characterization of some equine vector-borne diseases and associated arthropods in Egypt. Acta Trop. 2022, 227, 106274. [Google Scholar] [CrossRef]
- Selim, A.; Attia, K.A.; Alsubki, R.A.; Albohairy, F.; Kimiko, I.; Ben Said, M. The first study on the seroprevalence of Anaplasma spp. in small ruminants and assessment of associated risk factors in North Egypt. Vet. World 2022, 15, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Boussaadoun, M.A.; Gharbi, M.; Sayeh, L.; Soudani, M.C.; Darghouth, M.A. Epidemiological situation of bovine tropical theileriosis (Theileria annulata infection) in the Northwest Tunisia. J. Adv. Parasitol. 2015, 2, 69–74. [Google Scholar] [CrossRef]
- Prado, I.C.B.; Capuno, L.X.B., Jr.; Collera, P.D.; Cabralda, A.P.D.; De Ramos, K.A.S.; Bernardo, J.M.G.; Divina, B.P.; Masatani, T.; Tanaka, T.; Galay, R.L. Molecular Detection and Characterization of Babesia and Theileria in Cattle and Water Buffaloes from Southern Luzon, Philippines. Microorganisms 2022, 10, 678. [Google Scholar] [CrossRef]
- Adjou Moumouni, P.F.; Minoungou, G.L.B.; Dovonou, C.E.; Galon, E.M.; Efstratiou, A.; Tumwebaze, M.A.; Byamukama, B.; Vudriko, P.; Umemiya-Shirafuji, R.; Suzuki, H.; et al. A Survey of Tick Infestation and Tick-Borne Piroplasm Infection of Cattle in Oudalan and Séno Provinces, Northern Burkina Faso. Pathogens 2022, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Said, M.B.; Attia, K.A.; Alsubki, R.A.; Mohamed, A.A.; Kimiko, I.; Selim, A. Molecular epidemiological survey, genetic characterization and phylogenetic analysis of Anaplasma ovis infecting sheep in Northern Egypt. Acta Trop. 2022, 229, 106370. [Google Scholar] [CrossRef] [PubMed]
- Belkahia, H.; Ben Said, M.; El Hamdi, S.; Yahiaoui, M.; Gharbi, M.; Daaloul-Jedidi, M.; Mhadhbi, M.; Jedidi, M.; Darghouth, M.A.; Klabi, I.; et al. First molecular identification and genetic characterization of Anaplasma ovis in sheep from Tunisia. Small Rumin. Res. 2014, 121, 404–410. [Google Scholar] [CrossRef]
- Dahmani, M.; Davoust, B.; Sambou, M.; Bassene, H.; Scandola, P.; Ameur, T.; Raoult, D.; Fenollar, F.; Mediannikov, O. Molecular investigation and phylogeny of species of the Anaplasmataceae infecting animals and ticks in Senegal. Parasit. Vectors. 2019, 12, 495. [Google Scholar] [CrossRef]
- Abdullah, H.S.; Dyary, O.H. Molecular characterization and phylogenic analysis of Anaplasma spp. in small ruminants from Sulaymaniyah governorate, Iraq. Iraqi J. Vet. Sci. 2022, 36, 15–20. [Google Scholar] [CrossRef]
- Aung, A.; Kaewlamuna, W.; Narapakdeesakula, D.; Pooferya, J.; Kaewthamasorna, M. Molecular detection and characterization of tick-borne parasites in goats and ticks from Thailand. Ticks Tick Borne Dis. 2022, 13, 101938. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Faruque, M.R.; Rahman, M.M.; Chowdhury, M.Y.E. Epidemiology and molecular detection of Anaplasma spp. in goats from Chattogram district, Bangladesh. Vet. Med. Sci. 2022, 8, 1240–1249. [Google Scholar] [CrossRef]
- Nguyen, A.H.L.; Tiawsirisup, S.; Kaewthamasorn, M. Molecular detection and genetic characterization of Anaplasma marginale and Anaplasma platys-like (Rickettsiales: Anaplasmataceae) in water buffalo from eight provinces of Thailand. BMC Vet. Res. 2020, 16, 380. [Google Scholar] [CrossRef] [PubMed]
- Bursakov, S.A.; Kovalchuk, S.N. Co-infection with tick-borne disease agents in cattle in Russia. Ticks Tick Borne Dis. 2019, 10, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Abanda, B.; Paguem, A.; Abdoulmoumini, M.; Kingsley, T.M.; Renz, A.; Eisenbarth, A. Molecular identification and prevalence of tick-borne pathogens in zebu and taurine cattle in North Cameroon. Parasit. Vectors 2019, 12, 448. [Google Scholar] [CrossRef]
- Lu, M.; Tian, J.; Pan, X.; Qin, X.; Wang, W.; Chen, J.; Guo, W.; Li, K. Identification of Rickettsia spp., Anaplasma spp., and an Ehrlichia canis-like agent in Rhipicephalus microplus from Southwest and South-Central China. Ticks Tick Borne Dis. 2022, 13, 101884. [Google Scholar] [CrossRef]
- Orkun, Ö. Comprehensive screening of tick-borne microorganisms indicates that a great variety of pathogens are circulating between hard ticks (Ixodoidea: Ixodidae) and domestic ruminants in natural foci of Anatolia. Ticks Tick Borne Dis. 2022, 13, 102027. [Google Scholar] [CrossRef]
- Ghafar, A.; Cabezas-Cruz, A.; Galon, C.; Obregon, D.; Gasser, R.B.; Moutailler, S.; Jabber, A. Bovine ticks harbour a diverse array of microorganisms in Pakistan. Parasites Vectors 2020, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Pena, A.; Bouattour, A.; Camicas, J.L.; Walker, A.R. Ticks of Domestic Animals in the Mediterranean Region. A guide to Identification of Species; University of Zaragoza: Zaragoza, Spain, 2004. [Google Scholar]
- Kumsa, B.; Laroche, M.; Almeras, L.; Mediannikov, O.; Raoult, D.; Parola, P. Morphological, molecular and MALDI-TOF mass spectrometry identification of ixodid 688 tick species collected in Oromia, Ethiopia. Parasitol. Res. 2016, 115, 4199–4210. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.; Oliveira, A.C.; Granada, S.; Nachum-Biala, Y.; Gilad, M.; Lopes, A.P.; Sousa, S.R.; Vilhena, H.; Baneth, G. Molecular investigation of tick-borne pathogens in dogs from Luanda, Angola. Parasit. Vectors 2016, 9, 252. [Google Scholar] [CrossRef]
- Qablan, M.A.; Oborník, M.; Petrželková, K.J.; Sloboda, M.; Shudiefat, M.F.; Hořín, P.; Lukeš, J.; Modrý, D. Infections by Babesia caballi and Theileria equi in Jordanian equids: Epidemiology and genetic diversity. Parasitology 2013, 140, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- El-Naga, T.R.A.; Barghash, S.M. Blood Parasites in Camels (Camelus dromedarius) in Northern West Coast of Egypt. J. Bacteriol. Parasitol. 2016, 7, 258. [Google Scholar]
- Hall, T.; Biosciences, I.; Carlsbad, C. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]

| Region | Examined Animals | Hemopathogens | Overall Prevalence | χ2 | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Piroplasma | Anaplasmataceae | Co-Infection | |||||||||
| Positive | % | Positive | % | Positive | % | Positive | % | ||||
| Al-Faiyum | 47 | 1 | 2.12 | 16 | 34.04 | 0 | 0 | 17 | 36.17 | 13.235 | <0.001 ** |
| Al-Minufia | 49 | 0 | 0 | 2 | 4.08 | 0 | 0 | 2 | 4.08 | - | - |
| Matruh | 57 | 2 | 3.5 | 17 | 29.82 | 1 | 1.75 | 19 | 33.33 | 11.842 | 0.001 ** |
| Beni-Suef | 26 | 5 | 19.23 | 2 | 7.69 | 4 | 15.38 | 7 | 26.92 | 1.286 | 0.257 # |
| Al-Beheira | 28 | 1 | 3.57 | 3 | 10.71 | 0 | 0 | 4 | 14.29 | 1.000 | 0.317 # |
| Al-Giza | 27 | 1 | 3.7 | 4 | 14.81 | 0 | 0 | 5 | 18.52 | 1.800 | 0.180 # |
| Total | 234 | 10 | 4.27 | 44 | 18.80 | 5 | 2.14 | 54 | 23.08 | 21.407 | <0.001 ** |
| Factor | Total Animals | Piroplasma | Anaplasmataceae | χ2 | p Value | |||
|---|---|---|---|---|---|---|---|---|
| Positive | % | Positive | % | |||||
| Animal species | Cattle | 112 | 10 | 8.93 | 28 | 25 | 8.526 | 0.004 ** |
| Buffaloes | 26 | 0 | 0 | 2 | 7.69 | - | - | |
| Sheep | 38 | 0 | 0 | 10 | 26.32 | - | - | |
| Goats | 28 | 0 | 0 | 3 | 10.71 | - | - | |
| Donkeys | 22 | 0 | 0 | 1 | 4.55 | - | - | |
| Horses | 8 | 0 | 0 | 0 | 0 | - | - | |
| χ2 | - | 38.923 | ||||||
| p value | - | <0.001 ** | ||||||
| Sex | Female | 168 | 10 | 5.95 | 32 | 19.05 | 11.524 | 0.001 ** |
| Male | 66 | 0 | 0 | 12 | 18.18 | - | - | |
| χ2 | - | 0.027 | ||||||
| p value | - | 0.869 # | ||||||
| Age | ≤1 | 54 | 3 | 5.56 | 5 | 9.26 | 5.000 | 0.480 # |
| >1 | 180 | 7 | 3.89 | 39 | 21.67 | 22.261 | <0.001 ** | |
| χ2 | 0.400 | 16.670 | ||||||
| p value | 0.527 # | <0.001 ** | ||||||
| Tick-infested animals | Yes | 36 | 6 | 16.67 | 15 | 41.67 | 3.857 | 0.050 * |
| No | 198 | 4 | 2.02 | 29 | 14.64 | 18.939 | <0.001 ** | |
| χ2 | 11.842 | 12.789 | ||||||
| p value | 0.001 ** | <0.001 ** | ||||||
| Region | Infested Animals | Anaplasmataceae Detection (Rhipicephalus annulatus) | ||
|---|---|---|---|---|
| Examined Number | Positive | % | ||
| Al-Faiyum | Cow (8) | 61 | 42 | 68.85 |
| Buffalo (1) | 10 | 10 | 100 | |
| Al-Monufia | Buffalo (4) | - | - | - |
| Matruh | Cow (2) | 5 | 3 | 60 |
| Beni-Suef | Cow (4) | 13 | 10 | 76.92 |
| Al-Beheira | Cow (1) | 6 | 4 | 66.67 |
| Total | 20 | 95 | 69 | 72.63 |
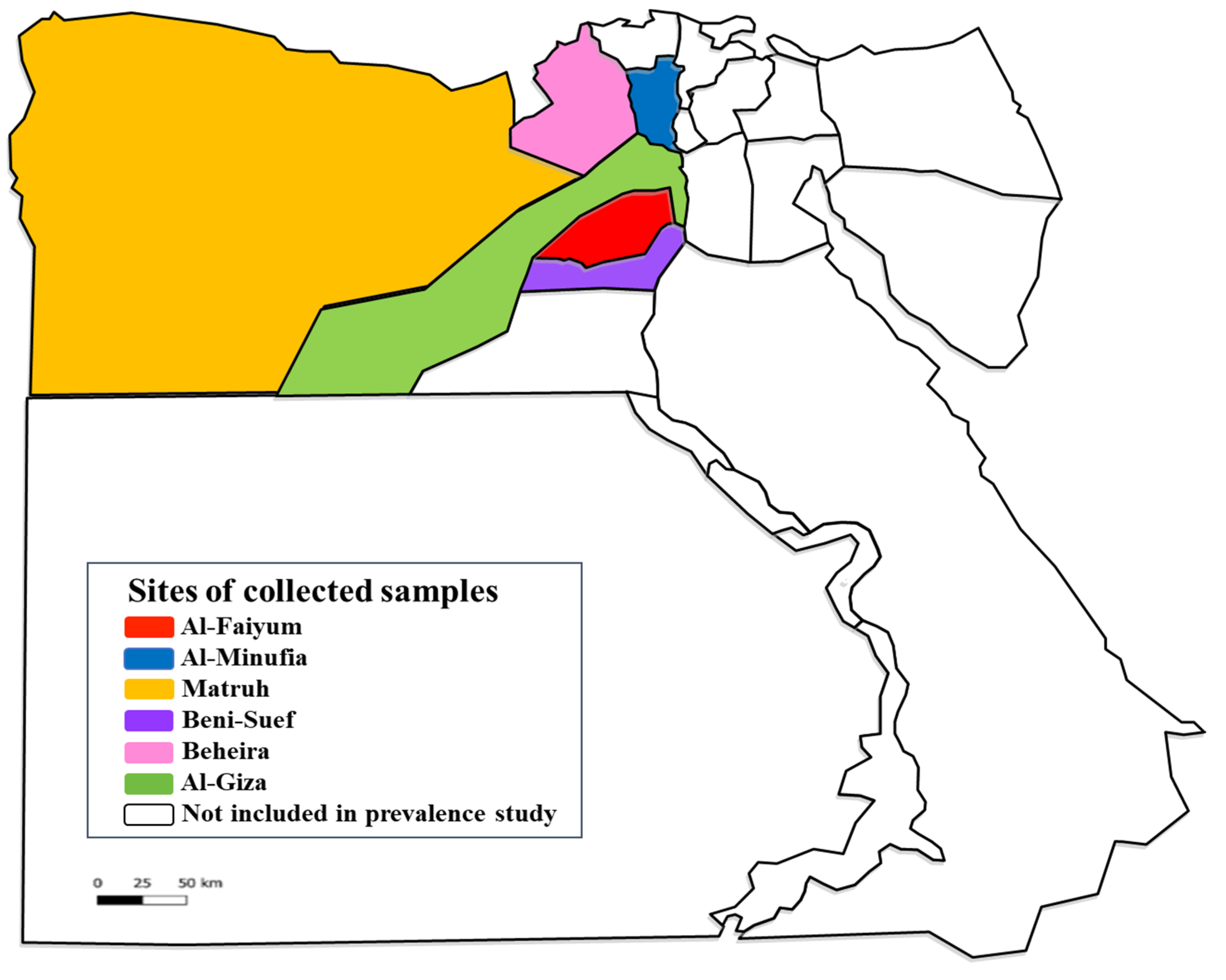
| Provinces | Geographic Coordinates | Animal Hosts | No. of Animals | Locations |
|---|---|---|---|---|
| Al-Faiyum | 29°18′35.8″ N, 30°50′30.48″ E | Cattle Buffaloes Sheep Donkeys | 31 6 6 4 | Households |
| Al-Minufia | 30°35′50.09″ N, 30° 59′15.48″ E | Cattle Buffaloes Sheep Goats Donkeys Horses | 22 12 6 2 5 2 | Households |
| Matruh | 31°21′10.44″ N, 27°14′14.10″ E | Cattle Buffaloes Sheep Goats Donkeys Horses | 11 1 17 20 6 2 | Households |
| Beni-Suef | 29°03′60.00″ N, 31°04′60.00″ E | Cattle Donkeys Horses | 21 3 2 | Households |
| Al-Beheira | 30°50′53.16″ N, 30°20′36.78″ E | Cattle Buffaloes Sheep Goats Donkeys Horses | 13 5 5 2 2 1 | Households |
| Al-Giza | 29°58′27.00″ N, 31°08′2.21″ E | Cattle Buffaloes Sheep Goats Donkeys Horses | 14 2 4 4 2 1 | Households |
| Pathogens | Targeted Gene | Primers | Tm | References |
|---|---|---|---|---|
| Piroplasma T. annulata B. bigemina | 18S rRNA (969 bp) 18S rRNA (360 bp) SS rRNA (689 bp) | piro18S-F1-GCGAATGGCTCATTAIAACA piro18S-F4-CACATCTAAGGAAGGCAGCA TBM-CTTCAGCACCTTGAGAGAAATC Equi-R-TGCCTTAAACTTCCTTGCGAT Bg3-TAGTTGTATTTCAGCCTCGCG Bg4-AACATCCAAGCAGCTAHTTAG | 58 °C 58 °C 57 °C | Dahmana et al. (2019) [2] Qablan et al. (2013) [60] El-Naga and Barghash, et al. (2016) [61] |
| Anaplasmataceae (Anaplasma and Ehrlichia) | 16S rRNA (500 bp) | ECB-CGTATTACCGCGGCTGCTGGCA ECC-AGAACGAACGCTGGCGGCAAGC | 65 °C | Cardoso et al. (2016) [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Shafy, S.; Abdullah, H.H.A.M.; Elbayoumy, M.K.; Elsawy, B.S.M.; Hassan, M.R.; Mahmoud, M.S.; Hegazi, A.G.; Abdel-Rahman, E.H. Molecular Epidemiological Investigation of Piroplasms and Anaplasmataceae Bacteria in Egyptian Domestic Animals and Associated Ticks. Pathogens 2022, 11, 1194. https://doi.org/10.3390/pathogens11101194
Abdel-Shafy S, Abdullah HHAM, Elbayoumy MK, Elsawy BSM, Hassan MR, Mahmoud MS, Hegazi AG, Abdel-Rahman EH. Molecular Epidemiological Investigation of Piroplasms and Anaplasmataceae Bacteria in Egyptian Domestic Animals and Associated Ticks. Pathogens. 2022; 11(10):1194. https://doi.org/10.3390/pathogens11101194
Chicago/Turabian StyleAbdel-Shafy, Sobhy, Hend H. A. M. Abdullah, Mohamed K. Elbayoumy, Bassma S. M. Elsawy, Mohamed R. Hassan, Mona S. Mahmoud, Ahmed G. Hegazi, and Eman H. Abdel-Rahman. 2022. "Molecular Epidemiological Investigation of Piroplasms and Anaplasmataceae Bacteria in Egyptian Domestic Animals and Associated Ticks" Pathogens 11, no. 10: 1194. https://doi.org/10.3390/pathogens11101194
APA StyleAbdel-Shafy, S., Abdullah, H. H. A. M., Elbayoumy, M. K., Elsawy, B. S. M., Hassan, M. R., Mahmoud, M. S., Hegazi, A. G., & Abdel-Rahman, E. H. (2022). Molecular Epidemiological Investigation of Piroplasms and Anaplasmataceae Bacteria in Egyptian Domestic Animals and Associated Ticks. Pathogens, 11(10), 1194. https://doi.org/10.3390/pathogens11101194








