Outcomes of Patients Receiving a Kidney Transplant or Remaining on the Transplant Waiting List at the Epicentre of the COVID-19 Pandemic in Europe: An Observational Comparative Study
Abstract
1. Introduction
2. Results
2.1. Transplant Waiting-List
2.1.1. Demographic and Clinical Characteristics of Patients on the Transplant Waiting List
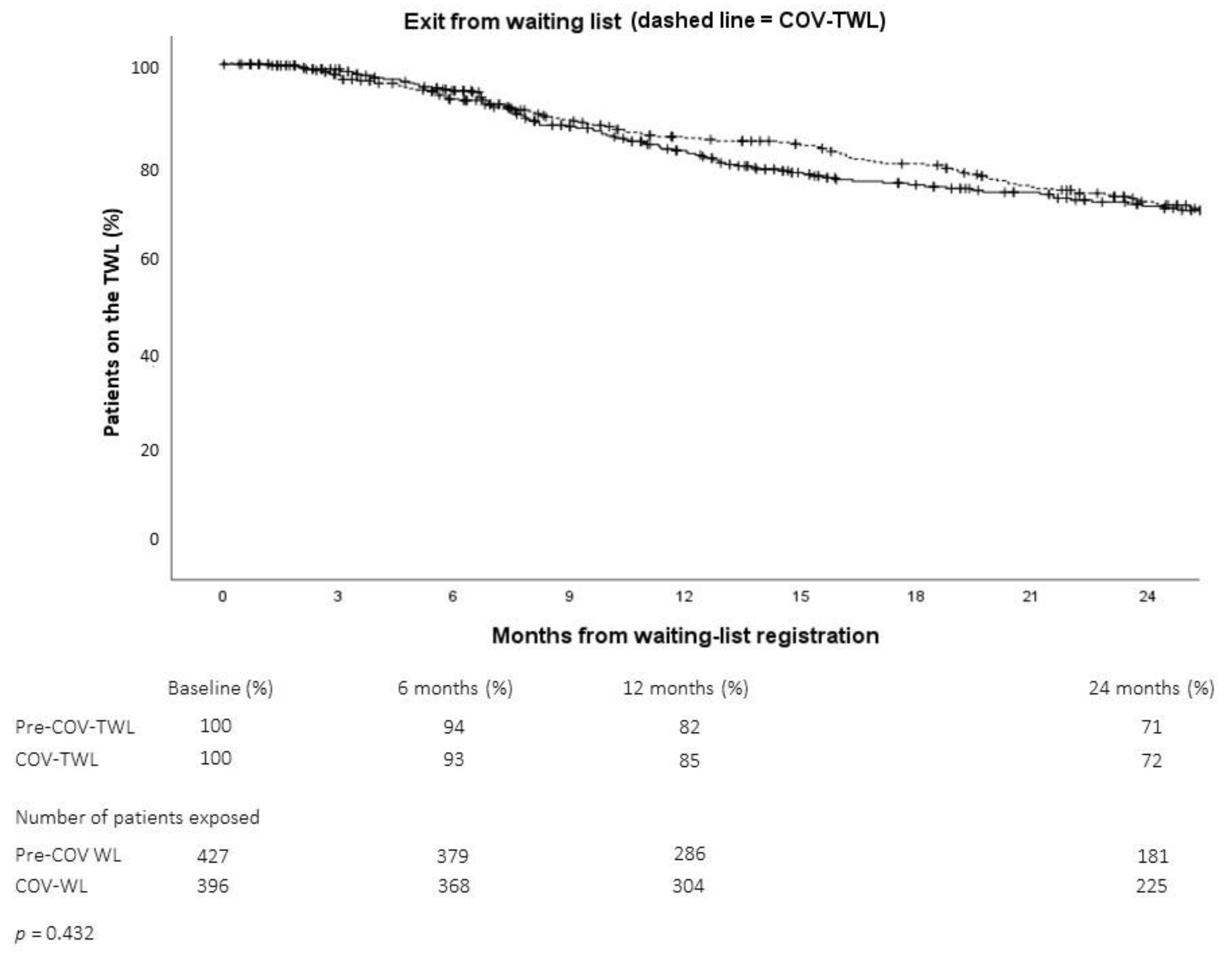
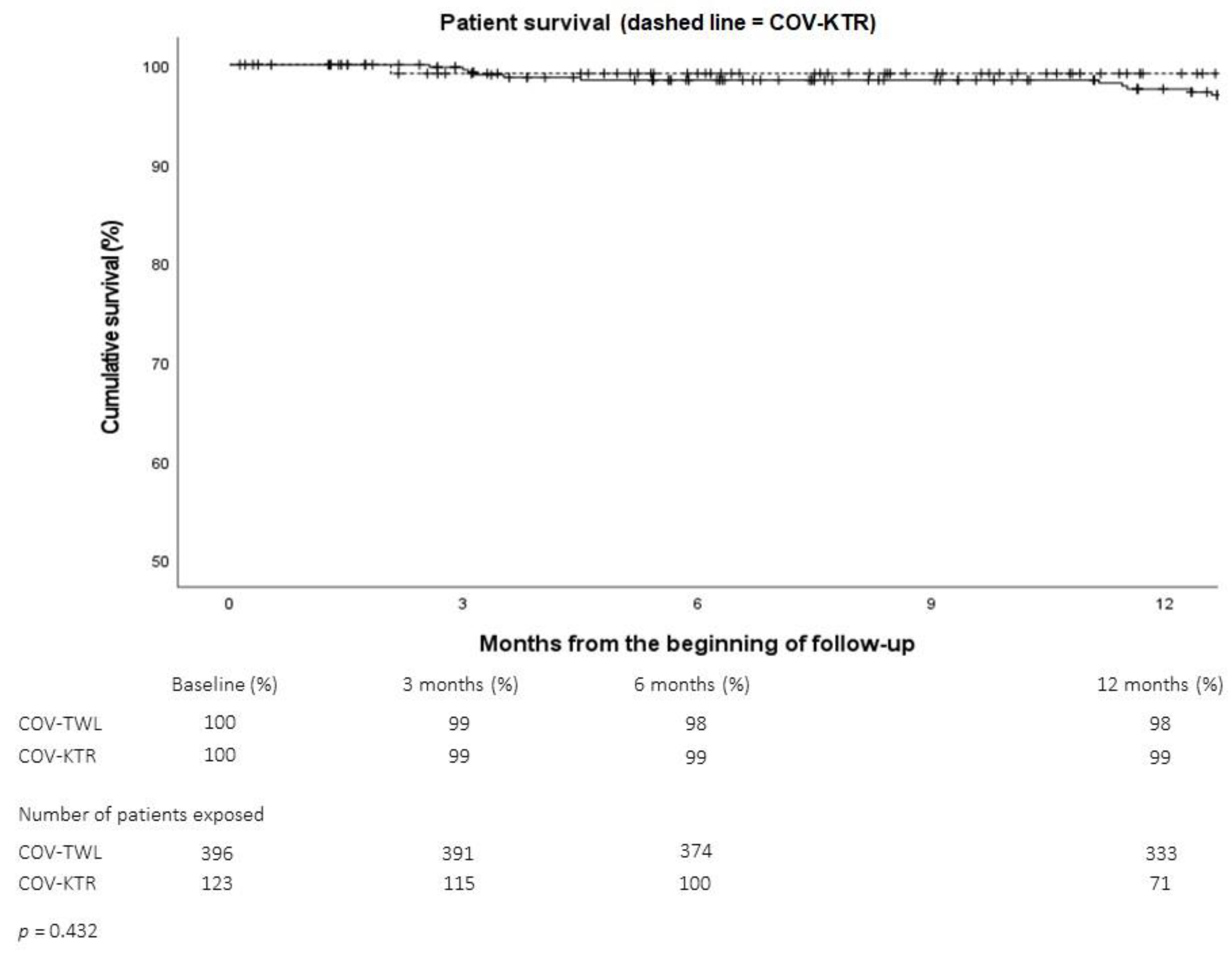
2.1.2. Outcomes of Patients on the Transplant Waiting List
2.2. Kidney Transplants
2.2.1. Donors’ Characteristics
2.2.2. Recipients’ Characteristics
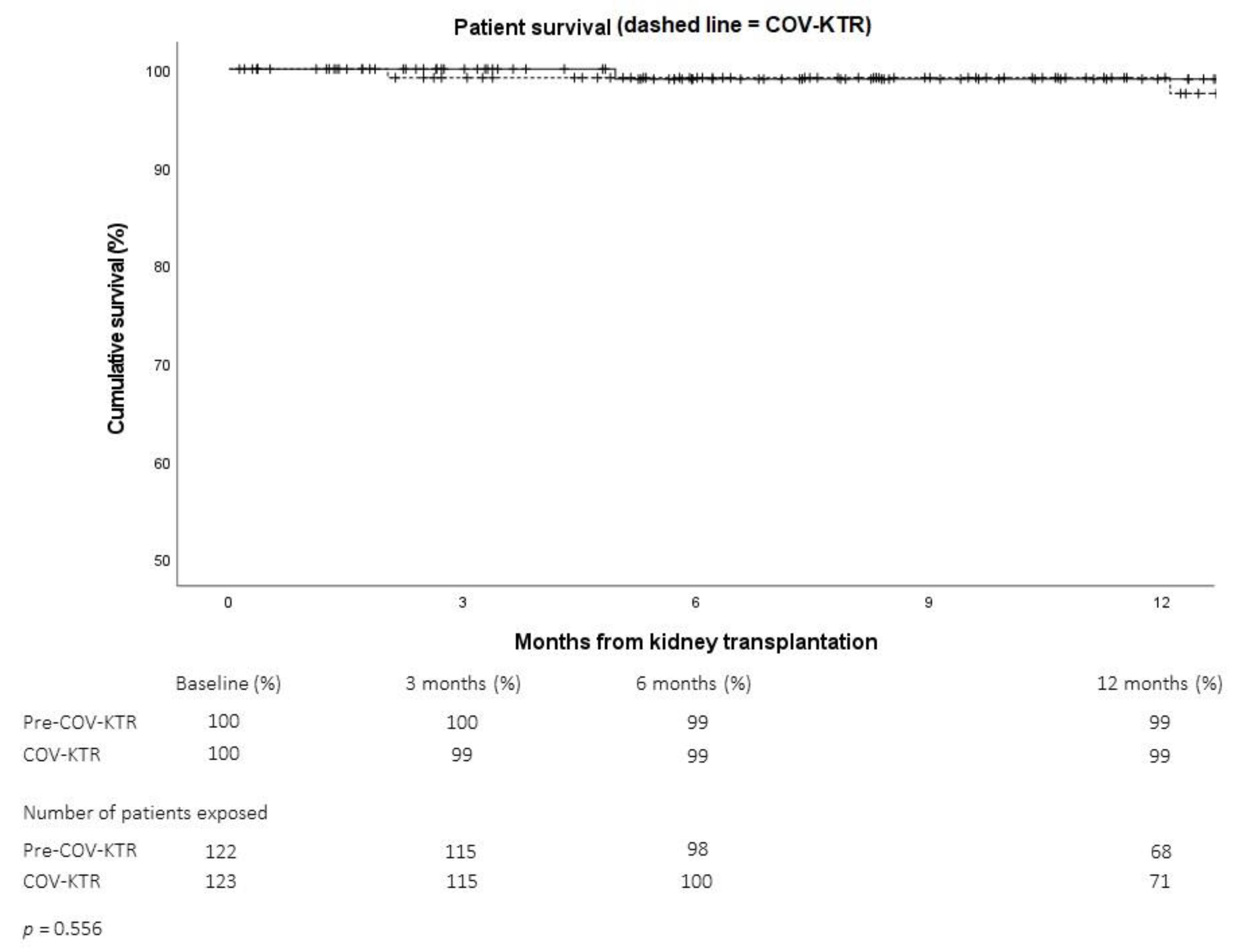
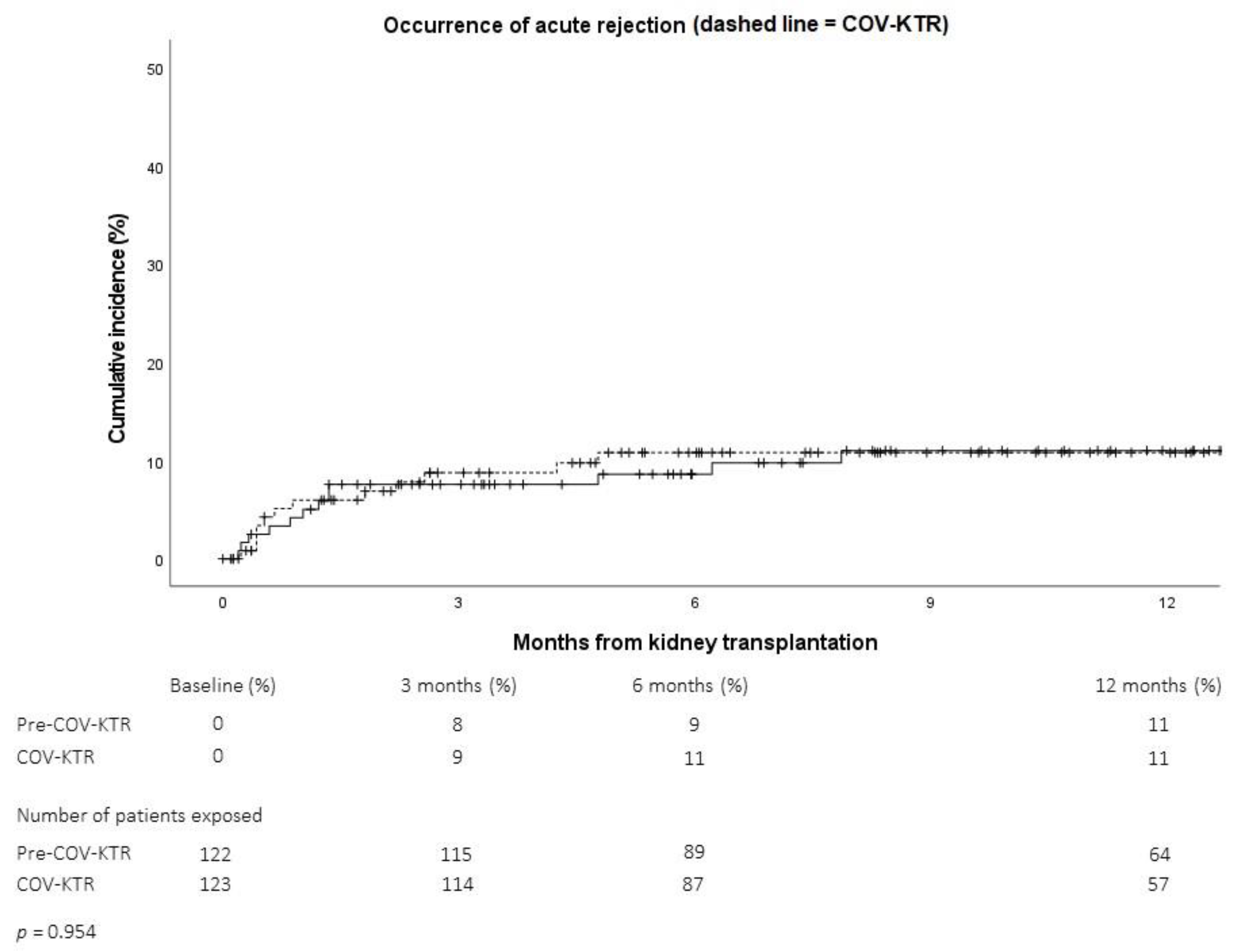
2.2.3. Kidney Transplant Outcomes
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen-Crowe, B.; Sutherland, M.; McKenney, M.; Elkbuli, A. A Closer Look Into Global Hospital Beds Capacity and Resource Shortages During the COVID-19 Pandemic. J. Surg. Res. 2021, 260, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Lugli, G.; Ottaviani, M.M.; Botta, A.; Ascione, G.; Bruschi, A.; Cagnazzo, F.; Zammarchi, L.; Romagnani, P.; Portaluri, T. The Impact of the SARS-CoV-2 Pandemic on Healthcare Provision in Italy to non-COVID Patients: A Systematic Review. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022012. [Google Scholar] [PubMed]
- O’Rielly, C.; Ng-Kamstra, J.; Kania-Richmond, A.; Dort, J.; White, J.; Robert, J.; Brindle, M.; Sauro, K. Surgery and COVID-19: A rapid scoping review of the impact of the first wave of COVID-19 on surgical services. BMJ Open 2021, 11, e043966. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Pesenti, A.; Cecconi, M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast During an Emergency Response. JAMA 2020, 323, 1545–1546. [Google Scholar] [CrossRef] [PubMed]
- Truog, R.D.; Mitchell, C.; Daley, G.Q. The Toughest Triage—Allocating Ventilators in a Pandemic. N. Engl. J. Med. 2020, 382, 1973–1975. [Google Scholar] [CrossRef]
- Malhotra, A.; Wu, X.; Fleishon, H.B.; Duszak, R., Jr.; Silva, E., 3rd; McGinty, G.B.; Bender, C.; Williams, B.; Pashley, N.; Stengel, C.J.B.; et al. Initial Impact of COVID-19 on Radiology Practices: An ACR/RBMA Survey. J. Am. Coll. Radiol. 2020, 17, 1525–1531. [Google Scholar] [CrossRef]
- Rossi, A.; Prochowski Iamurri, A.; Cerchione, C.; Gentili, N.; Danesi, V.; Altini, M.; Paganelli, G.; Barone, D. Radiology imaging management in an Italian cancer center (IRST IRCCS) during the COVID-19 pandemic. Insights Imaging 2020, 11, 129. [Google Scholar] [CrossRef]
- Angelico, R.; Trapani, S.; Manzia, T.M.; Lombardini, L.; Tisone, G.; Cardillo, M. The COVID-19 outbreak in Italy: Initial implications for organ transplantation programs. Am. J. Transplant. 2020, 20, 1780–1784. [Google Scholar] [CrossRef]
- Patriti, A.; Baiocchi, G.L.; Catena, F.; Marini, P.; Catarci, M.; FACS on behalf of the Associazione Chirurghi Ospedalieri Italiani (ACOI). Emergency general surgery in Italy during the COVID-19 outbreak: First survey from the real life. World J. Emerg. Surg. 2020, 15, 36. [Google Scholar] [CrossRef]
- Andreata, M.; Faraldi, M.; Bucci, E.; Lombardi, G.; Zagra, L. Operating room efficiency and timing during coronavirus disease 2019 outbreak in a referral orthopaedic hospital in Northern Italy. Int. Orthop. 2020, 44, 2499–2504. [Google Scholar] [CrossRef]
- D’Antiga, L. Coronaviruses and Immunosuppressed Patients: The Facts During the Third Epidemic. Liver Transpl. 2020, 26, 832–834. [Google Scholar] [CrossRef]
- Gori, A.; Dondossola, D.; Antonelli, B.; Mangioni, D.; Alagna, L.; Reggiani, P.; Bandera, A.; Rossi, G. Coronavirus disease 2019 and transplantation: A view from the inside. Am. J. Transplant. 2020, 20, 1939–1940. [Google Scholar] [CrossRef] [PubMed]
- Monaco, A.; Manzia, T.M.; Angelico, R.; Iaria, G.; Gazia, C.; Al Alawi, Y.; Fourtounas, K.; Tisone, G.; Cacciola, R. Awareness and Impact of Non-pharmaceutical Interventions During Coronavirus Disease 2019 Pandemic in Renal Transplant Recipients. Transplant. Proc. 2020, 52, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
- Boyarsky, B.J.; Po-Yu Chiang, T.; Werbel, W.A.; Durand, C.M.; Avery, R.K.; Getsin, S.N.; Jackson, K.R.; Kernodle, A.B.; Van Pilsum Rasmussen, S.E.; Massie, A.B.; et al. Early impact of COVID-19 on transplant center practices and policies in the United States. Am. J. Transplant. 2020, 20, 1809–1818. [Google Scholar] [CrossRef]
- Kute, V.B.; Tullius, S.G.; Rane, H.; Chauhan, S.; Mishra, V.; Meshram, H.S. Global Impact of the COVID-19 Pandemic on Solid Organ Transplant. Transplant. Proc. 2022, 54, 1412–1416. [Google Scholar] [CrossRef] [PubMed]
- Azzi, Y.; Brooks, A.; Yaffe, H.; Greenstein, S. COVID-19 and the Response of Transplant Centers: The Global Response with an Emphasis on the Kidney Recipient. Curr. Transplant. Rep. 2021, 8, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, Q.; Haberfellner, F.; Büttner-Herold, M.; Torrez, C.; Haller, B.; Assfalg, V.; Renders, L.; Amann, K.; Heemann, U.; Schmaderer, C.; et al. The Kidney Donor Profile Index (KDPI) Correlates With Histopathologic Findings in Post-reperfusion Baseline Biopsies and Predicts Kidney Transplant Outcome. Front. Med. 2022, 9, 875206. [Google Scholar] [CrossRef]
- Zhong, Y.; Schaubel, D.E.; Kalbfleisch, J.D.; Ashby, V.B.; Rao, P.S.; Sung, R.S. Reevaluation of the Kidney Donor Risk Index. Transplantation 2019, 103, 1714–1721. [Google Scholar] [CrossRef]
- Karpinski, J.; Lajoie, G.; Cattran, D.; Fenton, S.; Zaltzman, J.; Cardella, C.; Cole, E. Outcome of kidney transplantation from high-risk donors is determined by both structure and function. Transplantation 1999, 67, 1162–1167. [Google Scholar] [CrossRef]
- Available online: https://optn.transplant.hrsa.gov/data/allocation-calculators/epts-calculator/ (accessed on 15 September 2022).
- Available online: https://www.trapianti.salute.gov.it/imgs/C_17_cntPubblicazioni_139_allegato.pdf (accessed on 15 September 2022).
- Available online: http://sito.regione.campania.it/burc/pdf08/burc14or_08/del316_08.pdf (accessed on 15 September 2022).
- Khan, M.; Adil, S.F.; Alkhathlan, H.Z.; Tahir, M.N.; Saif, S.; Khan, M.; Khan, S.T. COVID-19: A Global Challenge with Old History, Epidemiology and Progress So Far. Molecules 2020, 26, 39. [Google Scholar] [CrossRef]
- Younes, N.; Al-Sadeq, D.W.; Al-Jighefee, H.; Younes, S.; Al-Jamal, O.; Daas, H.I.; Yassine, H.; Nasrallah, G.K. Challenges in Laboratory Diagnosis of the Novel Coronavirus SARS-CoV-2. Viruses 2020, 12, 582. [Google Scholar] [CrossRef] [PubMed]
- Favi, E.; Leonardis, F.; Manzia, T.M.; Angelico, R.; Alalawi, Y.; Alfieri, C.; Cacciola, R. “Salus Populi Suprema Lex”: Considerations on the Initial Response of the United Kingdom to the SARS-CoV-2 Pandemic. Front. Public Health 2021, 9, 646285. [Google Scholar] [CrossRef]
- Maringe, C.; Spicer, J.; Morris, M.; Purushotham, A.; Nolte, E.; Sullivan, R.; Rachet, B.; Aggarwal, A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol. 2020, 21, 1023–1034. [Google Scholar] [CrossRef]
- Horton, R. Offline: COVID-19 and the NHS—“A national scandal”. Lancet 2020, 395, 1022. [Google Scholar] [CrossRef]
- COVIDSurg Collaborative. Elective surgery cancellations due to the COVID-19 pandemic: Global predictive modelling to inform surgical recovery plans. Br. J. Surg. 2020, 107, 1440–1449. [Google Scholar]
- NasrAllah, M.M.; Osman, N.A.; Elalfy, M.; Malvezzi, P.; Rostaing, L. Transplantation in the era of the COVID-19 pandemic: How should transplant patients and programs be handled? Rev. Med. Virol. 2021, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fiocco, A.; Ponzoni, M.; Caraffa, R.; Carrozzini, M.; Bagozzi, L.; Nadali, M.; Bifulco, O.; Toscano, G.; Fraiese, A.P.; Bottio, T.; et al. Heart transplantation management in northern Italy during COVID-19 pandemic: Single-centre experience. ESC Heart Fail. 2020, 7, 2003–2006. [Google Scholar] [CrossRef]
- Hardman, G.; Sutcliffe, R.; Hogg, R.; Mumford, L.; Grocott, L.; Mead-Regan, S.J.; Nuttall, J.; Dunn, S.; Seeley, P.; Clark, S.; et al. The impact of the SARS-CoV-2 pandemic and COVID-19 on lung transplantation in the UK: Lessons learned from the first wave. Clin. Transplant. 2021, 35, e14210. [Google Scholar] [CrossRef]
- Khairallah, P.; Aggarwal, N.; Awan, A.A.; Vangala, C.; Airy, M.; Pan, J.S.; Murthy, B.V.R.; Winkelmayer, W.C.; Ramanathan, V. The impact of COVID-19 on kidney transplantation and the kidney transplant recipient—One year into the pandemic. Transpl. Int. 2021, 34, 612–621. [Google Scholar] [CrossRef]
- Rombolà, G.; Brunini, F. COVID-19 and dialysis: Why we should be worried. J. Nephrol. 2020, 33, 401–403. [Google Scholar] [CrossRef]
- Martino, F.; Plebani, M.; Ronco, C. Kidney transplant programmes during the COVID-19 pandemic. Lancet Respir. Med. 2020, 8, e39. [Google Scholar] [CrossRef]
- Zidan, A.; Alabbad, S.; Ali, T.; Nizami, I.; Haberal, M.; Tokat, Y.; Kamel, R.; Said, H.; Abdelaal, A.; Elsharkawy, M.; et al. Position Statement of Transplant Activity in the Middle East in Era of COVID-19 Pandemic. Transplantation 2020, 104, 2205–2207. [Google Scholar] [CrossRef]
- Imam, A.; Tzukert, K.; Merhav, H.; Imam, R.; Abu-Gazala, S.; Abel, R.; Elhalel, M.D.; Khalaileh, A. Practical recommendations for kidney transplantation in the COVID-19 pandemic. World J. Transplant. 2020, 10, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Boedecker, S.C.; Klimpke, P.; Kraus, D.; Runkel, S.; Galle, P.R.; Koch, M.; Weinmann-Menke, J. COVID-19-Importance for Patients on the Waiting List and after Kidney Transplantation-A Single Center Evaluation in 2020-2021. Pathogens 2021, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.M.; Weiner, D.E.; Aweh, G.; Salenger, P.; Johnson, D.S.; Lacson, E., Jr. Epidemiology and Outcomes of COVID-19 in Home Dialysis Patients Compared with In-Center Dialysis Patients. J. Am. Soc. Nephrol. 2021, 32, 1569–1573. [Google Scholar] [CrossRef]
- COVID-19 Task Force Committee of the Japanese Association of Dialysis Physicians; Japanese Society for Dialysis Therapy; Japanese Society of Nephrology; Kikuchi, K.; Nangaku, M.; Ryuzaki, M.; Yamakawa, T.; Hanafusa, N.; Sakai, K.; Kanno, Y.; et al. COVID-19 of dialysis patients in Japan: Current status and guidance on preventive measures. Ther. Apher. Dial. 2020, 24, 361–365. [Google Scholar] [PubMed]
- Ikizler, T.A.; Kliger, A.S. Minimizing the risk of COVID-19 among patients on dialysis. Nat. Rev. Nephrol. 2020, 16, 311–313. [Google Scholar] [CrossRef]
- Rostoker, G.; Issad, B.; Fessi, H.; Massy, Z.A. Why and how should we promote home dialysis for patients with end-stage kidney disease during and after the coronavirus 2019 disease pandemic? A French perspective. J. Nephrol. 2021, 34, 985–989. [Google Scholar] [CrossRef]
- Hilbrands, L.B.; Duivenvoorden, R.; Vart, P.; Franssen, C.F.M.; Hemmelder, M.H.; Jager, K.J.; Kieneker, L.M.; Noordzij, M.; Pena, M.J.; Vries, H.; et al. COVID-19-related mortality in kidney transplant and dialysis patients: Results of the ERACODA collaboration. Nephrol. Dial. Transplant. 2020, 35, 1973–1983. [Google Scholar] [CrossRef]
- Gennuso, K.P.; Blomme, C.K.; Givens, M.L.; Pollock, E.A.; Roubal, A.M. Deaths of Despair(ity) in Early 21st Century America: The Rise of Mortality and Racial/Ethnic Disparities. Am. J. Prev. Med. 2019, 57, 585–591. [Google Scholar] [CrossRef]
- Azzi, Y.; Bartash, R.; Scalea, J.; Loarte-Campos, P.; Akalin, E. COVID-19 and Solid Organ Transplantation: A Review Article. Transplantation 2021, 105, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Angelico, R.; Blasi, F.; Manzia, T.M.; Toti, L.; Tisone, G.; Cacciola, R. The Management of Immunosuppression in Kidney Transplant Recipients with COVID-19 Disease: An Update and Systematic Review of the Literature. Medicina 2021, 57, 435. [Google Scholar] [CrossRef] [PubMed]
- Alberici, F.; Delbarba, E.; Manenti, C.; Econimo, L.; Valerio, F.; Pola, A.; Maffei, C.; Possenti, S.; Piva, S.; Latronico, N.; et al. Management of Patients on Dialysis and With Kidney Transplantation During the SARS-CoV-2 (COVID-19) Pandemic in Brescia, Italy. Kidney Int. Rep. 2020, 5, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Kindrachuk, J.; Ork, B.; Hart, B.J.; Mazur, S.; Holbrook, M.R.; Frieman, M.B.; Traynor, D.; Johnson, R.F.; Dyall, J.; Kuhn, J.H.; et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob. Agents Chemother. 2015, 59, 1088–1099. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, Y.; Shen, J.; Huang, Y.; Martin, W.; Cheng, F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Zaidan, M.; Legendre, C. Solid Organ Transplantation in the Era of COVID-19: Lessons from France. Transplantation 2021, 105, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Alfieri, C.M.; Cerutti, R.; Lunghi, G.; Messa, P. COVID-19 and Acute Kidney Injury: A Systematic Review and Meta-Analysis. Pathogens 2020, 9, 1052. [Google Scholar] [CrossRef]
- Kremer, D.; Pieters, T.T.; Verhaar, M.C.; Berger, S.P.; Bakker, S.J.L.; van Zuilen, A.D.; Joles, J.A.; Vernooij, R.W.M.; van Balkom, B.W.M. A systematic review and meta-analysis of COVID-19 in kidney transplant recipients: Lessons to be learned. Am. J. Transplant. 2021, 21, 3936–3945. [Google Scholar] [CrossRef]
- Goffin, E.; Candellier, A.; Vart, P.; Noordzij, M.; Arnol, M.; Covic, A.; Lentini, P.; Malik, S.; Reichert, L.J.; Sever, M.S.; et al. COVID-19-related mortality in kidney transplant and haemodialysis patients: A comparative, prospective registry-based study. Nephrol. Dial. Transplant. 2021, 36, 2094–2105. [Google Scholar] [CrossRef]
- Nahi, S.L.; Shetty, A.A.; Tanna, S.D.; Leventhal, J.R. Renal allograft function in kidney transplant recipients infected with SARS-CoV 2: An academic single center experience. PLoS ONE 2021, 16, e0252979. [Google Scholar] [CrossRef]
- Xu, J.; Han, M.; Huang, C.; Liao, Y.; Wang, D.; Zhu, X.; Wang, C.; Huang, J.; Guo, Z.; Chen, G.; et al. Single-center experience of organ transplant practice during the COVID-19 epidemic. Transpl. Int. 2021, 34, 1812–1823. [Google Scholar] [CrossRef] [PubMed]


| Variables | Whole Population (N = 823) | Pre-COV-TWL (N = 427) | COV-TWL (N = 396) | p |
|---|---|---|---|---|
| Sex (males) | 494 (60.0) | 251 (58.5) | 243 (61.4) | 0.477 |
| Age (years) | 53 (44–61) | 52 (44–61) | 53 (45–62) | 0.171 |
| Ethnicity: | ||||
| Caucasian | 707 (85.9) | 366 (85.7) | 341 (86.1) | 0.920 |
| Afro-Caribbean | 40 (4.9) | 20 (4.7) | 20 (5.1) | 0.872 |
| Other | 76 (9.2) | 41 (9.6) | 35 (8.8) | 0.720 |
| Renal replacement therapy | 741 (90.0) | 388 (90.9) | 353 (89.1) | 0.418 |
| Haemodialysis | 590/741 (79.6) | 310/388 (79.9) | 280/353 (79.3) | 0.856 |
| Dialysis vintage (months) | 37 (17–59) | 34 (16–57) | 40 (19–62) | 0.074 |
| Previous kidney transplant | 200 (24.3) | 111 (26.0) | 89 (22.5) | 0.256 |
| Primary kidney disease: | ||||
| Primary or secondary glomerulonephritis | 417 (50.7) | 210 (49.2) | 207 (52.3) | 0.403 |
| Diabetic nephropathy | 41 (5.0) | 19 (4.4) | 22 (5.6) | 0.523 |
| Polycystic kidney disease | 112 (13.6) | 61 (14.3) | 51 (12.9) | 0.611 |
| Hypertensive nephrosclerosis | 81 (9.8) | 35 (8.2) | 46 (11.6) | 0.103 |
| Tubulo-interstitial disease | 37 (4.5) | 19 (4.4) | 18 (4.5) | 1.000 |
| Genetic kidney disease | 35 (4.3) | 21 (4.9) | 14 (3.5) | 0.389 |
| Uropathy | 84 (10.2) | 45 (10.5) | 39 (9.8) | 0.818 |
| Thrombotic microangiopathy | 40 (4.9) | 21 (4.9) | 19 (4.8) | 1.000 |
| Ischaemia | 7 (0.9) | 5 (1.2) | 2 (0.5) | 0.452 |
| Unknown | 17 (2.1) | 14 (3.3) | 3 (0.8) | 0.013 |
| Pre-existing conditions: | ||||
| Arterial hypertension | 713 (88.5) | 363 (87.9) | 350 (89.1) | 0.660 |
| Diabetes mellitus | 103 (12.8) | 53 (12.8) | 50 (12.7) | 1.000 |
| Chronic obstructive pulmonary disease | 144 (17.9) | 74 (17.9) | 70 (17.8) | 1.000 |
| Coronary artery disease | 134 (16.6) | 67 (16.2) | 67 (17.1) | 0.777 |
| Obesity (BMI ≥ 30 kg/m2) | 90 (11.2) | 45 (10.9) | 45 (11.5) | 0.824 |
| CMV IgG positivity | 701 (87.1) | 366 (88.6) | 335 (85.5) | 0.207 |
| EBV IgG positivity | 727 (90.3) | 375 (90.8) | 352 (89.8) | 0.636 |
| HSV IgG positivity | 655 (81.4) | 339 (82.1) | 316 (80.6) | 0.651 |
| VZV IgG positivity | 778 (96.6) | 401 (97.1) | 377 (96.2) | 0.558 |
| HBV viremia | 8 (1.0) | 4 (1.0) | 4 (1.0) | 1.000 |
| HCV viremia | 14 (1.7) | 8 (1.9) | 6 (1.5) | 0.790 |
| Follow-up (months) | 13 (6–24) | 12 (6–24) | 15 (6–24) | <0.001 |
| Variables | Whole Population (N = 245) | Pre-COV Donors (N = 122) | COV Donors (N = 123) | p |
|---|---|---|---|---|
| Donor sex (male) | 140 (57.1) | 68 (55.7) | 72 (58.5) | 0.699 |
| Donor age (years) | 55 (46–61) | 54 (44–61) | 55 (48–62) | 0.419 |
| Type of donor: | ||||
| DBD | 178 (72.7) | 82 (67.2) | 96 (78.0) | 0.063 |
| DCD | 19 (7.8) | 13 (10.7) | 6 (4.9) | 0.100 |
| ECD | 98 (40.0) | 48 (39.3) | 50 (40.7) | 0.896 |
| LD | 48 (19.6) | 27 (22.1) | 21 (17.1) | 0.338 |
| Donor risk factors: | ||||
| Cerebrovascular accident | 99 (40.4) | 26 (37.7) | 53 (43.1) | 0.435 |
| Arterial hypertension | 79 (32.2) | 37 (30.3) | 42 (34.1) | 0.585 |
| Last SCr > 1.5 mg/dL | 32 (13.1) | 18 (14.8) | 14 (11.4) | 0.455 |
| ICU admission | 197 (80.4) | 95 (77.9) | 102 (82.9) | 0.338 |
| ICU stay (days) | 3 (1–5) | 3 (1–5) | 3 (2–5) | 0.885 |
| Donor ethnicity: | ||||
| Caucasian | 236 (96.3) | 122 (100) | 114 (92.7) | 0.003 |
| Afro-Caribbean | 1 (0.4) | 0 (0.0) | 1 (0.8) | 1.000 |
| Other | 8 (3.3) | 0 (0.0) | 8 (6.5) | 0.007 |
| Cold ischemia time (minutes) | 780 (623–960) | 760 (630–940) | 790 (620–1010) | 0.456 |
| KDPI | 63 (34–85) | 61 (31–84) | 63 (35–87) | 0.471 |
| KDRI | 1.12 (0.85–1.45) | 1.12 (0.82–1.44) | 1.12 (0.86–1.49) | 0.558 |
| Karpinski score * | 3 (3–4) | 4 (3–4) | 3 (3–4) | 0.416 |
| Variables | Whole Population (N = 245) | Pre-COV-KTR (N = 122) | COV-KTR (N = 123) | p |
|---|---|---|---|---|
| Recipient sex (male) | 139 (56.7) | 71 (58.2) | 68 (55.3) | 0.699 |
| Recipient age (years) | 52 (43–59) | 52 (45–60) | 50 (39–58) | 0.115 |
| Recipient ethnicity: | ||||
| Caucasian | 220 (89.8) | 111 (91.0) | 109 (88.6) | 0.674 |
| Afro-Caribbean | 6 (2.4) | 1 (0.8) | 5 (4.1) | 0.213 |
| Other | 19 (7.8) | 10 (8.2) | 9 (7.3) | 0.816 |
| Renal replacement therapy | 230 (96.7) | 118 (96.7) | 112 (91.1) | 0.107 |
| Haemodialysis | 189/230 (82.2) | 92/118 (78.0) | 97/112 (86.6) | 0.120 |
| Dialysis vintage (months) | 35 (16–56) | 34 (16–62) | 37 (15–56) | 0.728 |
| Primary kidney disease: | ||||
| Primary or secondary glomerulonephritis | 113 (46.1) | 56 (45.9) | 57 (46.3) | 1.000 |
| Diabetic nephropathy | 8 (3.3) | 5 (4.1) | 3 (2.4) | 0.500 |
| Polycystic kidney disease | 41 (16.7) | 25 (20.5) | 16 (13.0) | 0.127 |
| Hypertensive nephrosclerosis | 17 (6.9) | 7 (5.7) | 10 (8.1) | 0.616 |
| Tubulointerstitial disease | 9 (3.7) | 3 (2.5) | 6 (4.9) | 0.500 |
| Genetic or congenital kidney disease | 27 (11.0) | 13 (10.7) | 14 (11.4) | 1.000 |
| Uropathy | 30 (12.2) | 12 (9.8) | 18 (14.6) | 0.330 |
| Thrombotic microangiopathy | 17 (6.9) | 7 (5.7) | 10 (8.1) | 0.616 |
| Other | 3 (1.2) | 2 (1.6) | 1 (0.8) | 0.622 |
| Pre-existing comorbidities: | ||||
| Arterial hypertension | 211 (86.1) | 106 (86.9) | 105 (85.4) | 0.854 |
| Diabetes mellitus | 24 (9.8) | 19 (15.6) | 5 (4.1) | 0.002 |
| Chronic obstructive pulmonary disease | 34 (13.9) | 19 (15.6) | 15 (12.2) | 0.466 |
| Coronary artery disease | 29 (11.8) | 11 (9.0) | 18 (14.6) | 0.235 |
| Obesity (BMI ≥ 30 kg/m2) | 17 (6.9) | 10 (8.2) | 7 (5.7) | 0.464 |
| CMV IgG positivity | 209 (85.3) | 109 (89.3) | 100 (81.3) | 0.104 |
| EBV IgG positivity | 212 (86.5) | 107 (87.7) | 105 (85.4) | 0.709 |
| HSV IgG positivity | 196 (80.0) | 96 (78.7) | 100 (81.3) | 0.635 |
| VZV IgG positivity | 237 (96.7) | 118 (96.7) | 119 (96.7) | 1.000 |
| HBV viremia | 5 (2.0) | 1 (0.8) | 4 (3.3) | 0.370 |
| HCV viremia | 3 (1.2) | 1 (0.8) | 2 (1.6) | 1.000 |
| Previous kidney transplant | 56 (22.9) | 33 (27.0) | 23 (18.7) | 0.130 |
| Last PRA (%) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.184 |
| N° Baseline DSA | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.095 |
| Baseline DSA | 14 (5.7) | 10 (8.2) | 4 (3.3) | 0.107 |
| HLA mismatch | 4 (3–5) | 4 (3–5) | 4 (3–5) | 0.076 |
| EPTS score | 28 (16–54) | 33 (18–58) | 23 (12–46) | 0.009 |
| Italian Recipient Case Mix Index | 3 (2–4) | 3 (2–4) | 3 (2–4) | 0.114 |
| Length of hospitalisation | 13 (10–21) | 15 (10–20) | 13 (10–22) | 0.751 |
| ICU admission | 58 (23.7) | 26 (21.3) | 31 (25.2) | 0.546 |
| ICU stay (days) | 1 (1–2) | 1 (1–4) | 1 (1–1) | 0.043 |
| Induction immunosuppression: | ||||
| Anti-IL2R monoclonal antibodies | 126 (51.4) | 56 (45.9) | 70 (56.9) | 0.097 |
| rATG | 133 (54.3) | 75 (61.5) | 58 (47.2) | 0.029 |
| Methylprednisolone | 245 (100) | 122 (100) | 123 (100) | 1.000 |
| Anti-CD20 monoclonal antibodies | 15 (6.1) | 9 (7.4) | 6 (4.9) | 0.439 |
| Anti-C5 monoclonal antibodies | 27 (11.0) | 7 (5.7) | 20 (16.3) | 0.013 |
| Plasma exchange | 23 (9.4) | 15 (12.3) | 8 (6.5) | 0.131 |
| Polyclonal human immunoglobulins | 19 (7.8) | 13 (10.7) | 6 (4.9) | 0.100 |
| Maintenance immunosuppression #: | ||||
| Tacrolimus | 242/242 (100.0) | 120/120 (100.0) | 122/122 (100.0) | 1.000 |
| MMF/MPA | 237/242 (97.9) | 116/120 (96.7) | 121/122 (99.2) | 0.211 |
| Prednisone | 237/242 (97.9) | 117/120 (97.5) | 120/122 (98.4) | 0.682 |
| Tacrolimus trough levels (ng/mL): | ||||
| After 1 month | 9.1 (7.5–11.1) | 9.1 (7.6–10.9) | 9.0 (7.4–13.8) | 0.695 |
| After 3 months | 8.4 (7.1–10.2) | 9.1 (7.1–10.2) | 8.2 (7.1–10.0) | 0.248 |
| After 6 months | 8.4 (6.9–10.0) | 8.9 (7.3–10.1) | 8.0 (6.7–9.6) | 0.090 |
| After 9 months | 7.9 (6.7–9.4) | 7.7 (6.5–8.6) | 8.3 (6.8–9.8) | 0.120 |
| After 12 months | 7.7 (6.5–9.3) | 8.1 (6.8–9.6) | 7.5 (6.4–9.1) | 0.231 |
| Follow-up (months) | 12.2 (5.7–17.8) | 12.4 (5.6–17.7) | 12.0 (5.9–18.0) | 0.889 |
| Variables | Whole Population (N = 245) | Pre-COV-KTR (N = 122) | COV-KTR (N = 123) | p |
|---|---|---|---|---|
| PNF | 4 (1.2) | 2 (1.6) | 2 (1.6) | 1.000 |
| DGF | 61 (24.9) | 27 (22.1) | 34 (27.6) | 0.376 |
| DGF duration (days) | 7 (4–12) | 7 (5–11) | 6 (4–12) | 0.641 |
| CCI | 23 (0–42) | 23 (9–42) | 21 (0–42) | 0.236 |
| SCr at discharge | 1.46 (1.14–1.94) | 1.34 (1.10–1.73) | 1.60 (1.20–2.08) | 0.003 |
| SCr after 1 month | 1.5 (1.18–1.88) | 1.32 (1.07–1.63) | 1.65 (1.28–2.06) | <0.001 |
| SCr after 3 months | 1.44 (1.16–1.81) | 1.33 (1.11–1.60) | 1.61 (1.26–2.06) | <0.001 |
| SCr after 6 months | 1.44 (1.17–1.80) | 1.32 (1.15–1.60) | 1.61 (1.19–2.08) | <0.001 |
| SCr after 9 months | 1.41 (1.12–1.78) | 1.35 (1.07–1.56) | 1.49 (1.18–1.98) | 0.003 |
| SCr after 12 months | 1.38 (1.12–1.70) | 1.30 (1.06–1.57) | 1.46 (1.18–2.01) | 0.008 |
| N° of hospital re-admissions | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.789 |
| SARS-CoV-2 cases | 22 (9.0) | 0 (0.0) | 22 (17.9) | <0.001 |
| Variables | NON-COVID KTR (N = 204) | COVID KTR (N = 41) | p |
|---|---|---|---|
| Recipient sex (male) | 108 (52.9) | 31 (75.6) | 0.009 |
| Recipient age (years) | 52 (43–59) | 52 (32–61) | 0.469 |
| Recipient ethnicity: | |||
| Caucasian | 185 (90.7) | 35 (85.4) | 0.393 |
| Afro-Caribbean | 6 (2.9) | 0 (0.0) | 0.593 |
| Other | 13 (6.4) | 6 (14.6) | 0.102 |
| Renal replacement therapy | 193 (94.6) | 37 (90.2) | 0.288 |
| Haemodialysis | 157/193 (81.3) | 32/37 (86.5) | 0.639 |
| Dialysis vintage (months) | 35 (15–54) | 44 (21–72) | 0.234 |
| Primary kidney disease: | |||
| Primary or secondary glomerulonephritis | 90 (44.1) | 24 (58.5) | 0.122 |
| Diabetic nephropathy | 8 (3.9) | 0 (0.0) | 0.359 |
| Polycystic kidney disease | 38 (18.6) | 3 (7.3) | 0.106 |
| Hypertensive nephrosclerosis | 14 (6.9) | 3 (7.3) | 1.000 |
| Tubulo-interstitial disease | 8 (3.9) | 1 (2.4) | 1.000 |
| Genetic or congenital kidney disease | 20 (9.8) | 7 (17.1) | 0.178 |
| Uropathy | 26 (12.7) | 4 (9.8) | 0.795 |
| Thrombotic microangiopathy | 16 (7.8) | 1 (2.4) | 0.320 |
| Other | 3 (1.5) | 0 (0.0) | 1.000 |
| Pre-existing conditions: | |||
| Arterial hypertension | 178 (87.3) | 33 (80.5) | 0.320 |
| Diabetes mellitus | 20 (9.8) | 4 (9.8) | 1.000 |
| Chronic obstructive pulmonary disease | 32 (15.7) | 2 (4.9) | 0.083 |
| Coronary artery disease | 23 (11.3) | 6 (14.6) | 0.596 |
| Obesity (BMI ≥ 30 kg/m2) | 15 (7.4) | 2 (4.9) | 0.745 |
| CMV IgG positivity | 173 (84.8) | 36 (87.8) | 0.810 |
| EBV IgG positivity | 180 (88.2) | 32 (78.0) | 0.128 |
| HSV IgG positivity | 166 (81.4) | 30 (73.2) | 0.283 |
| VZV IgG positivity | 198 (97.1) | 39 (95.1) | 0.624 |
| HBV viremia | 3 (1.5) | 2 (4.9) | 0.196 |
| HCV viremia | 2 (1.0) | 1 (2.4) | 0.424 |
| Previous kidney transplantation | 46 (22.5) | 10 (24.4) | 0.839 |
| Last PRA (%) | 0 (0–0) | 0 (0–0) | 0.411 |
| Baseline DSA | 0 (0–0) | 0 (0–0) | 0.778 |
| HLA mismatch | 4 (3–5) | 4 (3–5) | 0.560 |
| Length of hospitalisation | 14 (10–21) | 13 (9–23) | 0.644 |
| ICU admission ICU stay (days) | 46 (22.5) 1 (1–1) | 11 (26.8) 1 (1–3) | 0.548 0.332 |
| Induction immunosuppression: | |||
| Anti-IL2R monoclonal antibodies | 108 (52.9) | 20 (48.8) | 0.732 |
| rATG | 112 (54.9) | 21 (51.2) | 0.732 |
| Methylprednisolone | 203 (99.5) | 41 (100) | 1.000 |
| Anti-CD20 monoclonal antibodies | 10 (4.9) | 3 (7.3) | 0.461 |
| Anti-C5 monoclonal antibodies | 25 (12.3) | 2 (4.9) | 0.272 |
| Plasma exchange | 19 (9.3) | 6 (14.6) | 0.393 |
| Polyclonal human immunoglobulins | 15 (7.4) | 4 (9.8) | 0.534 |
| Maintenance immunosuppression: | |||
| Tacrolimus | 201 (98.5) | 41 (100) | 1.000 |
| MMF/MPA | 194 (95.1) | 41 (100) | 0.221 |
| Prednisone | 196 (96.1) | 41 (100) | 0.359 |
| Tacrolimus trough levels (ng/mL): | |||
| After 1 month | 9.1 (7.7–11.2) | 9.2 (7.3–13.4) | 0.768 |
| After 3 months | 8.3 (7.0–9.8) | 9.2 (8.1–11.7) | 0.007 |
| After 6 months | 8.3 (6.8–10.0) | 8.7 (7.8–10.1) | 0.359 |
| After 9 months | 7.9 (6.7–9.4) | 8.1 (6.1–9.5) | 0.636 |
| After 12 months | 7.8 (6.5–9.2) | 7.4 (6.5–9.9) | 0.997 |
| Follow-up (months) | 20.7 (8.7–36.3) | 19.6 (11.3–30.5) | 0.920 |
| Patient | Months from KT | Hospital Admission | Symptoms | Treatment | Recipient Outcome | Allograft Outcome | |||
|---|---|---|---|---|---|---|---|---|---|
| CNI ↓ | MMF ↓ | MP ↑ | Other | ||||||
| 1 | 32 | Yes | Pneumonia + RF | Yes | Yes | Yes | Abx, O2 | Alive | Stable |
| 2 | 41 | Yes | FLS + RF | No | Yes | Yes | Remdesivir, O2 | Alive | Stable |
| 3 | 19 | No | Fever | No | Yes | No | Abx | Alive | Stable |
| 4 | 17 | No | Fever | No | No | No | - | Alive | Impaired |
| 5 | 15 | No | Fever | No | Yes | No | - | Alive | Stable |
| 6 | 21 | No | FLS | No | Yes | No | - | Alive | Stable |
| 7 | 15 | Yes | RF + AKI + A/A + PE | Yes | Yes | Yes | Mo-Ab, O2 | Deceased | - |
| 8 | 22 | Yes | Pneumonia + AKI + RF | Yes | No | No | - | Deceased | - |
| 9 | 24 | No | Asymptomatic | No | Yes | No | - | Alive | Stable |
| 10 | 22 | Yes | FLS + AKI | No | Yes | Yes | - | Alive | Stable |
| 11 | 10 | Yes | Pneumonia | No | Yes | Yes | Abx, HCQ | Alive | Stable |
| 12 | 10 | No | Asymptomatic | No | No | No | - | Alive | Impaired |
| 13 | 29 | No | FLS | No | Yes | Yes | - | Alive | Stable |
| 14 | 29 | No | Para-flu syndrome | No | Yes | Yes | - | Alive | Stable |
| 15 | 11 | No | Asymptomatic | Yes | No | No | - | Alive | Impaired |
| 16 | 27 | No | FLS | No | No | Yes | Mo-Ab, Abx | Alive | Stable |
| 17 | 5 | No | FLS | No | Yes | No | Abx, HCQ | Alive | Stable |
| 18 | 25 | No | FLS | No | Yes | Yes | - | Alive | Stable |
| 19 | 10 | Yes | Pneumonia + RF | Yes | Yes | Yes | - | Deceased | - |
| 20 | 10 | Yes | Pneumonia | No | No | Yes | O2 | Alive | Impaired |
| 21 | 8 | No | Asymptomatic | No | Yes | No | - | Alive | Stable |
| 22 | 19 | No | FLS | No | Yes | No | - | Alive | Stable |
| 23 | 18 | No | Para-flu syndrome + A/A | No | Yes | Yes | - | Alive | Stable |
| 24 | 5 | Yes | Pneumonia | No | Yes | Yes | - | Alive | Impaired |
| 25 | 16 | No | FLS + A/A | No | Yes | No | - | Alive | Stable |
| 26 | 7 | No | FLS | No | Yes | Yes | - | Alive | Stable |
| 27 | 2 | No | Asymptomatic | No | Yes | No | - | Alive | Stable |
| 28 | 10 | No | Asymptomatic | No | Yes | No | Abx | Alive | Stable |
| 29 | 1 | Yes | FLS + AKI | Yes | Yes | No | - | Alive | Impaired |
| 30 | 15 | No | FLS | No | Yes | No | Mo-Ab | Alive | Stable |
| 31 | 1 | Yes | Asymptomatic | No | Yes | No | - | Alive | Stable |
| 32 | 1 | Yes | Pneumonia + AKI+ RF | No | Yes | No | O2 | Alive | Stable |
| 33 | 11 | No | FLS | No | Yes | Yes | - | Alive | Impaired |
| 34 | 10 | Yes | FLS | No | Yes | Yes | - | Alive | Stable |
| 35 | 1 | No | FLS | No | Yes | Yes | - | Alive | Stable |
| 36 | 8 | No | FLS | No | Yes | Yes | - | Alive | Stable |
| 37 | 6 | No | FLS | No | Yes | Yes | - | Alive | Stable |
| 38 | 7 | Yes | Pneumonia + A/A | No | Yes | Yes | O2, LMWH | Alive | Stable |
| 39 | 5 | Yes | FLS + AKI + A/A | No | Yes | No | - | Alive | Impaired |
| 40 | 1 | No | Para-flu syndrome | No | Yes | Yes | - | Alive | Stable |
| 41 | 1 | No | Asymptomatic | No | Yes | Yes | - | Alive | Stable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perego, M.; Iesari, S.; Gandolfo, M.T.; Alfieri, C.; Delbue, S.; Cacciola, R.; Ferraresso, M.; Favi, E. Outcomes of Patients Receiving a Kidney Transplant or Remaining on the Transplant Waiting List at the Epicentre of the COVID-19 Pandemic in Europe: An Observational Comparative Study. Pathogens 2022, 11, 1144. https://doi.org/10.3390/pathogens11101144
Perego M, Iesari S, Gandolfo MT, Alfieri C, Delbue S, Cacciola R, Ferraresso M, Favi E. Outcomes of Patients Receiving a Kidney Transplant or Remaining on the Transplant Waiting List at the Epicentre of the COVID-19 Pandemic in Europe: An Observational Comparative Study. Pathogens. 2022; 11(10):1144. https://doi.org/10.3390/pathogens11101144
Chicago/Turabian StylePerego, Marta, Samuele Iesari, Maria Teresa Gandolfo, Carlo Alfieri, Serena Delbue, Roberto Cacciola, Mariano Ferraresso, and Evaldo Favi. 2022. "Outcomes of Patients Receiving a Kidney Transplant or Remaining on the Transplant Waiting List at the Epicentre of the COVID-19 Pandemic in Europe: An Observational Comparative Study" Pathogens 11, no. 10: 1144. https://doi.org/10.3390/pathogens11101144
APA StylePerego, M., Iesari, S., Gandolfo, M. T., Alfieri, C., Delbue, S., Cacciola, R., Ferraresso, M., & Favi, E. (2022). Outcomes of Patients Receiving a Kidney Transplant or Remaining on the Transplant Waiting List at the Epicentre of the COVID-19 Pandemic in Europe: An Observational Comparative Study. Pathogens, 11(10), 1144. https://doi.org/10.3390/pathogens11101144







